Abstract
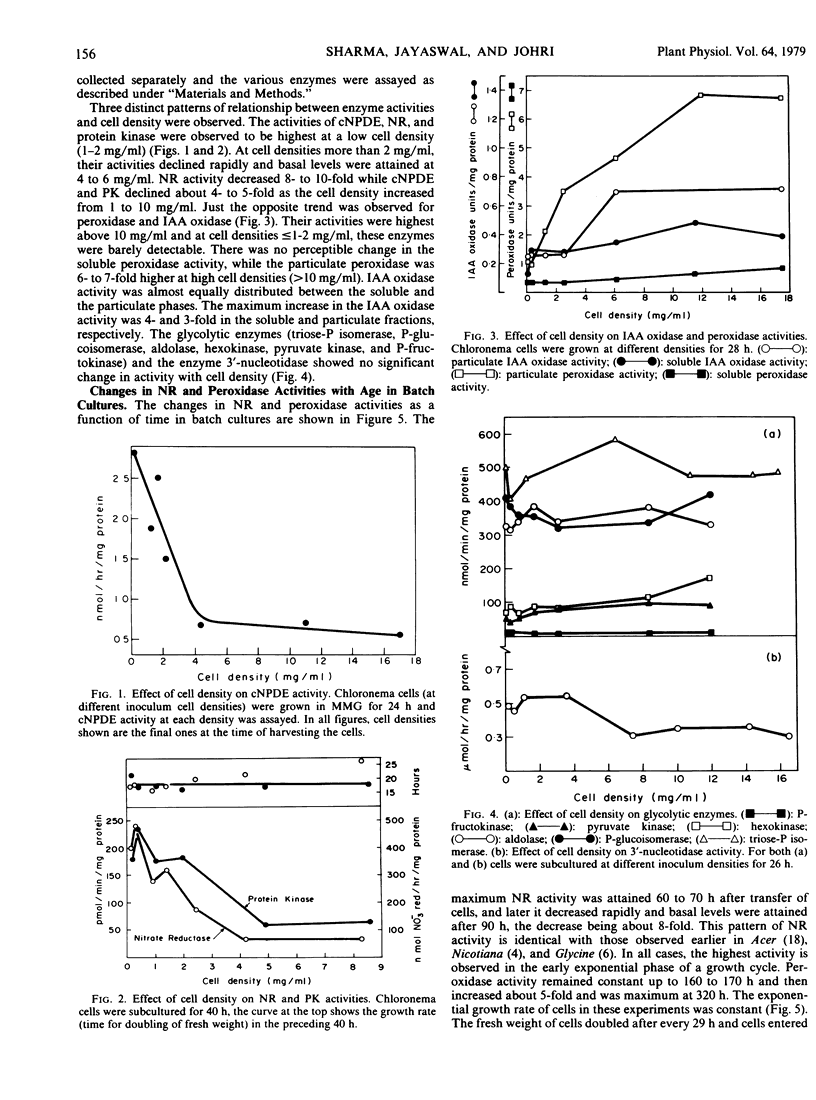
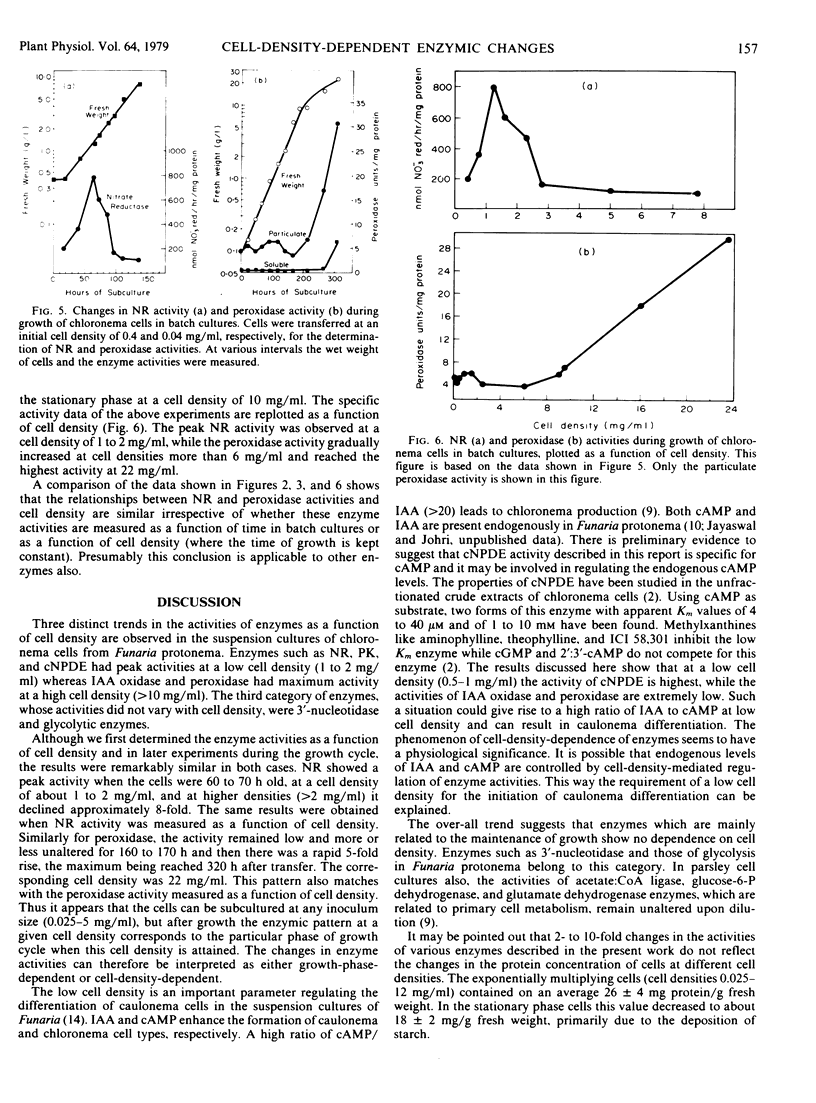
In the growing chloronema cell suspension cultures of the moss Funaria hygrometrica Hedw., activities of several enzymes have been found to be cell-density-dependent. Cyclic nucleotide phosphodiesterase (cNPDE), nitrate reductase (NR), and protein kinase showed highest activity at a low cell density (1 to 2 milligrams per milliliter) while indoleacetic acid (IAA) oxidase and peroxidase were highest at a high cell density (>10 milligrams per milliliter). 3′-Nucleotidase and the glycolytic enzymes (aldolase, hexokinase, phosphofructokinase, phosphoglucoisomerase, pyruvate kinase, and triose phosphate isomerase) showed no significant dependence on the cell density. Alternatively, if the NR and peroxidase activities were determined as a function of time in batch cultures, their levels were maximal 60 to 70 and 320 hours after subculture, respectively, the corresponding cell densities being 1 to 2 and 23 milligrams per milliliter. The relationship between cell density and NR and peroxidase activities is the same, whether these enzymes are measured in batch cultures during a growth cycle or in the cells cultured at different initial inoculum densities for a constant time. Conventionally enzymic changes have been correlated with growth phases; however, it is felt that the pattern of enzymic activities can also be interpreted as cell-density-dependent.
