Abstract
Objective
The purpose of this study was to examine the overall prevalence of polypharmacy within the spinal cord injury (SCI) population, the level of polypharmacy with respect to seven classes of high-risk drugs commonly used to treat secondary conditions in the SCI population, and the overall risks for drug-related problems (DRP) related to polypharmacy.
Design
A retrospective case–control design.
Setting
A commercially available claims dataset that included patient cases from 4800 hospitals in the USA between 2007 and 2009.
Participants
Individuals with tetraplegia, paraplegia, and those with SCI but not specified as either tetraplegia or paraplegia as well as a control population of randomly selected, age- and sex-matched individuals without a diagnosis of SCI.
Outcome measures
The overall prevalence of polypharmacy, the prevalence of commonly prescribed high-risk medications, and the prevalence of reported DRPs.
Results
Overall, the patients in the SCI population were prescribed significantly more medications than their control counterparts. There was a higher rate of individuals being prescribed medications from multiple high-risk classes (e.g. analgesic-narcotics, anticonvulsant, antidepressant, and skeletal muscle relaxer), as well as multiple medications within each class (e.g. multiple analgesic-narcotics). The SCI group had a higher incidence of DRPs.
Conclusion
Our results are some of the first to demonstrate the extent of polypharmacy in individuals with SCI, including commonly prescribed high-risk medications, leading to a higher rate of DPRs. The higher rate of polypharmacy and DRPs can impact rehabilitation goals and community integration following neurologic injury.
Keywords: Polypharmacy, Medications
Introduction
Polypharmacy involves complex treatment regimens requiring multiple medications within a given patient and healthcare professionals are challenged with balancing the potential benefits and risks of using multiple medications. The use of multiple medications is a common standard of care in management of complications following spinal cord injury (SCI). Patients with SCI often require long-term healthcare management of secondary complications (e.g. spasticity, urinary tract infections, pain, and pressure sores), and are also at increased risk for several chronic comorbidities like diabetes and heart disease over their lifetime, and very often at younger ages than their non-SCI counterparts.1 Treatment protocols to manage chronic secondary complications following SCI are designed to address pain, spasticity, spasms, bowel and bladder dysfunction, depression, and anxiety.2–5 The classes of medications commonly prescribed for these conditions include: (1) sedative-hypnotic, non-barbiturates, (2) anxiolytics, (3) antispasmodics, (4) serotoninergic systems agents (e.g. select serotonin reuptake inhibitors [SSRIs] and serotonin-norepinephrine reuptake [SNRIs] inhibitors), (5) analgesic-narcotics, (6) anticonvulsants, (7) skeletal muscle relaxants, and (8) tricyclic antidepressants. The complexity of treatment regimens following SCI places patients at increased risk for drug-related problems (DRPs) including adverse drug events and medication errors secondary to drug–drug, drug–disease, or drug–nutrient interactions.6–8 Several recent studies have indicated that using medications to control spasticity can significantly impact the person's ability to ambulate, and use of psychotropic medications may actually decrease the life expectancy of individuals with SCI.9–11
Polypharmacy risks in patients with SCI may result from overlapping treatment options as well as a consequence of altered drug disposition in these patients. Fundamental assumptions of pharmacokinetics and pharmacodynamics derived from clinical studies on able-bodied individuals do not apply to this population.12,13 In many cases, the standard dosing schedules may not achieve the anticipated concentration of the drug, leading to under treatment or potentially supratherapeutic levels leading to adverse events.6,14,15 In addition, medications required for managing comorbidities following SCI often have overlapping pharmacological mechanisms or targets creating even higher risks for DRPs.12,16
To date, the majority of studies addressing the problem of polypharmacy have been in the geriatric population where the risk for adverse drug reactions has been shown to be associated with the number of drugs taken.17,18 Of the few studies that have examined polypharmacy in the SCI population, a recent retrospective study of 175 patients with chronic SCI reported over 300 drugs being administered in more than 19 classes of medications.19 In addition, a recent study examined the combination use of opoid and anticonvulsant therapy for neuropathic pain in the SCI population.12 Although outcomes improved, over 53% of the patients showed at least one treatment-related adverse event. Even with these recent studies, there remains a dearth of studies examining the impact of polypharmacy in the SCI population. The purpose of this study was to examine the overall prevalence of polypharmacy within the SCI population, the level of polypharmacy with respect to seven classes of drugs commonly used to treat secondary conditions in the SCI population, and the overall risks for DRP related to polypharmacy.
