Abstract
[Purpose] The aim of this double-blind, randomized and placebo-controlled study is to investigate the effects of Transcutaneous Electrical Nerve Stimulation for reducing the side effects of Chemotherapy-induced Peripheral Neuropathy in cancer patients undergoing chemotherapy with oxaloplatin or paclitaxel. [Subjects and Methods] Twenty-four patients were randomly allocated into two groups: active or placebo stimulation. All patients were assessed for pain, numbness/tingiling, frequency of symptoms, and quality of life. The transcutaneous Electrical Nerve Stimulation device was applied daily with modulating frequencies ranging between 7 Hz and 65 Hz in distal limb regions during three cycles of chemotherapy (45 days). The other stimulation parameters were: pulse duration of 200 μsec, intensity at the highest tolerable level, and increases in intensity when it diminished. [Results] The data showed no difference between active or placebo groups in terms of pain, numbness/tingling, frequency of symptoms or impact on daily life activities. [Conclusion] These results suggest that Transcutaneous Electrical Nerve Stimulation applied in the frequency variation mode was not proven to be effective to improve the symptoms of Chemotherapy-induced Peripheral Neuropathy during chemotherapy cycles. There was no worsening of symptoms in subsequent cycles of the onset of symptoms of the disease.
Keywords: Transcutaneous electric nerve stimulation, Chemotherapy-induced peripheral neuropathy, Physical therapy
INTRODUCTION
The use of chemotherapy drugs is the basis for the systemic treatment of cancer1). While these drugs kill tumor cells, they can also cause adverse effects during their use, such as nausea, vomiting, diarrhea, constipation, alopecia, fatigue, skin alterations, and neurotoxicity, among others.
Chemotherapy-induced peripheral neuropathy (CIPN) is a nuisance and a limiting side effect for patients, since the symptoms of pain, dysesthesia, and paresthesia generated by chemotherapy can mean a reduction in the functionality and quality of life for cancer patients. Moreover, these side effects may compromise the treatment of the disease and lead to reduced doses of drugs or treatment discontinuation2, 3).
The current recommendations of the American Society of Clinical Oncology (ASCO)4) point out that there are not yet any strongly recommended drugs available to prevent CIPN, given the lack of consistent and high-quality research on the issue. Chu’s5) review about the effects of antidepressant drugs and anticonvulsants for the control of CIPN confirm ASCO’s data. Therefore, the treatment of these disorders could ideally be done without the use of drugs by applying non-pharmacological treatments, which would have the advantage of not compromising the different body systems already burdened by chemotherapy.
Transcutaneous Electrical Nerve Stimulation (TENS) has arisen as an alternative to drug treatment for the control of peripheral neuropathies. The rationale for the use of TENS is based on studies showing that electrical stimulation has shown a positive effect on rat’s nerve regeneration, revealing a higher number of myelinated fibers, axon density, and blood vessels in the total nerve area compared to control groups6). When using Transcutaneous Electrical Stimulation in mice’s peripheral nerve injuries, the density, fiber diameter, and degree of myelination are also similar to animals that have not suffered injuries7).
A case-control study evaluated the effect of TENS on peripheral neuropathic pain induced by chemotherapy in patients with different types of cancer, pointing out beneficial effects in reducing pain during TENS application without generating side effects8). Silva9) used TENS in the treatment of intercostobrachial pain developed after breast cancer surgery to show that, in addition to pain relief, TENS produced changes in somatotopic organization in the parietal cortex after application, causing increased attention to painful processes. This increased attention would cause an inhibition in the transmission of painful stimuli.
Other symptoms of CIPN, such as paresthesia and decreased quality of life of these patients, have not been evaluated in previous controlled studies. Therefore, this study aims to evaluate the effects of TENS on symptoms of numbness and tingling and the frequency of symptoms and its impact on the daily life activities of patients with CIPN.
