Abstract
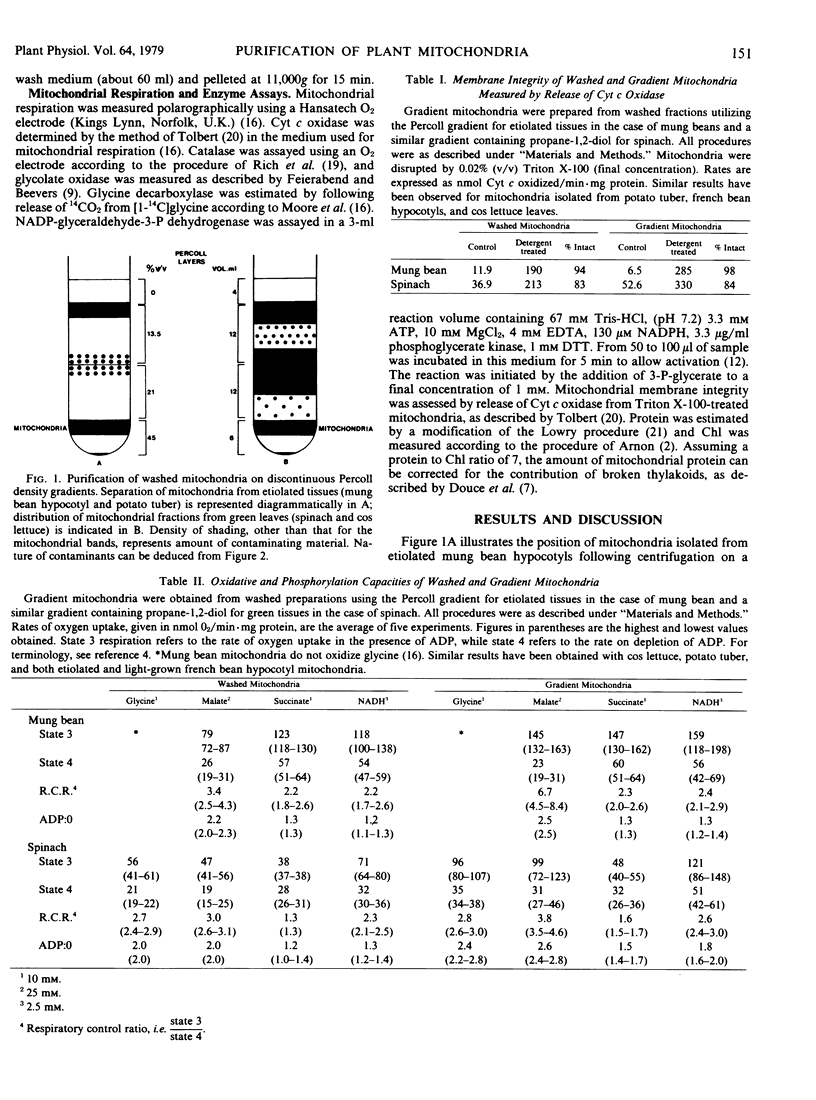
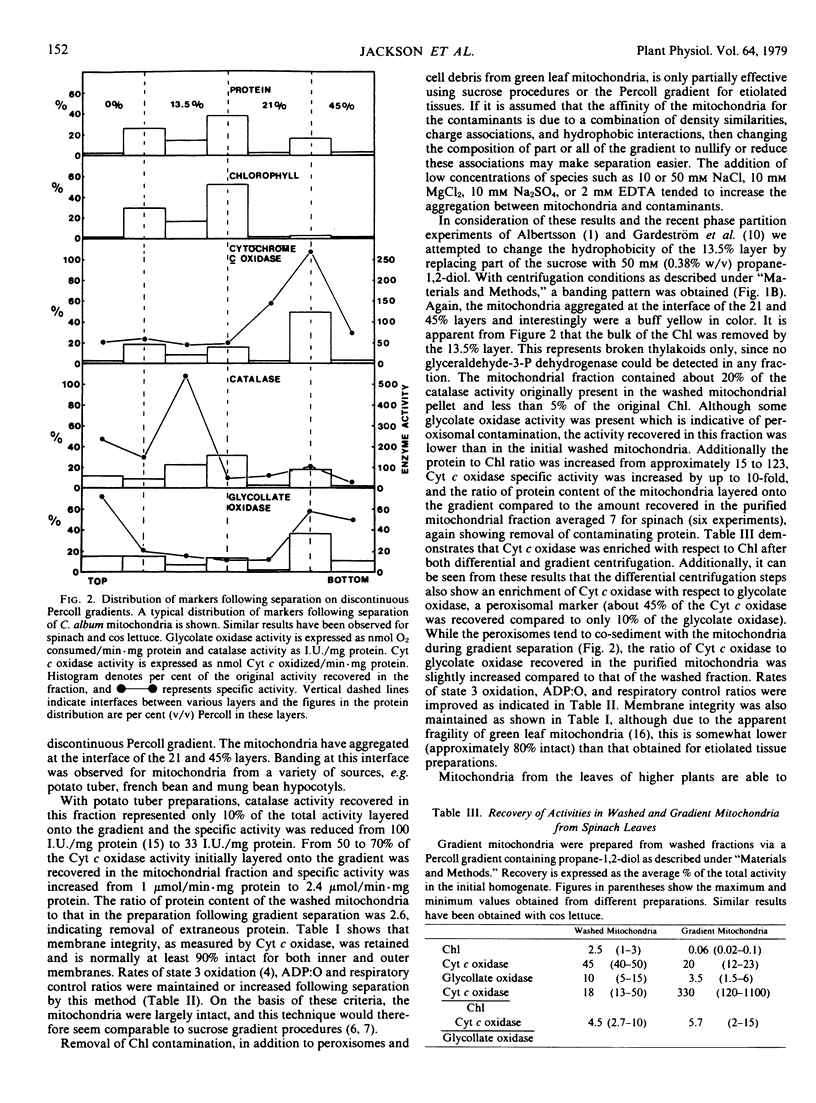
Discontinuous Percoll density gradients have been developed for the purification of mitochondria, permitting rapid separation under isosmotic and low viscosity conditions. Mitochondria from several etiolated tissues have been successfully separated from contaminating subcellular structures by this method. For potato tuber the ratio of washed to purified mitochondrial protein was 2.6, similar to the increase in specific activity of cytochrome c oxidase following separation. The purification of mitochondria from green leaf tissues on Percoll gradients has reduced chlorophyll contamination of spinach mitochondria from about 70 micrograms chlorophyll per milligram protein to approximately 8 micrograms chlorophyll per milligram protein.
The ratio of protein content of the washed mitochondria compared to that in the purified preparation was 7 for spinach and respiratory activity was retained. The physiological integrity and oxidative properties of washed and gradient mitochondria are compared.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Collot M., Wattiaux-De Coninck S., Wattiaux R. Deterioration of rat-liver mitochondria during isopycnic centrifugation in an isoosmotic medium. Eur J Biochem. 1975 Feb 21;51(2):603–608. doi: 10.1111/j.1432-1033.1975.tb03962.x. [DOI] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J., Beevers H. Developmental studies on microbodies in wheat leaves : I. Conditions influencing enzyme development. Plant Physiol. 