Methods
Using a retrospective case–control design, we identified patients from a commercially available claims dataset that included patient cases from 4800 hospitals in the USA between 2007 and 2009. Using ICD 9 codes (344, 806, 952), SCI cases were identified. These codes include individuals with tetraplegia, paraplegia, and those with SCI but not specified as either tetraplegia or paraplegia. Only patients with these codes who were 18 years of age and older were included in this study. A control population was randomly selected and included age- and sex-matched individuals without a diagnosis of SCI. Individuals with SCI and controls needed to be in the dataset for at least 1 year and data for a 3-year period were collected for each subject where available.
Polypharmacy measures
While the exact number of medications that constitute polypharmacy has varied from study to study, several studies have defined polypharmacy as the use of five or more concomitant agents.8,20,21 For the purpose of this study, polypharmacy was defined as five or more different, concomitant medications during the study period. Medications in the dataset were identified by codes from the National Council on Prescription Drugs Program (NCPDP) standards and the top 100 prescribed drugs for both the SCI population and the control group were examined. The overall prevalence of polypharmacy was examined by comparing the total number of drugs taken by patients in each of the groups as well as the number of individuals taking five or more medications (our definition of polypharmacy) in each of the groups. In an effort to manage the variance associated with the longitudinal nature of the data and the number of drugs a patient was prescribed at any given time, the maximum number of different drugs was recorded. This decision assumed patients with claims for the same prescription more than once on a given day, or with larger supply, adjusted dose, or identical medication claims within 3 days were duplicative or representing variance in refill timeline for maintenance drugs. We also assumed that a patient would not be taking two simultaneous prescriptions of the same drug and would most likely take the prescription that was a higher dosage or the script that lasted the longest.
In addition to the overall prevalence of polypharmacy, we also identified seven classes of medications most commonly used to treat secondary complications in the SCI population as high-risk combinations based on their potential for overlapping effects that could lead to DRPs. These classes included: (1) sedative-hypnotic, non-barbiturates, (2) antianxiety, (3) serotoninergic system agents (SSRIs and SNRIs), (4) analgesic-narcotics, (5) anticonvulsants, (6) skeletal muscle relaxants, and (7) tricyclic antidepressants. These classes of medications are also commonly used in the general population to treat other conditions. For this analysis we compared those individuals with SCI and controls, who met our criteria for polypharmacy, that were prescribed medications from the high-risk classes.
Overall polypharmacy DRPs analysis
DRPs were defined using the Supplementary Classification of External Causes of Injury and Poisoning Codes (E-codes) found in the ICD-9-CM. This list included 169 individual codes for adverse events related to specific drug classes as well as an additional six codes covering poisoning related to several specific classes of drugs such as sedatives, analgesics, barbiturates, etc. These codes did not specifically state if the DRP was nausea, drowsiness etc., only that they were serious enough for a healthcare provider to document them. Risks for DRPs from polypharmacy regimens were recorded when medication duplication, published contraindicated drug interactions, or metabolic interactions existed. Drugs in the top 100 listings with FDA Black Box warnings were also identified as placing patients at risk for DRPs when included in a polypharmacy profile. Contraindications and Black Box warnings were determined using Lexicomp, Epocrates, and Micromedex resources. The overall number of DRPs was examined in the SCI and control group with respect to overall polypharmacy as well as with respect to the high-risk combination. The average incidences of visits for DRPs to any healthcare settings per 1000 persons over 1, 2, and 3 years were calculated for patients with SCI and control patients with polypharmacy regimens.
Analysis
Descriptive analysis (mean, SD) was used to compare the SCI and control groups with respect to the overall prevalence of polypharmacy in each group as well as the overall percentages of individuals treated with high-risk combination regimens. The overall mean number of medications as well as the mean number of high-risk medications in the SCI and control groups was compared using a t-test with significance set at P < 0.05. Since the number of individuals in both the SCI and control groups was so large, we also calculated the 95% confidence intervals (CIs) for comparison between groups. The comparison of overall prevalence of DRPs and as well as the DRPs associated with high-risk medication use were conducted using t-test with P < 0.05. In addition, odds ratio (OR) were used to compare the incidence of DRP between the SCI and control groups as well as polypharmacy and non-polypharmacy groups.