SUBJECTS AND METHODS
This two-arm clinical trial was randomized, placebo-controlled, and double-blind. It was conducted in cancer patients who developed symptoms of CIPN after the first chemotherapy cycle. The patients were recruited in Barretos Cancer Hospital, Institute of Cancer of the State of São Paulo, and São Francisco University Hospital. This study was approved by the Research Ethics Committee of the Faculty of Medicine, University of São Paulo, under protocol nº 341/13. It was registered at ClinicalTrials.gov before the research began, under registration number NTC 02107417. All participants were informed about the objectives of the study and signed a consent form to participate in the study.
The study included patients between the ages of 18 and 70 who met the following inclusion criteria: being subjected to chemotherapy treatment with drugs with a high to moderate degree of neurotoxicity, experiencing neuropathic pain and/or peripheral sensory neuropathy of grade I or grade II on the Common Terminology Criteria for Adverse Events (CTCAE) scale, exhibiting symptoms of peripheral neuropathy after the first cycles of chemotherapy with at least two points on the visual analog scale of paresthesia and/or pain, and having a present performance status of ECOG ≤2 (Karnofsky≥50%).
Exclusion criteria were: having sensitivity disorders before chemotherapy treatment, having skin lesions on the site of application of the electrodes, exhibiting cognitive difficulties or trouble understanding how to fill out questionnaires, or being cardiac pacemaker users or diabetes mellitus patients.
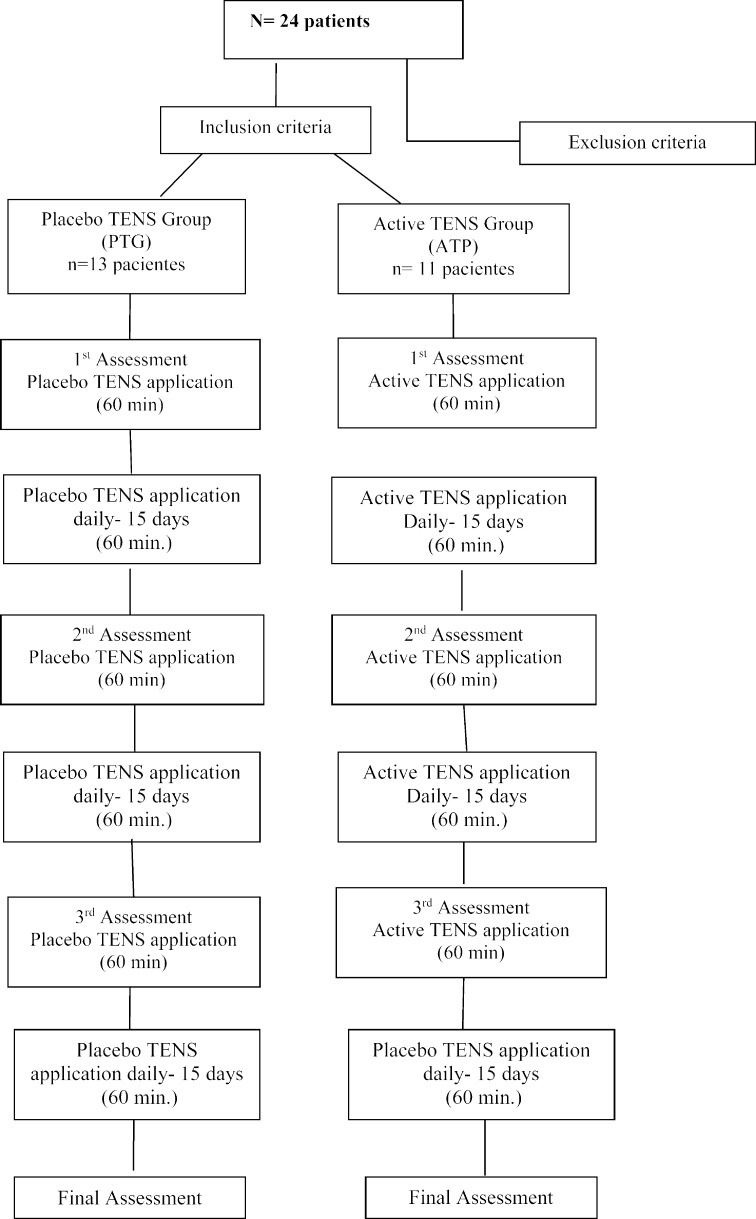
The study sample consisted of 24 patients with diagnoses of breast and colorectal cancer undergoing a chemotherapy treatment containing in its protocol the chemotherapeutic agents paclitaxel or oxaliplatin. Patients were divided into 2 groups: active TENS (GAT) and placebo TENS (GPT). Patients underwent TENS application for 45 days, daily. The flowchart of the research is shown in Fig. 1.