In moss protonema, the dependence of cNPDE, IAA oxidase, and peroxidase on cell density may play an important role in modulating the endogenous levels of IAA and cAMP, both of which regulate the differentiation of specific cell types (Johri and Desai 1973 Nature New Biol 245: 223-224; and Handa and Johri 1976 Nature 259: 480-482).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Russell T. R., Carchman R. A., Pastan I. Interrelationship between adenylate cyclase activity, adenosine 3':5' cyclic monophosphate phosphodiesterase activity, adenosine 3':5' cyclic monophosphate levels, and growth of cells in culture. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3802–3805. doi: 10.1073/pnas.70.12.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. E. Multisample enzyme extraction from cultured plant cell suspensions. Plant Physiol. 1971 Jan;47(1):38–42. doi: 10.1104/pp.47.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P. Regulation of nitrate reductase in cultured tobacco cells. Biochim Biophys Acta. 1966 May 5;118(2):299–310. doi: 10.1016/s0926-6593(66)80038-3. [DOI] [PubMed] [Google Scholar]
- Fischer U., Amrhein N. Cyclic nucleotide phosphodiesterase of Chlamydomonas reinhardtii. Biochim Biophys Acta. 1974 Apr 25;341(2):412–420. doi: 10.1016/0005-2744(74)90234-4. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K., Ebel J., Ortmann R., Sutter A., Wellmann E., Grisebach H. Regulation of enzyme activities related to the biosynthesis of flavone glycosides in cell suspension cultures of parsley (Petroselinum hortense). Biochim Biophys Acta. 1971 Jul 20;244(1):7–15. doi: 10.1016/0304-4165(71)90114-0. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K., Wellmann E. Light-independent induction of enzymes related to phenylpropanoid metabolism in cell suspension cultures from parsley. Biochim Biophys Acta. 1973 May 28;304(3):702–706. doi: 10.1016/0304-4165(73)90215-8. [DOI] [PubMed] [Google Scholar]
- Handa A. K., Johri M. M. Cyclic adenosine 3':5'-monophosphate in moss protonema: a comparison of its levels by protein kinase and gilman assays. Plant Physiol. 1977 Mar;59(3):490–496. doi: 10.1104/pp.59.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Jaworski E. G. Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1274–1279. doi: 10.1016/s0006-291x(71)80010-4. [DOI] [PubMed] [Google Scholar]
- Keates R. A. Cyclic nucleotide-independent protein kinase from pea shoots. Biochem Biophys Res Commun. 1973 Sep 18;54(2):655–661. doi: 10.1016/0006-291x(73)91473-3. [DOI] [PubMed] [Google Scholar]
- Keegstra K., Albersheim P. The Involvement of Glycosidases in the Cell Wall Metabolism of Suspension-cultured Acer pseudoplatanus Cells. Plant Physiol. 1970 Jun;45(6):675–678. doi: 10.1104/pp.45.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu E. H., Lamport D. T. An Accounting of Horseradish Peroxidase Isozymes Associated with the Cell Wall and Evidence that Peroxidase Does Not Contain Hydroxyproline. Plant Physiol. 1974 Dec;54(6):870–876. doi: 10.1104/pp.54.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra P. K., Lobo Z. A kinetic study of glycolytic enzyme synthesis in yeast. J Biol Chem. 1971 Jan 25;246(2):475–488. [PubMed] [Google Scholar]
- Pledger W. J., Thompson J., Strada S. J. Effects of growth conditions on cyclic nucleotide phosphodiesterases of cultured fibroblasts. J Cyclic Nucleotide Res. 1975;1(6):251–259. [PubMed] [Google Scholar]
- RAY P. M. The destruction of indoleacetic acid. II. Spectrophotometric study of the enzymatic reaction. Arch Biochem Biophys. 1956 Sep;64(1):193–216. doi: 10.1016/0003-9861(56)90254-5. [DOI] [PubMed] [Google Scholar]
- Stoker M. G., Rubin H. Density dependent inhibition of cell growth in culture. Nature. 1967 Jul 8;215(5097):171–172. doi: 10.1038/215171a0. [DOI] [PubMed] [Google Scholar]
- Widnell C. C. Purification of rat liver 5'-nucleotidase as a complex with sphingomyelin. Methods Enzymol. 1974;32:368–374. doi: 10.1016/0076-6879(74)32037-x. [DOI] [PubMed] [Google Scholar]