Results
There were a total of 13160 individuals with SCI in the dataset for the years 2007–2009. Of these 13160 individuals, 7399 (56%) met our criteria for polypharmacy (prescribed at least five medications concomitantly; Fig. 1 and Table 1). The remaining 5761 cases did not meet our definition of polypharmacy and were excluded from the remainder of the analysis. Within the polypharmacy SCI group, 2533 individuals were classified as having tetraplegia, 3095 individuals were classified as having paraplegia, and 1771 individuals were categorized as non-specific SCI without either designation. There was a relatively equal distribution between males (n = 3565) and females (n = 3834). Comparison of the incidence of polypharmacy by SCI type (paraplegia, tetraplegia, and complex) showed no difference; 65% of individuals with paraplegia, 61% with tetraplegia, and 66% of those with complex SCI met the criteria for polypharmacy. In addition, the majority of individuals in each of the SCI groups were in the 5–9 medications category (62% of those with paraplegia, 57% of those with tetraplegia, and 59% with complex SCI). The percentage of individuals on higher numbers of medications was comparable between groups. The only exception was a small number of individuals (<1%), in both the paraplegia and tetraplegia groups, that had been prescribed 34–39 medications concomitantly.
Figure 1.
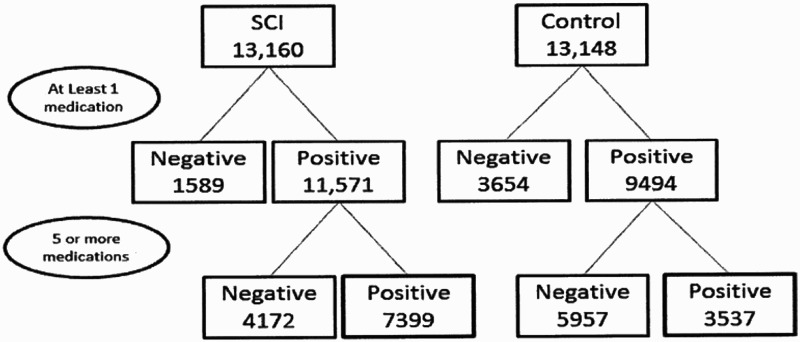
Sample size flow diagram.
Table 1.
Incidence of polypharmacy in SCI and control populations
| SCI |
Control |
||||
|---|---|---|---|---|---|
| Number of drugs prescribed | Number of patients | % of Total SCI population | Number of drugs prescribed | Number of patients | % of Total control population |
| 5–9 | 4401 | 33% | 5–9 | 2607 | 20% |
| 10–14 | 1962 | 15% | 10–14 | 725 | 5.5% |
| 15–19 | 754 | 6% | 15–19 | 155 | 1.2% |
| 20 or more | 282 | 2% | 20 or more | 50 | 0.3% |
| Total | 7399 | 56% | 3537 | 27% | |
Table1 demonstrates a significantly higher number of individuals with SCI were on five or more medications compared with the non-SCI group (11.07 ± 3.58 vs. 7.88 ± 2.95; X ± SD, P < 0.001). Twenty-three percent of the total SCI population was on 10 or more medications compared to only 7% in the control group.
The control group contained 13 148 individuals, of whom 3537 (27%) met our criteria for polypharmacy with a relatively equal distribution between males (n = 1603) and females (n = 1934). Overall, the patients in the SCI population that met our criteria for polypharmacy were prescribed significantly more medications than their control counterparts (11.07 ± 3.58 vs. 7.88 ± 2.95; P < 0.0001). When examining the CIs, we are 95% confident the individuals with SCI were prescribed on average 3.19 (3.05–3.32) more medications than the controls.
High-risk drug analysis
Of the 7399 individuals within the SCI group that met the polypharmacy criteria, 92% (n = 6831) had been prescribed at least one medication from the high-risk classes of interest vs. 44% (n = 2507) in the control group (Table 2). Overall, this difference in high-risk medication prescription between the SCI and control groups was statistically significant (5.49 ± 4.33 vs. 3 ± 2.51; P < 0.0001). At 95% confidence, the individuals with SCI were prescribed on average 2.49 (CI: 2.31–2.67) more high-risk medications than the controls.
Table 2.