Fig. 1.
The flow diagram of the research
The entry of patients in each experimental group was performed randomly by the site www.random.org before the start of treatment. Patients were subjected to the following TENS (Ibramed, Neurodyn Portátil) parameters: symmetrical biphasic waveform with modulating frequencies ranging from 7 Hz to 65 Hz, a pulse width of 200 µs at the highest tolerable current intensity with an application time of 60 minutes. The stimulation was performed with individual self-adhesive electrodes with an area of 5 cm2 in the region where the patient had symptoms of neuropathy, typically, the ends of the upper limbs and/or lower limbs. In the application of active TENS, the patient was instructed to increase the stimulation intensity each time the intensity reduced during the 60 minutes of application10). In TENS placebo application, patients received the same schedule of active TENS, but the equipment was produced to apply the current for only 30 seconds and then the intensity began to decrease during the next 15 seconds until reaching zero11).
The evaluations were performed by a blinded evaluator on the experimental groups.
We used the following instruments for data collection:
-Sociodemographic and clinical data form to characterize the sample profile with the following data: gender, age, race, education, type of cancer, occupational status and disease status. TNM Rating (T, refers to the size and extent of the main tumor; N refers to the number of nearby lymph nodes that have cancer; M refers to metastasis), treatments conducted (surgical, radiotherapy, hormonal, chemotherapy, other types of medication, acupuncture) and affected segment by neuropathy (upper limb and/or lower limb) were also used to create the profiles.
-Common Terminology Criteria for Adverse Events−CTCAE version 4.02 of 20090. Items of the questionnaire used to assess the symptoms of CIPN were paresthesia (grade I, II, and III), peripheral sensory neuropathy (I, II, III, IV, and V), and dysesthesia (grade I, II, and II).
-Visual analog scale for pain and paresthesia assessment (VAS).
-Performance Status scale ECOG (Eastern Cooperative Oncology Group) to assess the functional capacity of oncologic patients, with the following scores and activity levels: 0) Completely active; able to perform all their activities without restriction. (Karnofsky 90–100%); 1) Restriction of rigorous physical activities, but able to perform light work and of a sedentary nature (Karnofsky 70–80%); 2) Able to perform all self-care, but unable to carry out any work activity but able to remain standing approximately 50% of waking hours (Karnofsky 60–50%); 3) Capable of only limited self-care, confined to bed or chair more than 50% of waking hours Karnofsky 30–40%); 4) Completely unable to perform self-care, totally confined to bed (Karnofsky 10–20%)12).
-Chemotherapy Induced Neurotoxicity Questionnaire (CINQ) for the evaluation of symptoms of pain and dysesthesia and paresthesia of upper and lower limbs. CINQ is an instrument containing 20 items to evaluate the symptoms divided into 2 categories: symptoms of acute and chronic neuropathy in the lower extremities (10 items) and symptoms of acute and chronic neuropathy in upper extremities (10 items). This questionnaire evaluates the frequency of symptoms (CINQa) and the symptoms’ interference with patients’ daily activities (CINQb).
Questionnaires were administered before the treatment (Assessment 1: Ax1) and after 15 (Ax2), 30 (Ax3), and 45 (Ax4) days of treatment.
