Abstract
Objectives
Research has established that the amount of inherent tension a peripheral nerve tract is exposed to influences nerve excursion and joint range of movement (ROM). The effect that spinal posture has on sciatic nerve excursion during neural mobilisation exercises has yet to be determined. The purpose of this research was to examine the influence of different sitting positions (slump-sitting versus upright-sitting) on the amount of longitudinal sciatic nerve movement during different neural mobilisation exercises commonly used in clinical practice.
Methods
High-resolution ultrasound imaging followed by frame-by-frame cross-correlation analysis was used to assess sciatic nerve excursion. Thirty-four healthy participants each performed three different neural mobilisation exercises in slump-sitting and upright-sitting. Means comparisons were used to examine the influence of sitting position on sciatic nerve excursion for the three mobilisation exercises. Linear regression analysis was used to determine whether any of the demographic data represented predictive variables for longitudinal sciatic nerve excursion.
Results
There was no significant difference in sciatic nerve excursion (across all neural mobilisation exercises) observed between upright-sitting and slump-sitting positions (P = 0.26). Although greater body mass index, greater knee ROM and younger age were associated with higher levels of sciatic nerve excursion, this model of variables offered weak predictability (R2 = 0.22).
Discussion
Following this study, there is no evidence that, in healthy people, longitudinal sciatic nerve excursion differs significantly with regards to the spinal posture (slump-sitting and upright-sitting). Furthermore, although some demographic variables are weak predictors, the high variance suggests that there are other unknown variables that may predict sciatic nerve excursion. It can be inferred from this research that clinicians can individualise the design of seated neural mobilisation exercises, using different seated positions, based upon patient comfort and minimisation of neural mechanosensitivity with the knowledge that sciatic nerve excursion will not be significantly influenced.
Keywords: Neural mobilisation, Neurodynamics, Sciatic nerve, Ultrasound imaging, Ultrasonography, Peripheral nervous system, Physiotherapy, Physical therapy
Introduction
Limb and spinal position may influence the inherent tension of a peripheral nerve tract and therefore influence nerve excursion and joint range of movement (ROM) as the body moves. For example, a cadaver study, which examined the straight-leg raise (SLR) test, found there was less excursion of the tibial nerve1 when the nerve was pre-tensioned with ankle dorsiflexion, and the reverse when the ankle was plantarflexed and the nerve tract unloaded. In vivo studies of the median2,3 and ulnar nerves4 found that when the nerve tract was pre-tensioned less nerve excursion occurred following wrist movement; the reverse trend was observed when the nerve tract was unloaded. Furthermore, Coppieters et al.5 observed less in vivo median nerve excursion during elbow extension when the cervical nerve roots and brachial plexus were pre-tensioned (via contralateral side-flexion of the cervical spine compared to ipsilateral side-flexion).
There are numerous examples of the influence that limb position has upon neural tension during neurodynamic testing and neural mobilisation, however, there is less information regarding the effects of spinal position. Analysis of cadavers has concluded that cervical- and thoracic flexion-induced cephalic movement of the spinal cord and lumbo-sacral nerve roots with an associated increase in tension of these structures.6,7 Furthermore, the addition of cervical flexion to the slump test has shown significant decreases in knee extension, assumed to occur from increased tension imposed upon the sciatic nerve tract and lumbo-sacral nerve roots.8,9
The influence that spinal flexion may have upon the outcome of slump-based neurodynamic tests is yet to be determined. It has been shown that spinal flexion (slump) compared to spinal neutral (upright), during the femoral nerve neurodynamic test, resulted in significant differences in both hip extension ROM and pain intensity.10 The mechanical influences of a flexed spine (slump) versus a neutral spine (upright) have yet to be determined for the seated slump test.
A loss of mobility of the lumbo-sacral nerve roots and/or sciatic nerve has been implicated in clinical conditions such as lumbar nerve root adhesion11,12 and sciatic nerve entrapment.13,14 Theoretical concepts regarding neural mobilisation have advocated for their use to influence peripheral nerve movement (i.e. sliding and elongation).3,5,15 Furthermore, neural mobilisation exercises performed in slumped sitting16,17 have been used in research targetting movement of the lumbo-sacral nerve roots and/or sciatic nerve. The mechanical influence such exercises may have would be clinically advantageous for conditions whereby sciatic nerve excursion is impaired. Given the fact that increased nerve tension directly influences available nerve excursion,18 the effect of joint position and spinal posture during neural mobilisation exercises also needs to be considered, in order to maximise nerve excursion.
