Abstract
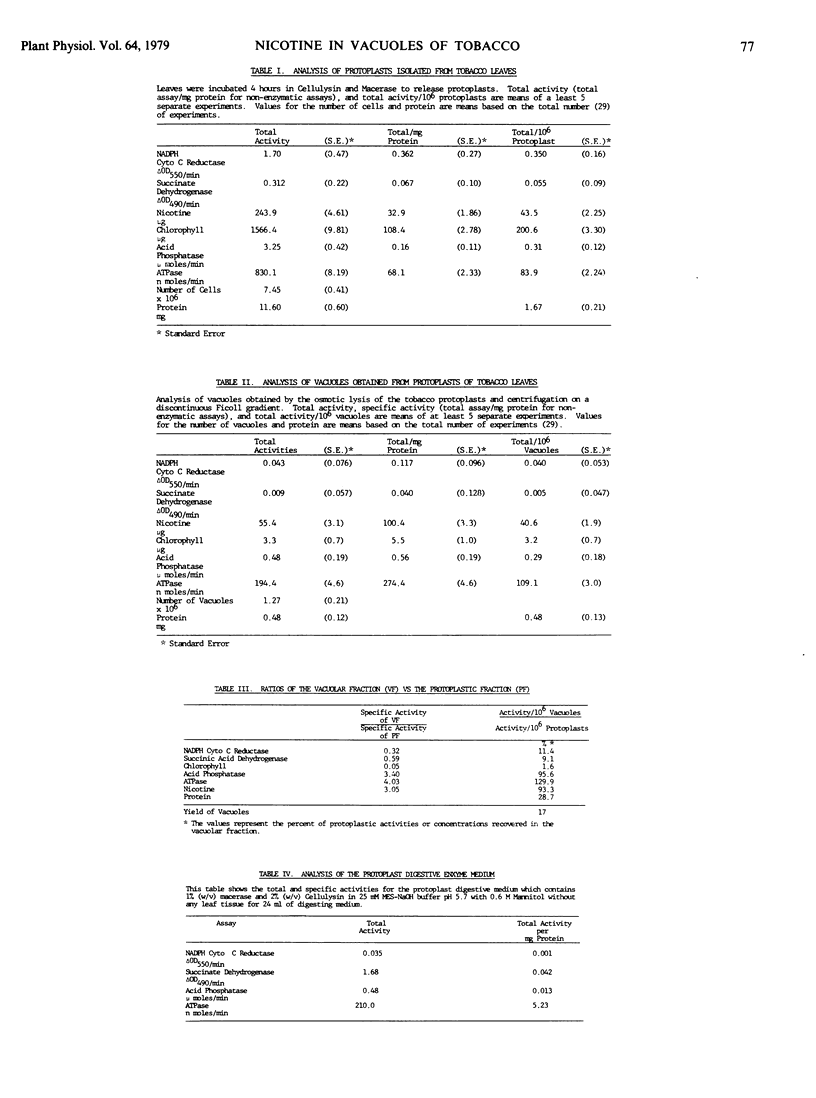
Nicotine was shown to be associated with mature vacuoles isolated from protoplasts of Nicotiana rustica. The vacuolar preparations also contained high levels of acid phosphatase, ATPase, and approximately 30% of the soluble protoplastic protein. The contamination of the vacuolar isolate by chlorophyll, succinate dehydrogenase, and NADPH cytochrome c reductase (markers for chloroplasts, mitochondria, and endoplasmic reticulum) was low. The enzymic activity associated with the vacuoles was not due to the exogenously supplied digestive enzymes used in the preparation of the protoplast. The relatively easy isolation of tobacco vacuoles makes this an excellent system for biochemical investigations of the vacuole.