The data were presented using measures of central tendency (mean and median) and dispersion (standard deviation) for quantitative variables and relative percentages for categorical variables. Pearson’s χ2 test was used to verify the association between the categories of the study and, when necessary, resorted to Fisher’s exact test. Comparisons of the VAS scores between groups were performed using the nonparametric statistical Mann-Whitney U test and comparison of VAS along with the assessments by Friedman ANOVA. The Q-Cochran test was used to determine differences in the CINQ responses among the evaluations (Ax1, Ax2, Ax3 and Ax4). The comparison of the sums of scores of CINQ questions among the assessments was done using hierarchical linear models adjusted for restricted maximum likelihood. The statistical tests were performed at a significance level of 0.05. The data analyses were performed in R program version 3.1.1 for Windows.
RESULTS
The mean age in PTG was 46.3 ± 13.7 and 52.7 ± 9 in ATG, without significative differences between them. Assessing the functional status of the active TENS group patients, we found that 10 patients were between 0 and 1 ECOG degrees, with no or some functional limitations, while only 1 had more marked limitations related to the inability to work. In the placebo TENS group, 8 patients had ECOG 0–1 and 5 ECOG 2.
The sample characteristics are summarized in Table 1. The experimental groups showed no significant differences with regard to gender, marital status, race, education, employment status, or reductions in their occupational activity due to the treatment.
Table 1. Patient characteristics.
| Placebo TENS (n=13) | Active TENS (n=11) | Total (n=24) | ||||
|---|---|---|---|---|---|---|
| % | N | N | % | N | % | |
| Gender | ||||||
| Female | 2 | 15.4 | 3 | 27.3 | 5 | 20.8 |
| Male | 11 | 84.6 | 8 | 72.7 | 19 | 79.2 |
| Marital status | ||||||
| Single | 4 | 30.8 | 0 | 0.0 | 4 | 16.7 |
| Married | 8 | 61.5 | 10 | 90.9 | 18 | 75.0 |
| Divorced | 1 | 7.7 | 1 | 9.1 | 2 | 8.3 |
| Widowed | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Race/ethnicity | ||||||
| White | 6 | 46.2 | 9 | 81.8 | 15 | 62.5 |
| Asiatic | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Black | 3 | 23.1 | 0 | 0.0 | 3 | 12.5 |
| Admixture | 4 | 30.7 | 2 | 18.2 | 6 | 25.0 |
| Education | ||||||
| Elementary or less | 4 | 30.8 | 8 | 72.7 | 12 | 50.0 |
| Middle school | 6 | 46.2 | 1 | 9.1 | 7 | 29.2 |
| High school | 2 | 15.4 | 1 | 9.1 | 3 | 12.5 |
| College or higher | 1 | 7.6 | 1 | 9.1 | 2 | 8.3 |
| Works | ||||||
| Yes | 3 | 23.1 | 5 | 45.5 | 8 | 33.3 |
| No | 10 | 76.9 | 6 | 54.5 | 16 | 66.7 |
| Stopped working | ||||||
| Yes | 9 | 69.2 | 6 | 54.5 | 15 | 62.5 |
| No | 4 | 30.8 | 5 | 45.5 | 9 | 37.5 |
Fisher’s Exact test
Table 2 shows the disease characteristics among groups in relation to cancer type and disease stage. No significant differences were observed between the groups in terms of these characteristics (p>0.05).