The use of neural mobilisation exercises, which use modifications of the slump test have been advocated for people with low-back related leg pain.15–17,19–21 Furthermore, clinicians have also been asked to consider varying spinal posture (slump versus upright) in sitting based neural mobilisation exercises.20 As indicated earlier, it is possible that the degree of spinal flexion utilised during seated based exercises may impact upon relative neural tension and therefore nerve mechanics. Therefore, the aim of this study was to determine whether different spinal postures (slump-sitting versus upright-sitting) altered the amount of longitudinal sciatic nerve movement during neural mobilisation exercises commonly used in clinical practice. It was hypothesised that exercises performed in slump-sitting would induce less longitudinal sciatic nerve excursion compared to those in upright-sitting due to potentially greater tension being imposed on the sciatic nerve and lumbo-sacral nerve roots in slump-sitting.
Methods
Design
A controlled laboratory study involving a single group, observational, repeated measures comparison was conducted. The dependent variable was longitudinal sciatic nerve excursion. The independent variable was the spinal posture adopted for each of the two sitting positions (slump-sitting and upright-sitting) used for the neural mobilisation exercises.
Participants
Thirty-eight healthy participants over the age of 18 years (Table 1) were recruited into the study by means of advertisements posted on university and community noticeboards. Participants were excluded if they had a history of major trauma or surgery to the lumbar, hip, buttock (glutaeal) or hamstring (posterior thigh) regions; symptoms consistent with sciatic nerve impairment (i.e. paraesthesia, weakness, etc.); or a positive slump test as described by Butler.19 Participants were also excluded if they had a neurological condition or other systemic disorders (e.g. diabetes) that might alter the function of the peripheral nervous system.
Table 1.
Participant demographic details
| Demographic details | Males (n = 18) | Females (n = 16) | T-test |
|---|---|---|---|
| Mean ( ± standard deviation) | Mean ( ± standard deviation) | P-value | |
| Age (year) | 27.4 (4.4) | 32.5 (13.09) | 0.16 |
| Height (cm) | 182.0 (5.0) | 164.9 (5.0) | 0.82 |
| Weight (kg) | 87.0 (10.5) | 63.0 (7.0) | 0.12 |
| Body Mass Index (BMI) (kg m− 2) | 26.3 (2.6) | 23.2 (2.4) | 0.71 |
| Knee Range of motion (°) | 64.7 (12.2) | 64.8 (20.5) | 0.05 |
Intervention
For all neural mobilisation exercises, knee extension was performed passively via a Biodex system 3, (Biodex Medical, Shirley, NY, USA) isokinetic dynamometer. To standardise knee ROM, participants were positioned in slump-sitting (Fig. 1) with the cervical spine in full, comfortable flexion. For the leg to be tested (randomly chosen), the knee was passively extended from 90° flexion until the participant experienced a 4 out of 10 feeling of hamstring stretch discomfort on a numeric rating scale (NRS) (0 equivalent to ‘no stretch,’ 10 equivalent to the ‘worst imaginable stretch’). The level of 4/10 was chosen to represent a moderate level of stretch discomfort, and has been used in previous research.22,23
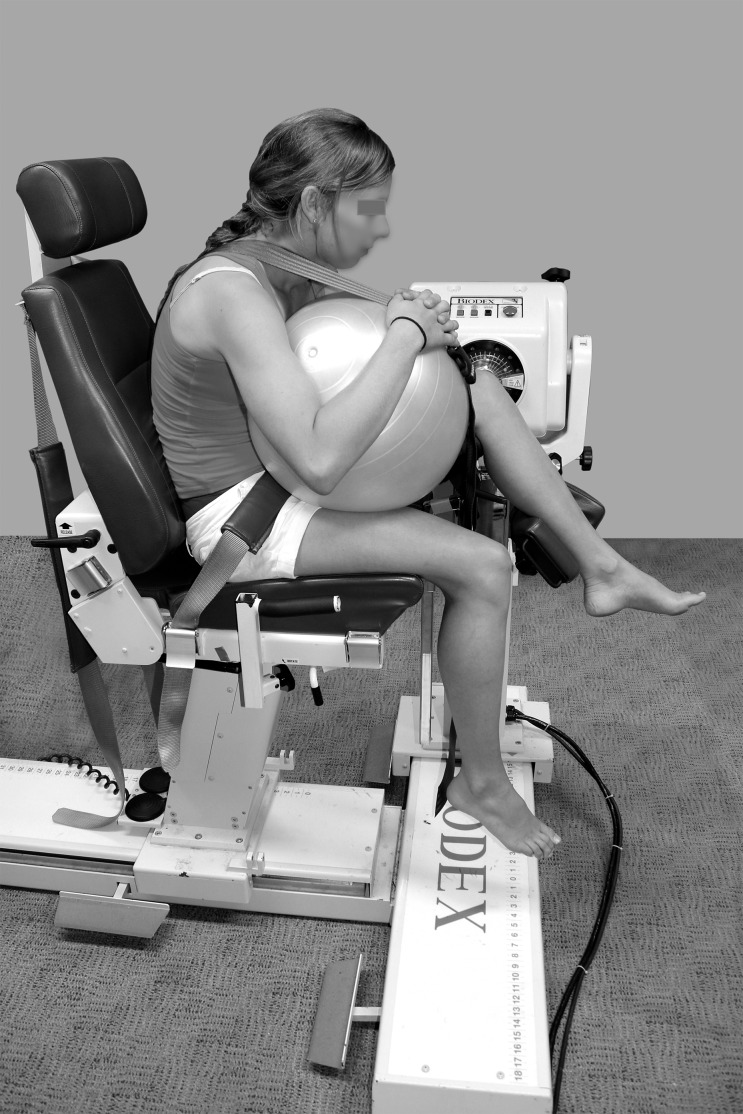
Figure 1.