Full text
PDF
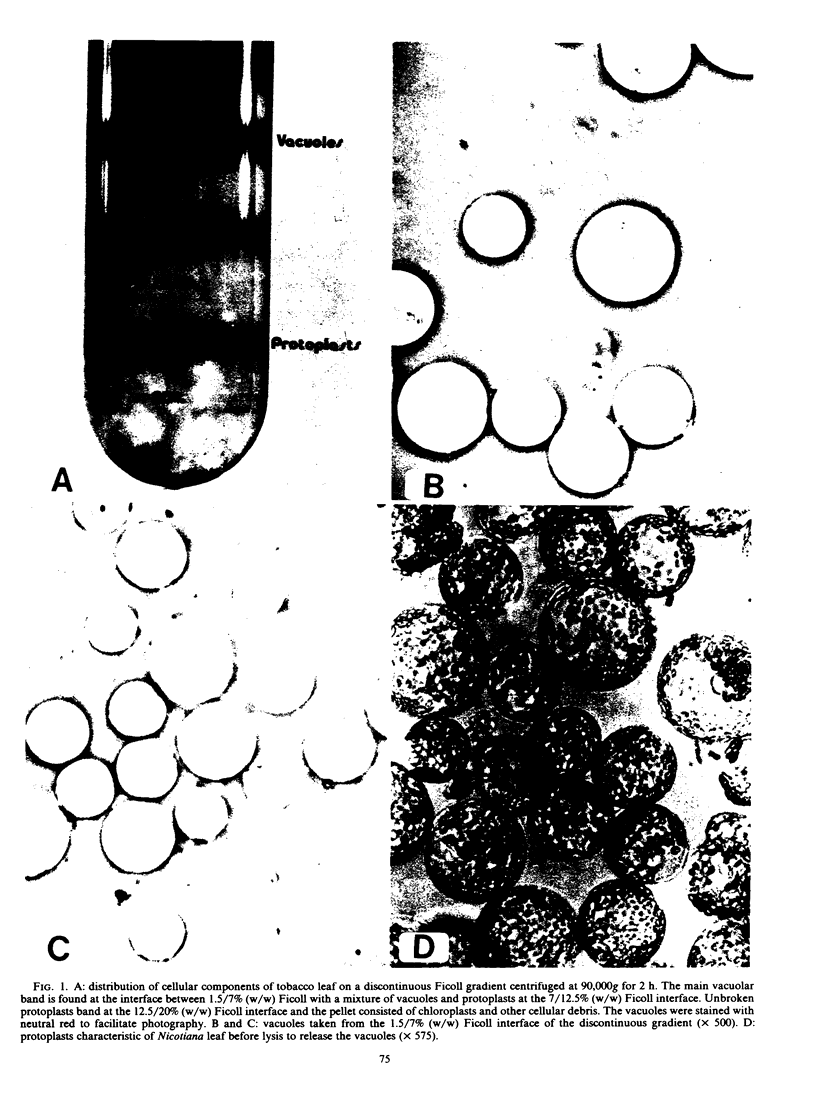


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butcher H. C., Wagner G. J., Siegelman H. W. Localization of Acid hydrolases in protoplasts: examination of the proposed lysosomal function of the mature vacuole. Plant Physiol. 1977 Jun;59(6):1098–1103. doi: 10.1104/pp.59.6.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Müller H., Holzer H. Compartmentation of the tryptophan-synthase-proteolyzing system in Saccharomyces cerevisiae. Eur J Biochem. 1974 Oct 1;48(1):117–117. doi: 10.1111/j.1432-1033.1974.tb03748.x. [DOI] [PubMed] [Google Scholar]
- Leigh R. A., Branton D. Isolation of Vacuoles from Root Storage Tissue of Beta vulgaris L. Plant Physiol. 1976 Nov;58(5):656–662. doi: 10.1104/pp.58.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Wagner G. J., Siegelman H. W., Hind G. Membrane-bound ATPase of intact vacuoles and tonoplasts isolated from mature plant tissue. Biochim Biophys Acta. 1977 Feb 14;465(1):110–117. doi: 10.1016/0005-2736(77)90359-5. [DOI] [PubMed] [Google Scholar]
- Matern H., Betz H., Holzer H. Compartmentation of inhibitors of proteinases A and B and carboxypeptidase Y in yeast. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1051–1057. doi: 10.1016/0006-291x(74)90419-7. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A., Conn E. E., Lin C. H., Shimada M. Localization of Cinnamic Acid 4-Monooxygenase and the Membrane-bound Enzyme System for Dhurrin Biosynthesis in Sorghum Seedlings. Plant Physiol. 1977 Oct;60(4):629–634. doi: 10.1104/pp.60.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. A., Conn E. E. Presence of the cyanogenic glucoside dhurrin in isolated vacuoles from sorghum. Plant Physiol. 1978 Feb;61(2):154–157. doi: 10.1104/pp.61.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M., Ryan C. A. Immunological Identification of Proteinase Inhibitors I and II in Isolated Tomato Leaf Vacuoles. Plant Physiol. 1977 Jul;60(1):61–63. doi: 10.1104/pp.60.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemken A. Isolation of vacuoles from yeasts. Methods Cell Biol. 1975;12:99–109. doi: 10.1016/s0091-679x(08)60954-1. [DOI] [PubMed] [Google Scholar]