Incidence of high-risk medication prescription
| SCI |
Control |
||||
|---|---|---|---|---|---|
| Number of distinct high-risk classes | Number of subjects | % of Total SCI group with polypharmacy (n = 7399) | Number of distinct high-risk classes | Number of subjects | % of Total control group with polypharmacy (n = 5761) |
| 1 | 1738 | 24% | 1 | 1129 | 20% |
| 2 | 1881 | 25% | 2 | 790 | 14% |
| 3 | 1692 | 23% | 3 | 358 | 6% |
| 4 | 841 | 11% | 4 | 157 | 3% |
| 5 | 520 | 7% | 5 | 58 | 1% |
| 6 | 147 | 2% | 6 | 15 | <1% |
| 7 | 12 | <1% | 7 | 0 | 0% |
| Total | 6831 | 92% | 2507 | 44% | |
Table 2 demonstrated a significantly higher percentage of individuals with SCI, who were classified as being on polypharmacy, were prescribed medications from more than one or more of the high-risk classes of medications (5.49 ± 4.33 vs. 3 ± 2.51; X ± SD, P < 0.0001). Sixty-eight percent of the SCI polypharmacy group was on two or more high-risk medications compared to 24% of controls.
The SCI group had a higher percentage of individuals who were prescribed drugs from more than one class compared to the control population (68 vs. 24%; Table 2). In addition, the SCI group had a higher percentage of individuals prescribed medications from three or more of the seven classes of interest compared with the non-SCI group (43 vs. 10%). The two drug classes with the largest number of individuals (in both the SCI and control groups) were the analgesic-narcotics and anticonvulsants. In the SCI population, 18% individuals had been prescribed more than one analgesic-narcotic medication compared to 4% of the control population and individuals with SCI were 5.5 times more likely to be on two or more narcotic than controls (CI: 4.56–6.58; P < 0.05).
Incidence of DRPs
Of the population that were on five or more medications, individuals with SCI had a significantly higher percentage of reported DRPs compared to control (8.6 vs. 6.14%; P < 0.05). The OR comparison between the SCI and control groups was 1.52 (CI: 1.3–1.79; P < 0.0001). In other words, individuals with SCI were 1.52 times more likely to have a DRP compared to those in the control group. To further determine the impact of polypharmacy on the incidence of DRP, we also compared the polypharmacy groups with the non-polypharmacy groups (those individuals taking less than five medications). In the SCI population, individuals with polypharmacy had a significantly higher percentage of reported DRP compared to those individuals with SCI that were on less than five medications (8.6 vs. 2.13%; P < 0.05). In addition, examination of the OR determined that polypharmacy was 3.7 times (CI: 2.71–5.12) more likely to be the reason for DRPs in the SCI vs. control groups. With respect to the percentage of individuals within the different high-risk class of medications, the three classes with the highest percentage of individuals with DRPs were analgesic-narcotics, anticonvulsants, and serotoninergics (Table 3).
Table 3.
Incidence of DRPs in the polypharmacy groups
| SCI |
Control |
|||
|---|---|---|---|---|
| High-risk drug class | Number of subjects on class at time of DRP | % of subjects with a DRP | Number of subjects on class at time of DRP | % of subjects with a DRP |
| Narcotics | 273 | 43% | 30 | 14% |
| Anticonvulsants | 191 | 30% | 25 | 11% |
| Serotoninergics | 187 | 29% | 40 | 18% |
| Antianxiety | 147 | 23% | 19 | 9% |
| Skeletal muscle relaxants | 105 | 16% | 8 | 4% |
| Sedatives | 87 | 14% | 14 | 6% |
| Tricyclic antidepressants | 31 | 5% | 8 | 4% |
| Total | 637 | 217 | ||
Table 3 demonstrated the percentage of individuals who had a reported DRP and were taking a medication within one of the high-risk classes of drugs (8.6 vs. 2.13; P < 0.05). For example, 43% of individuals with SCI who were on a narcotic also reported a DRP compared to 14% of controls.
Examination of the incidence of DRPs over time determined that over a 1-year period of time, there was a 41 of 1000 incidence rate for DRPs in the SCI population compared with 27 of 1000 in the control group. For individuals who were followed over 2 years, the incidence rate in the SCI population was 69 of 1000 compared to 58 of 1000 in the control group. For individuals who were followed over 3 years, the incidence rates for DRPs in the SCI population were 92 of 1000 compared with 74 of 1000 in the control group.