Slump-sitting position. Photograph reprinted with permission.
Two sitting positions used for the neural mobilisation exercises were investigated. For slump-sitting (Fig. 1) participants were positioned on the Biodex and asked to adopt a slumped spinal posture.15 The slump position was maintained through contact of the sternum against a 45 cm diameter ball placed on the participant's lap. A seatbelt was utilised to maintain this position. The upright-sitting position differed (Fig. 2) as all participants relaxed into the back-rest of the Biodex.
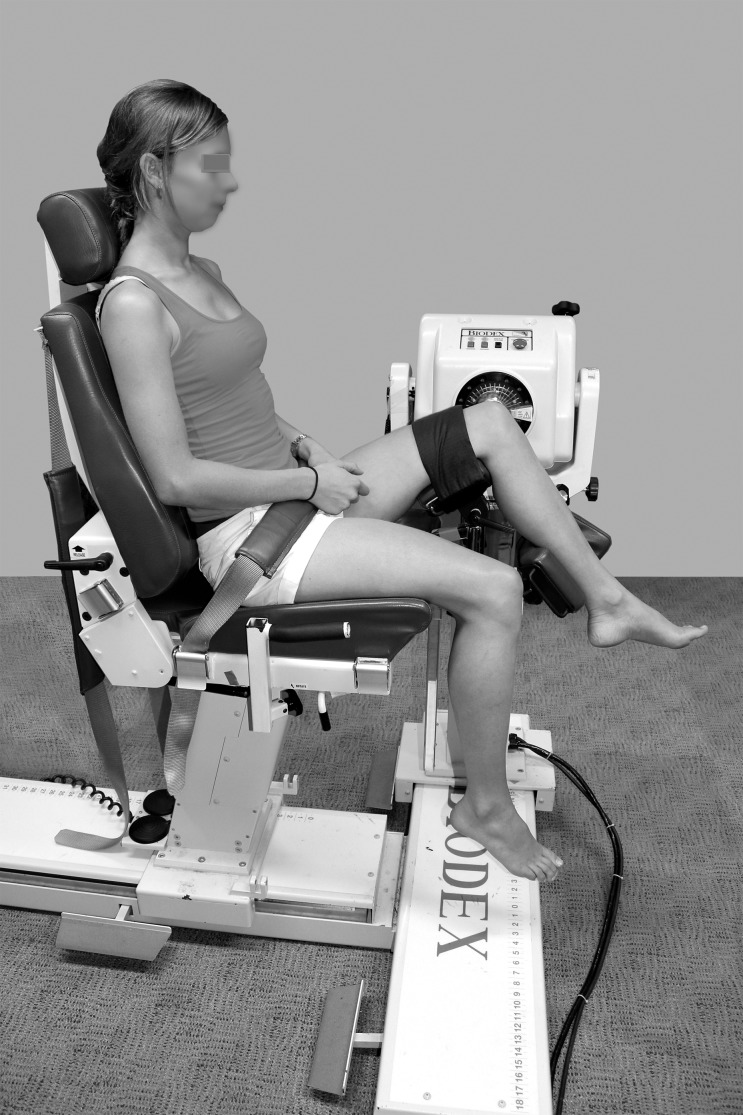
Figure 2.
Upright-sitting position. Photograph reprinted with permission.
In both positions, the hip of the tested leg was set at 90° flexion, as measured by a universal goniometer. Belts were used across the pelvis and the thigh of the tested leg to further maintain the position.