Table 2. Distribution of characteristics of the type and cancer stage according treatment groups.
| TENS Placebo (n=13) | TENS Ativo (n=11) | Total (n=24) | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Type of tumor | ||||||
| Breast | 9 | 69.2 | 4 | 36.4 | 13 | 54.2 |
| Colorectal | 4 | 30.8 | 7 | 63.6 | 11 | 45.8 |
| T | ||||||
| T1 | 0 | 0.0 | 1 | 9.1 | 1 | 4.2 |
| T2 | 6 | 46.2 | 2 | 18.2 | 8 | 33.3 |
| T3 | 6 | 46.2 | 7 | 63.6 | 13 | 54.2 |
| T4 | 1 | 7.6 | 1 | 9.1 | 2 | 8.3 |
| N | ||||||
| N0 | 4 | 30.8 | 5 | 45.5 | 9 | 37.5 |
| N1 | 6 | 46.2 | 5 | 45.5 | 11 | 45.8 |
| N2 | 3 | 23.0 | 1 | 9.0 | 4 | 16.7 |
| N3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| M | ||||||
| M0 | 6 | 46.2 | 9 | 81.8 | 15 | 62.5 |
| M1 | 5 | 38.5 | 2 | 18.2 | 7 | 29.2 |
| M3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Mx | 2 | 15.3 | 0 | 0.0 | 2 | 8.3 |
χ2 test Pearson, *p>0.01
The data relating to the type of chemotherapy performed, different types of therapeutic treatments undertaken by the patient, and the region in which the patient developed neuropathy are presented in Table 3. No significant differences were observed in the distribution of the characteristics between the groups in any of the variables described (p>0.05).
Table 3. Distribution of the type of treatments performed by patients and the body region affected by CIPN between groups.
| Placebo TENS | Active TENS | Total | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Chemotherapy | ||||||
| Adjuvant | 10 | 76.9 | 7 | 63.6 | 17 | 70.8 |
| Neo-adjuvant | 2 | 15.4 | 4 | 36.4 | 6 | 25.0 |
| Paliative | 1 | 7.7 | 0 | 0.0 | 1 | 4.2 |
| Surgery | ||||||
| Yes | 11 | 84.6 | 7 | 63.6 | 18 | 75.0 |
| No | 2 | 15.4 | 4 | 36.4 | 6 | 25.0 |
| Radiotherapy | ||||||
| Yes | 2 | 15.4 | 2 | 18.2 | 4 | 16.7 |
| No | 11 | 84.6 | 9 | 81.8 | 20 | 83.3 |
| Hormone therapy | ||||||
| Yes | 2 | 15.4 | 2 | 18.2 | 4 | 16.7 |
| No | 11 | 84.6 | 9 | 81.8 | 20 | 83.3 |
| Immunotherapy | ||||||
| Yes | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| No | 13 | 100.0 | 11 | 100.0 | 24 | 100.0 |
| Acupuncture | ||||||
| Yes | 1 | 7.7 | 0 | 0.0 | 1 | 4.2 |
| No | 12 | 92.3 | 11 | 100.0 | 23 | 95.8 |
| Other medications | ||||||
| Yes | 7 | 53.8 | 7 | 63.6 | 14 | 58.3 |
| No | 6 | 46.2 | 4 | 36.4 | 10 | 41.7 |
| Involvement | ||||||
| MMSS | 9 | 69.2 | 7 | 63.6 | 16 | 66.7 |
| MMII | 4 | 30.8 | 4 | 36.4 | 8 | 33.3 |
Fisher’s Exact test, *p>0.05
Table 4 shows the behavior of CINQ scores over the application of TENS during the 45 treatment days for the placebo and active groups, indicating that there was no difference between groups during the treatment performed in any assessment carried out, both in terms of the frequency of symptoms (p=0.5906) and daily living activities (0.8565). Table 5 shows the comparison of VAS of pain and paresthesia intra- and inter-group during treatment with TENS in the experimental groups.