1972 Jan;49(1):28–32. doi: 10.1104/pp.49.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. The chloroplast at work. A review of modern developments in our understanding of chloroplast metabolism. Prog Biophys Mol Biol. 1978;33(1):1–54. doi: 10.1016/0079-6107(79)90024-5. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Smith E. W., Slayman C. W. Electron transport in Neurospora mitochondria. Studies on wild type and poky. J Biol Chem. 1972 Aug 10;247(15):4850–4858. [PubMed] [Google Scholar]
- Moore A. L., Jackson C., Halliwell B., Dench J. E., Hall D. O. Intramitochondrial localisation of glycine decarboxylase in spinach leaves. Biochem Biophys Res Commun. 1977 Sep 23;78(2):483–491. doi: 10.1016/0006-291x(77)90204-2. [DOI] [PubMed] [Google Scholar]
- Morgenthaler J. J., Price C. A. Photosynthetic activity of spinach chloroplasts after isopycnic centrifugation in gradients of silica. Plant Physiol. 1974 Oct;54(4):532–534. doi: 10.1104/pp.54.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich P. R., Boveris A., Bonner W. D., Jr, Moore A. L. Hydrogen peroxide generation by the alternate oxidase of higher plants. Biochem Biophys Res Commun. 1976 Aug 9;71(3):695–703. doi: 10.1016/0006-291x(76)90887-1. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E. Isolation of subcellular organelles of metabolism on isopycnic sucrose gradients. Methods Enzymol. 1974;31:734–746. doi: 10.1016/0076-6879(74)31077-4. [DOI] [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]