Three neural mobilisation exercises were randomly performed for each of the sitting positions. These included:
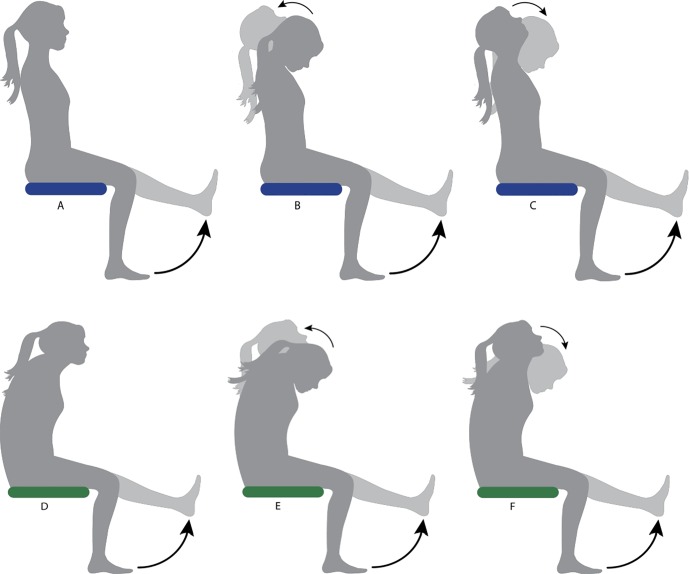
Single-joint mobilisation (knee): Passive knee extension, performed by the Biodex from 90° flexion to the pre-determined point of 4/10 stretch discomfort (moving of the sciatic nerve caudally via the tibial nerve). Each participant was instructed to look straight ahead to maintain consistency of cervical spine position (Fig. 3, Images A and D).
Figure 3.
Illustration of the three different neural mobilisation exercises performed in both sitting positions. Upright-sitting exercises are shown by images A–C and slump-sitting exercises are shown by images D–F. Images A and D represent single joint mobilisations, B and E represent slider mobilisations and C and F represent tensioner mobilisations.
Slider mobilisation: Simultaneous passive knee extension performed by the Biodex from 90° flexion to the pre-determined ROM of 4/10 stretch (moving of the sciatic nerve caudally via the tibial nerve) and active cervical spine extension from full comfortable cervical flexion to full comfortable cervical extension (unloading of nervous system cranially) (Fig. 3, B and E).
Tensioner mobilisation: Simultaneous passive knee extension performed by the Biodex from 90° flexion to the pre-determined ROM of 4/10 stretch discomfort (see above) (moving of the sciatic nerve caudally via the tibial nerve) and active cervical flexion from full comfortable cervical extension to full comfortable cervical flexion (loading of nervous system cranially) (Fig. 3, C and F).
Knee joint motion occurred at an angular velocity of 20°/s. Two repetitions of each movement were performed as familiarisation trials, allowing the participant to practice timing of active cervical spine movement in coordination with passive knee extension. A further three repetitions of each movement were performed for data collection with a 1-min rest between each mobilisation exercise. For all of the exercises, each participant was instructed to allow their foot and ankle to remain relaxed during the movement of the knee joint.
Outcome measures
Longitudinal excursion of the sciatic nerve (measured in millimetres, mm) was assessed at the level of the posterior mid-thigh (half-way between the glutaeal and poplitaeal creases). The same experienced sonographer performed all ultrasound scans and was blinded to the analysis of all ultrasound imaging.
B-mode real-time ultrasound imaging was performed using a Philips iU22 (Philips Medical Systems Company, The Netherlands) ultrasound machine with a 12–5 MHz, 50 mm, linear array transducer. Each video sequence was converted off-line to a digital format (bitmaps). ImageJ (Version 1.42, National Institute of Health, Maryland, USA) digital image analysis software was used to calculate the scale conversion from pixels to millimetres.
Each video sequence was then analysed using frame-by-frame cross-correlation analysis. This method employs a cross-correlation algorithm to determine relative pixel movement, of grey scale speckle features, between successive frames in a sequence of ultrasound images.24 Pixel shift measurements for the nerve were offset against (subtracted from) pixel shift measurements from stationary structures (i.e. subcutaneous layers, bone, etc.) within the same ultrasound field. This method allows for any slight movement of the ultrasound transducer to be eliminated from the analysis. This method has been previously reported and proved to be highly reliable for the assessment of nerve motion.5,15,24,25
To be selected for analysis, each video sequence had to have clear pixilation and clear identification of the sciatic nerve. Two video sequences were then randomly chosen for each of the three neural mobilisation exercises per participant. During data collection and analyses, the researcher was blinded to the participant (including their relevant demographic data), the recording session, the sitting position and the neural mobilisation exercise.