Table 4. Comparison of mean and standard deviation of the frequency of symptoms (CINQa) and interference of symptoms in patients’ daily activities (CINQb) during treatment with TENS in the experimental groups.
| Placebo TENS | Active TENS | Time group | ||
|---|---|---|---|---|
| CINQa | Ax 1 | 13.53 (9.29) | 12.18 (8.28) | 0.59* |
| Ax 2 | 11.06 (5.02) | 8.80 (4.87) | ||
| Ax 3 | 9.63 (8.46) | 8.67 (4.33) | ||
| Ax 4 | 6.87 (6.40) | 7.00 (4.31) | ||
| CINQb | Ax 1 | 9.61 (7.73) | 11.27 (8.66) | 0.85* |
| Ax 2 | 8.18 (6.61) | 7.59 (4.67) | ||
| Ax 3 | 8.46 (8.28) | 6.43 (4.19) | ||
| Ax 4 | 5.50 (5.93) | 7.00 (4.58) | ||
*p-value related to hierarchical linear mixed effect models. Ax- (Assessment)
Table 5. Comparison of VAS of pain and numbness/paresthesia intra- and inter-group during TENS treatment in experimental groups.
| Placebo TENS | Active TENS | |
|---|---|---|
| Median (IQR) | Median (IQR) | |
| PAIN VAS AX1 | 0.0 (0.0–0.0) | 0.0 (0.0–5.0) |
| PAIN VAS AX2 | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) |
| PAIN VAS AX3 | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) |
| PAIN VAS AX4 | 0.0 (0.0–2.0) | 0.0 (0.0–1.0) |
| p value | #0.69 | #0.632 |
| VAS NUMB/PARES Ax1 | 2.0 (2.0–6.5) | 4.0 (1.0–7.0) |
| VAS NUMB/PARES Ax2 | 5.0 (0.0–8.0) | 1.0 (1.0–3.0) |
| VAS NUMB/PARES Ax3 | 1.0 (0.0–5.0) | 5.0 (1.0–7.0) |
| VAS NUMB/PARES Ax4 | 1.5 (0.0–3.8) | 2.0 (1.0–2.0) |
| p value | #0.24 | #0.01 |
IQR interquartile range (25th. 75th percentile);
#p values from Mann-Whitney U test for comparison between assessment
*p values from Friedman test for comparison between groups
DISCUSSION
The results of this study show that the use of TENS with modulating frequencies between 7 Hz to 65 Hz indicated no significant difference in the symptoms of paresthesia and the impact of these symptoms on the daily activities among the groups who received applications of active or placebo TENS. These symptoms, however, do not increase during the three cycles subsequent to the onset of chemotherapy neuropathy symptoms.
Painful symptoms were not prevalent in our study. These data corroborate the study of Wolf13) and Pachman14), indicating that numbness and tingling are the most frequent symptoms of CIPN. The hands were more affected than the feet by the symptoms of neuropathy, which was corroborated by Zanville’s study15). Ewetrz’s review16) states that one of the assumptions for the development of CIPN in hands and feet relates to the fact that these neurons have long axons and are highly dependent on transport proteins and other nutrients to work correctly. Taxanes can lead to disruption of microtubules and mitochondria, leading to a deficit in cell nutrition and subsequent cell death. Platinum-based drugs can also contribute to cell death by inhibiting ADN synthesis.
The effects of TENS on nerve regeneration in rats show a positive effect of low frequency TENS in this process, presenting density, fiber diameter, and a degree of myelination of neurons similar to the uninjured rats of the control group7). It has been hypothesized that these results relate to the effect of TENS in the modulation of Ca2 + levels and of injured neurons through voltage-dependent channels after nerve injury, factors that would contribute to a decrease in the frequency of the firing of these neurons and stimulate production of neurotrophic factors (BDNF, NGF, and VEGF) and other transcription factors17). In our study, we use modulating frequencies ranging between 7 and 65 Hz. As there are no studies evaluating the symptoms of paresthesia and the impact of symptoms on daily activities, we could not compare the results of our study with others to verify if other TENS frequencies would produce better effects on the outcomes.