Data analysis
A priori power analysis and sample size calculation was performed based on data from seven participants from of a previous pilot trial (unpublished) that utilised a similar research design to that of the current study. The dependent variable used was sciatic nerve excursion for different neural mobilisation exercises and the independent variable was neural mobilisation sitting position (slump-sitting or upright-sitting). The analysis was performed using G*Power 3 software26,27 with calculations based on a 30% difference being observed. This percentage change was chosen based on similar research15 and was considered to reflect a change that would be notable to clinicians. With power set at 0.8 and alpha of 0.05, the required number of participants was 30.
A Shapiro–Wilk test was used to examine the data for normaility. On the basis of normally distributed data, a paired T-test was utilised to examine the effect of slump-sitting and upright-sitting positions on longitudinal sciatic nerve excursion across the three mobilisation exercises.
The intrarater reliability of measuring longitudinal sciatic nerve excursion using the method described was also examined. A 2-way mixed intraclass correlation coefficient (ICC2,1), with 95% confidence intervals and standard error of measurement (SEM) were calculated to determine the reliability of measurement.
In order to better understand the influence of spinal position on sciatic nerve excursion in our cohort, further analysis was conducted to determine whether any of the demographic data represented predictive variables for the amount of longitudinal sciatic nerve excursion by means of a model fitting procedure using linear regression analysis. Variables that were significant to 10% were retained in the model. Statistical significance was defined as P < 0.05.
Results
Of the 38 participants enrolled in the study, data from four participants were excluded due to poor image quality. Of the 34 remaining participants, 16 were female and 18 were male. The descriptive demographic data for the 34 participants are shown in Table 1. Following the Shapiro–Wilk test, it was apparent that sciatic nerve excursion for this sample was normally distributed (P>0.05).
Sciatic nerve excursion (across all neural mobilisation exercises) was observed to be slightly greater for upright-sitting compared to slump-sitting (Table 2), however, this was not significant (P = 0.26). For all data, the movement of the sciatic nerve was in a distal direction (i.e. towards the knee).
Table 2.
Descriptive statistics for longitudinal sciatic nerve excursion. Values are represented in millimetres (mean ± standard deviation). All values indicate distal movement of the sciatic nerve (i.e. towards the knee)
| Sitting position | Slider mobilisation | Single-joint mobilisation | Tensioner mobilisation |
|---|---|---|---|
| Slump | 6.4 ± 2.7 | 6.2 ± 2.9 | 6.0 ± 2.9 |
| Upright | 6.9 ± 2.6 | 6.1 ± 2.5 | 6.4 ± 2.7 |
The reliability of measuring sciatic nerve excursion across two trials for the three neural mobilisations was excellent for both the slump position (ICC 0.86, 95% CI, 0.84–0.92; SEM, 0.25 mm) and the upright position (ICC 0.89, 95% CI, 0.85–0.92; SEM, 0.21 mm).
A linear mixed effects model was fit to predict sciatic nerve excursion using repeated measures of outcome data and several covariates (listed in Tables 1 and 2). A backwards stepwise selection procedure was used to determine significant predictors of sciatic nerve excursion from these covariates. Variables that were not statistically significant (P>0.10) (Table 3), along with height and weight (due to being highly correlated with BMI) were excluded from the final model. Age, BMI, knee ROM and sitting position were retained as significant predictors of sciatic nerve excursion (F(3, 403) = 38.9, P < 0.0005, R2 = 0.22). Greater BMI, knee ROM and younger age were associated with higher levels of sciatic nerve excursion (Table 3). Slump position was associated with less sciatic nerve excursion compared to the upright position, however this was not a statistically significant effect (P = 0.17, Table 3). The R2 value (R2 = 0.22) was low, indicating that this model offers weak predictability. More than 75% of the variability surrounding sciatic nerve movement was not accounted here, highlighting that important risk factors for sciatic nerve excursion are yet to be identified.
Table 3.
Output from repeated measures model
| Variable | Category | Beta (95% CI) | P-value |
|---|---|---|---|
| Constant | − 1.88 ( − 4.93, 1.17) | 0.227 | |
| Age (years) | − 0.08 ( − 0.10, − 0.06) | < 0.0001 | |
| Sex | Males | 0.15 (0.45,0.75) | 0.626 |
| Body mass index (BMI) (kg m− 2) | 0.30 (0.20, 0.40) | < 0.0001 | |
| Knee range of motion (°) | 0.04 (0.02, 0.05) | < 0.0001 | |
| Position | Slump | 0.34 ( − 0.14, 0.82) | 0.168 |
Discussion
To the authors' knowledge, this study is the first in vivo study, which has assessed the influence of spinal posture on lower limb peripheral nerve excursion during neural mobilisation exercises. The results of this study found no evidence to support the hypothesis that neural mobilisation exercises performed in slump-sitting induce less longitudinal sciatic nerve excursion, compared with those performed in upright-sitting. This hypothesis was formed based on the assumption that slump-sitting would result in greater tension being imposed on the sciatic nerve via the spinal cord and lumbo-sacral nerve roots. The lack of statistical significance in this study coupled, with the fact that the R2 statistic was low (22%), indicates that there is more than 75% variability in our study data that is unaccounted for by the variables in our model. This suggests that there are other variables (that are either unknown or not currently collected) that may better predict sciatic nerve excursion among healthy participants utilising neural mobilisation exercises in different sitting positions.
Previous research has demonstrated that the amount of inherent tension to which a peripheral nerve is exposed can have a direct influence on the amount of nerve excursion available when the limb moves.1–4 Although nerve tension was not quantified, the results of the current study suggest that the assumed increase in generalised neural tension applied to the spinal cord and lumbo-sacral nerve roots by slump-sitting did not alter sciatic nerve excursion.
The findings of this study regarding the influence of spinal position upon nerve excursion appear to contradict the conclusions of previous studies. For example, previous research has indicated that the position of the cervical spine5 or movement of the cervical spine28 significantly influenced median nerve excursion during upper limb neural mobilisation exercises. There are several explanations that need to be considered. In the first instance, previous research has examined nerve excursion in the upper limb.5,28 The kinematics and functional use of the upper quadrant (i.e. cervical spine, scapulao-thoracic region and upper limb) is very different to that of the lower quadrant (i.e. lumbar spine, pelvis and lower limb). This fact makes direct comparison of nerve movement between the upper and lower quadrants difficult and possibly inappropriate.
Furthermore, it could also be argued that any increase in neural tension imposed from slump-sitting may be greatest at or near the thoracic and lumbo-thoracic regions. These regions are distant to the axes of joint rotation used during the neural mobilisation exercises examined. This anatomic distance may have limited any increased neural tension induced with spinal slump upon excursion of the sciatic nerve at the posterior mid-thigh. Although this is speculative, there is some evidence to suggest that anatomical distance may be a factor in relative neural tension. For example, previous research has suggested that the addition of cervical flexion during the SLR test did not show any significant change in hip flexion at the first onset of manually perceived resistance, in either healthy individuals or those with a lumbar radiculopathy.29 Also the work of Ellis et al.15 concluded that the addition of cervical flexion to a seated neural mobilisation exercise did not significantly alter sciatic nerve excursion which was induced from knee extension. However, the argument for anatomic distance and relative neural tension is controversial. From their cadaver based research, Gilbert et al.1 concluded that the addition of ankle dorsiflexion to the SLR caused a significant decrease in displacement of the L5 and S1 nerve roots. In regard to nerve root strain, it was evident that ankle dorsiflexion resulted in greater strain, however this did not reach significance. Clearly there is further research to be done to fully understand the influence that anatomic location has upon relative neural tension.
Neural tension influences the amount of perceived stretch and is known to limit joint ROM.3,5,15,18 In order to avoid potential adverse effects from excessive nerve stretch in the current study, knee extension joint ROM was limited to elicit a sensation of 4/10 (NRS) stretch in full slump-sitting. This was done to allow participants to move through a greater ROM than an absolute ROM limit may have allowed. This would therefore induce more nerve excursion, while minimising potential adverse effects (e.g. increased neural mechanosensitivity) from excessive nerve stretch. The choice of the level of 4/10 represented a moderate level of stretch and has been used in previous research.22,23 However, by limiting knee ROM in this way it is likely it was not possible to apply adequate tension to the lumbosacral-sciatic nerve tract to take the sciatic nerve through its full range of excursion. Furthermore, in the upright-position (where the nervous system is believed to be exposed to less tension) knee ROM was kept to the same range as for slump-sitting. This may have further decreased the likelihood of moving the sciatic nerve through its full range of excursion. Although this pragmatic approach to knee ROM standardisation allowed maintenance of a consistent methodology, it may have contributed to the non-significant result and inability to support the original hypothesis.
Limitations
It is important to note that this study examined a healthy population. Foundation research, which gains an understanding of concepts such as nerve excursion, requires information pertaining to healthy individuals. However, this does create a limitation in that direct inferences to clinical populations cannot be made from this research.
The methods used to perform the neural mobilisation exercises in the current study are similar to those used in previous work.15 As was also noted in Ellis et al.,15 it was logistically difficult to use both passive knee and passive cervical spine movements for the neural mobilisation exercises. It is acknowledged that this is a methodological limitation, in that the active cervical spine movement presents a less rigorous control than the standardised passive knee movements that were used.