An interesting finding of our study is that there was no significant difference between the active TENS and TENS placebo groups in the studied variables. It was expected that the symptoms of paresthesia and pain would get worse during the chemotherapy cycles and this may not have happened due to the fact that patients expected to receive treatment for neuropathy symptoms resulting from chemotherapy. This effect was also observed in Rakel’s study11) for post-surgery pain due to total knee arthroplasty. Worsening neuropathy symptoms would be expected since the development of symptoms may occur after the first chemotherapy cycle, worsen after performing subsequent cycles, and even after the end of treatment18).
Pachman14, 19) points out that the development of neuropathy after the first treatment cycle with oxaloplatin is a risk factor for the worsening of these symptoms in subsequent cycles. Patients treated with paclitaxel also have worsening neuropathy symptoms over the course of chemotherapy cycles20). The fact that the patients in this study have not presented worsening symptoms may show a TENS placebo effect on CIPN. Studies show that TENS placebo operates effectively in modulating pain11, 21). As the current in placebo TENS is applied in the same way as in active stimulation, but for a period of 30 seconds and then decreasing for the next 15 seconds until reaching zero intensity, the fact that the stimulation performed with placebo TENS mimics the application of the active TENS may have contributed to this placebo effect. The application of TENS placebo thus carried out is important since many patients have heard that TENS produces a feeling of a “small electric shock” and the intensity decreases as the application time goes on. Some studies, conducting the placebo TENS without electrostimulation (patient doesn’t feel any sensation), show a significant difference between the group of active TENS and the placebo stimulation group in the studied parameters, in favor of the active TENS22, 23). Other studies should be performed with high or low frequency TENS to verify these effects.
Clinical studies show an efficiency of application of transcutaneous electrical stimulation on the quality of life of patients with CIPN24, 25); however, these studies were not placebo-controlled, not allowing for the analysis of the placebo effect on quality of life. Our study found no improvement in the impact of CIPN in the daily activities of patients between the active TENS groups and placebo group, suggesting that the placebo effect may also have contributed to the fact that there was no impairment in the performance of daily life activities of these patients over the course cycles of chemotherapy.
Our study has some limitations, such as not accompanying the patients throughout the chemotherapeutic treatment and not evaluating the effect of TENS on other types of cancer and neurotoxic drugs.
The application of TENS with modulating frequencies between 7 Hz to 65 Hz in patients with breast and colorectal cancer showed no significant difference in terms of the symptoms of paresthesia and the impact on daily life activities between the groups that received application of active TENS and those that received a placebo for chemotherapy treatments with oxaloplatin and paclitaxol. There was no worsening of symptoms in subsequent cycles of the onset of CIPN symptoms.
Acknowledgments
We would like to thank FAPESP (São Paulo Research Foundation, Process No. 13/13619-9) for supporting this project.
REFERENCES
- 1.Costa TC, Lopes M, Anjos AC, et al. : [Chemotherapy-induced peripheral neuropathies: an integrative review of the literature]. Rev Esc Enferm USP, 2015, 49: 335–345. [DOI] [PubMed] [Google Scholar]
- 2.Stubblefield MD, Burstein HJ, Burton AW, et al. : NCCN task force report: management of neuropathy in cancer. J Natl Compr Canc Netw, 2009, 7: S1–S26, quiz S27–S28. [DOI] [PubMed] [Google Scholar]
- 3.Addington J, Freimer M: Chemotherapy-induced peripheral neuropathy: an update on the current understanding. F1000 Res, 2016, 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hershman DL, Lacchetti C, Dworkin RH, et al. American Society of Clinical Oncology: Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol, 2014, 32: 1941–1967. [DOI] [PubMed] [Google Scholar]
- 5.Chu SH, Lee YJ, Lee ES, et al. : Current use of drugs affecting the central nervous system for chemotherapy-induced peripheral neuropathy in cancer patients: a systematic review. Support Care Cancer, 2015, 23: 513–524. [DOI] [PubMed] [Google Scholar]