A further limitation to consider was the use of the standardised 45 cm diameter ball to maintain the slump position. The potential exists that, depending on the relative size of each participant, the size of the ball dictated different degrees of spinal flexion and therefore may have influenced neural tension. The choice to use the 45 cm ball was a pragmatic one, based on previous research that had previously reported this method.15 However, it is acknowledged that this is a methodological limitation.
Finally, it is noted that the foot and ankle of the moving leg was not held in a standardised position. It is not standard clinical practice to restrict foot and ankle movement in neural mobilisation exercises, such as those used in this study. Furthermore, the intention was to maximise knee extension, within the set comfort parameters, in order to maximise the sciatic nerve excursion that this induced. It is acknowledged, however, that having the foot in a non-standardised position presents a confounding variable that may have influenced the amount of sciatic nerve excursion seen.
Clinical implications
The clinical implications of the research are that spinal posture has little effect on sciatic nerve excursion between different sitting-based neural mobilisation exercises for the sciatic nerve and associated tracts in healthy participants. Although the current study assessed healthy participants, it can be inferred from the results that clinicians are able to individualise seated neural mobilisation exercises with regard to lumbar position, to meet the requirements of individual patients, without compromising sciatic nerve excursion. For example, neural mobilisation exercises performed in upright-sitting utilising tolerable ranges of movement at the knee and cervical spine have the potential to elicit similar sciatic nerve excursion as slump-sitting exercises, with the additional benefit of creating less neural tension and potentially mitigating any related adverse effects.
Disclaimer Statements
Contributors RE was involved in conceptual design, methods design and development, data collection and analysis, manuscript writing and editing. SO was involved in methods design and development, data collection and analysis, manuscript writing and editing. JW was involved with data collection and analysis, manuscript writing and editing. PP was involved in methods development, statistical support, data analysis, manuscript writing and editing. WH was involved with conceptual design, methods design, manuscript writing and editing.
Funding Faculty of Health and Environmental Sciences (AUT University) Summer Student Assistant Grants.
Conflicts of interest There are no conflicts of interest for any of the authors through this research or during the submission process.
Ethics approval Ethical approval for this research was gained through the Auckland University of Technology Ethics Committee.
References
- 1.Gilbert KK, Brismée JM, Collins DL, James CR, Shah RV, Sawyer SF, et al. . Lumbosacral nerve root displacement and strain: part 2. A comparison of 2 straight leg raise conditions in unembalmed cadavers. Spine. 2007;32(14):1521–5. [DOI] [PubMed] [Google Scholar]
- 2.Dilley A, Lynn B, Greening J, DeLeon N. Quantitative in vivo studies of median nerve sliding in response to wrist, elbow, shoulder and neck movements. Clin Biomech. 2003;18(10):899–907. [DOI] [PubMed] [Google Scholar]
- 3.Coppieters M, Alshami A. Longitudinal excursion and strain in the median nerve during novel nerve gliding exercises for carpal tunnel syndrome. J Orthop Res. 2007;25(7):972–80. [DOI] [PubMed] [Google Scholar]
- 4.Dilley A, Summerhayes C, Lynn B. An in vivo investigation of ulnar nerve sliding during upper limb movements. Clin Biomech. 2007;22(7):774–9. [DOI] [PubMed] [Google Scholar]
- 5.Coppieters M, Hough A, Dilley A. Different nerve-gliding exercises induce different magnitudes of median nerve longitudinal excursion: an in vivo study using dynamic ultrasound imaging. J Orthop Sports Phys Ther. 2009;39(3):164–71. [DOI] [PubMed] [Google Scholar]
- 6.Breig A. Adverse mechanical tension in the central nervous system. Stockholm: Almqvist and Wiksell; 1978. [Google Scholar]
- 7.Breig A, Marions O. Biomechanics of the lumbosacral nerve roots. Acta Radiol. 1963;1:1141–60. [DOI] [PubMed] [Google Scholar]
- 8.Herrington L, Bendix K, Cornwell C, Fielden N, Hankey K. What is the normal response to structural differentiation within the slump and straight leg raise tests? Man Ther. 2008;13(4):289–94. [DOI] [PubMed] [Google Scholar]
- 9.Johnson EK, Chiarello CM. The slump test: the effects of head and lower extremity position on knee extension. J Orthop Sports Phys Ther. 1997;26(6):310–7. [DOI] [PubMed] [Google Scholar]
- 10.Lai W-H, Shih Y-F, Lin P-L, Chen W-Y, Ma H-L. Normal neurodynamic responses of the femoral slump test. Man Ther. 2012;17:126–32. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi S, Shizu N, Suzuki Y, Asai T, Yoshizawa H. Changes in nerve root motion and intraradicular blood flow during an intraoperative straight-leg-raising test. Spine. 2003;28(13):1427–34. [DOI] [PubMed] [Google Scholar]
- 12.Efstathiou MA, Stefanakis M, Savva C, Giakas G. Effectiveness of neural mobilization inpatients with spinal radiculopathy: a critical review. J Bodyw Mov Ther. 2015;19(2):205–12. [DOI] [PubMed] [Google Scholar]
- 13.Hernando MF, Cerezal L, Pérez-Carro L, Abascal F, Canga A. Deep gluteal syndrome: anatomy, imaging, and management of sciatic nerve entrapments in the subgluteal space. Skeletal Radiol. 2015;44(7):919–34. [DOI] [PubMed] [Google Scholar]
- 14.Martin HD, Shears SA, Johnson JC, Smathers AM, Palmer IJ. The endoscopic treatment of sciatic nerve entrapment/deep gluteal syndrome. Arthroscopy. 2011;27(2):172–81. [DOI] [PubMed] [Google Scholar]
- 15.Ellis RF, Hing WA, McNair PJ. Comparison of longitudinal sciatic nerve movement with different mobilization exercises: an in vivo study utilizing ultrasound imaging. J Orthop Sports Phys Ther. 2012;42(8):667–75. [DOI] [PubMed] [Google Scholar]
- 16.Cleland JA, Childs JD, Palmer JA, Eberhart S. Slump stretching in the management of non-radicular low back pain: a pilot clinical trial. Man Ther. 2006;11(4):279–86. [DOI] [PubMed] [Google Scholar]
- 17.Ali M, Ur Rehman SS, Ahmad S, Farooq MN. Effectiveness of slump neural mobilization technique for the management of chronic radicular low back pain. Rawal Med J. 2015;40(1):41–3. [Google Scholar]
- 18.Coppieters M, Butler D. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and considerations regarding their application. Man Ther. 2008;13(3):213–21. [DOI] [PubMed] [Google Scholar]
- 19.Butler DS. The sensitive nervous system. Adelaide: Noigroup Publications; 2000. [Google Scholar]
- 20.Shacklock MO. Clinical neurodynamics: a new system of neuromusculoskeletal treatment. Oxford: Butterworth Heinemann; 2005. [Google Scholar]
- 21.Cleland JA, Hunt G, Palmer J. Effectiveness of neural mobilization in the treatment of a patient with lower extremity neurogenic pain: a single-case design. J Man Manip Ther. 2004;12(3):143–52. [Google Scholar]
- 22.Mitchell UH, Myrer JW, Hopkins JT, Hunter I, Feland JB, Hilton SC. Acute stretch perception alteration contributes to the success of the PNF “contract-relax” stretch. J Sport Rehabil. 2007;16:85–92. [DOI] [PubMed] [Google Scholar]
- 23.Ellis RF, Milne A, Tuck K, Hing WA, McNair PJ. The reliability of measuring knee range of movement in response to submaximal pain created by increased neural tension during a modified slump test. New Zealand Manipulative Physiotherpists Association (NZMPA) Biennial Conference; 2009; Rotorua, New Zealand. [Google Scholar]
- 24.Dilley A, Greening J, Lynn B, Leary R, Morris V. The use of cross-correlation analysis between high-frequency ultrasound images to measure longitudinal median nerve movement. Ultrasound Med Biol. 2001;27(9):1211–8. [DOI] [PubMed] [Google Scholar]
- 25.Ellis RF, Hing WA, Dilley A, McNair PJ. Reliability of measuring sciatic and tibial nerve movement with diagnostic ultrasound during a neural mobilisation technique. Ultrasound Med Biol. 2008;34(8):1209–16. [DOI] [PubMed] [Google Scholar]
- 26.Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Methods. 1996;28(1):1–11. [DOI] [PubMed] [Google Scholar]
- 27.Faul F, Erdfelder E, Lang A, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. [DOI] [PubMed] [Google Scholar]
- 28.Brochwicz P, von Piekartz H, Zalpour C. Sonography assessment of the median nerve during cervical lateral glide and lateral flexion. Is there a difference in neurodynamics of asymptomatic people? Man Ther. 2013;18(3):216–9. [DOI] [PubMed] [Google Scholar]
- 29.Hall T, Zusman M, Elvey RL. Adverse mechanical tension in the nervous system? Analysis of straight leg raise. Man Ther. 1998;3(3):140–6. [Google Scholar]