- 6.Lu MC, Ho CY, Hsu SF, et al. : Effects of electrical stimulation at different frequencies on regeneration of transected peripheral nerve. Neurorehabil Neural Repair, 2008, 22: 367–373. [DOI] [PubMed] [Google Scholar]
- 7.Cavalcante Miranda de Assis D, Martins Lima Ê, Teixeira Goes B, et al. : The parameters of transcutaneous electrical nerve stimulation are critical to its regenerative effects when applied just after a sciatic crush lesion in mice. BioMed Res Int, 2014, 2014: 572949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith TJ, Coyne PJ, Parker GL, et al. : Pilot trial of a patient-specific cutaneous electrostimulation device (MC5-A Calmare®) for chemotherapy-induced peripheral neuropathy. J Pain Symptom Manage, 2010, 40: 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva JG, Santana CG, Inocêncio KR, et al. : Electrocortical analysis of patients with intercostobrachial pain treated with TENS after breast cancer surgery. J Phys Ther Sci, 2014, 26: 349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sluka KA, Bjordal JM, Marchand S, et al. : What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Phys Ther, 2013, 93: 1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakel BA, Zimmerman MB, Geasland K, et al. : Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: a randomized, blinded, placebo-controlled trial. Pain, 2014, 155: 2599–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Instituto Nacional de Câncer –I NCA: http://bvsms.saude.gov.br/bvs/publicacoes/inca/acoes._cap6.pdf 9 (Accessed May 13, 2014)
- 13.Wolf SL, Barton DL, Qin R, et al. : The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapy-induced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20 instrument, N06CA. Support Care Cancer, 2012, 20: 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pachman DR, Qin R, Seisler D, et al. : Comparison of oxaliplatin and paclitaxel-induced neuropathy (Alliance A151505). Support Care Cancer, 2016, (Aug): 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanville NR, Nudelman KN, Smith DJ, et al. : Evaluating the impact of chemotherapy-induced peripheral neuropathy symptoms (CIPN-sx) on perceived ability to work in breast cancer survivors during the first year post-treatment. Support Care Cancer, 2016, 24: 4779–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ewertz M, Qvortrup C, Eckhoff L: Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol, 2015, 54: 587–591. [DOI] [PubMed] [Google Scholar]
- 17.Gordon T: Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics, 2016, 13: 295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simão DA, Murad M, Martins C, et al. : Chemotherapy-induced peripheral neuropathy: review for clinical practice. Rev Dor, 2015, 6: 215–220. [Google Scholar]
- 19.Pachman DR, Qin R, Seisler DK, et al. : Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III Trial N08CB (Alliance). J Clin Oncol, 2015, 33: 3416–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein D, Von Hoff DD, Moore M, et al. : Development of peripheral neuropathy and its association with survival during treatment with nab-paclitaxel plus gemcitabine for patients with metastatic adenocarcinoma of the pancreas: a subset analysis from a randomised phase III trial (MPACT). Eur J Cancer, 2016, 52: 85–91. [DOI] [PubMed] [Google Scholar]
- 21.Vance CG, Rakel BA, Blodgett NP, et al. : Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: a randomized controlled trial. Phys Ther, 2012, 92: 898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eidy M, Fazel MR, Janzamini M, et al. : Preemptive analgesic effects of Transcutaneous Electrical Nerve Stimulation (TENS) on postoperative pain: a randomized, double-blind, placebo-controlled trial. Iran Red Crescent Med J, 2016, 18: e35050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Öncü E, Zincir H: The effect of transcutaneous electrical nerve stimulation in patients with acute exacerbation of chronic obstructive pulmonary disease: randomized controlled trial. J Clin Nurs, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Coyne PJ, Wan W, Dodson P, et al. : A trial of Scrambler therapy in the treatment of cancer pain syndromes and chronic chemotherapy-induced peripheral neuropathy. J Pain Palliat Care Pharmacother, 2013, 27: 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pachman DR, Weisbrod BL, Seisler DK, et al. : Pilot evaluation of Scrambler therapy for the treatment of chemotherapy-induced peripheral neuropathy. Support Care Cancer, 2015, 23: 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]