Discussion
Our study is one of the few that has examined polypharmacy in the SCI population and showed in fact a high incidence of polypharmacy in this population as well as increased risk of DRPs compared to a population of individuals without SCI. Fifty-six percent of the individuals with SCI in our study were treated with more than five medications concomitantly, placing them at higher risk for DRPs. This was evidenced by the incidence of DRPs being signficantly higher in individuals with SCI compared to those without SCI as well as those with SCI taking five or more medications vs. those taking less than five medications. Secondary complications requiring pharmacological management include chronic pain and individuals with SCI frequently receive combination therapies using two or more analgesics with different mechanisms of action. Neuropathic pain is typically treated with non-conventional agents including anticonvulsants (e.g. gabapentin) or antidepressants. In addition, while combination treatment may result in greater pain relief, clinical trials regarding different combinations of analgesics are limited, especially with respect to determining which combinations to use, the occurrence of additive or supra-additive effects, sequential or concurrent treatment, or the adverse-event profiles of these analgesics.22 A recent study followed 54 individuals with SCI over a 3-month period to evaluate the use of opoid and anticonvulsant combination therapy for treating SCI-induced neuropathic pain.12 While improved outcomes for these individuals were observed, over 53% of these individuals showed at least one treatment-releated adverse event. The results of this study are significantly higher than the 8.6% reported incidence of DRP in our study. Possible reasons for this disparity include the E-codes that were used in the current study likely do not cover all possible treatment-related problems. In addition, E-codes do not represent specific symptoms (e.g. nausea, dizziness, etc.) only that the event was serious enough to be documented by the healthcare provider. So the use of the E-codes could under-represent the actual number of DRP. In addition, the Barrera-Chacon study actually followed the indivduals over a 3-month period of time and therefore, more likely to report DRPs that would normally not be reported by the individuals who are only making routine follow-up care appointments or may not recognize the problems they are dealing with are related to the medications and not just a secondary effect of having a SCI. However, eventhough the overall numbers differ between our two studies, the overall results are the same in that individuals with SCI, who are on multiple medications, are at greater risk for DRPs.
The challenges of pharmacological management following SCI as comorbidities continue to evolve throughout recovery and the lifetime of the patient. Loss of neurologic function, changes in sensation, psychological health, and nutritional challenges each place the patient at risk of progressive and recurrent complications that require additional medications. Predicting optimal dosing regimens is also difficult because of the altered muscle mass and physiological changes affecting drug absorption, distribution, metabolism, and excretion.13 These endogenous changes are independent of the drug–drug interactions and clinical conditions that require personal attention to each regimen. Patients also frequently self-medicate using alternative medications and over the counter products further complicating interpretation of patient responses to prescribed medications. Increased usage of alcohol or drugs such as marijuana has been documented in the SCI population and can have a direct impact on cognition, function, and long-term quality of life.23–25 While the use of alcohol and drugs such as marijuana have been shown to be high in the SCI population, they would not be prescribed by healthcare providers and their use would not be represented in the database.
Drug combinations used for individuals with both SCI and traumatic brain injury (TBI), may inherently act on the cognitive and neurologic functions and require monitoring for impact on recovery and treatment outcome goals. In addition, several recent studies have indicated that using medications to control spasticity can significantly impact the person's ability to ambulate, and the use of psychotropic medications may actually decrease the life expectancy of individuals with SCI.9–11 Physicians, as well as consumers, need to be aware of the relationship between psychotropic prescription medication use and mortality and be cautious when prescribing these medications, particularly multiple medications for different symptoms (i.e. pain, spasticity, sleep, and depression).10
Study limitations
In the polypharmacy literature, there are a number of variables that can be covariates and which can affect the interpretation of our results (e.g. the number of medications prior to injury and psychiatric conditions). While these are very important variables, we did not have data related to the study subjects prior to their injury. These variables will be the focus of future studies. In addition, the DRP had to be serious enough for a healthcare provider to document it. Therefore, the use of the E-codes may not cover all possible treatment-related problems and their use could under-represent the actual number of DRPs. While the current study focused on classes of medications that work at the neuromuscular level, there are additional classes of medications that are extremely important to the SCI population (e.g. cardiac, anti-inflammatory, sexual dysfunction, etc.), and will be the focus of subsequent studies. In addition, DRPs related to the discontinuance of medications is a serious issue. Unfortunately, the complexity of correlating discontinuance of a medication with the occurance of a DRP, from the information provided in this national database, was beyond the scope of this intial study and will be examined in future studies. Finally, the current study was not able to account for medications obtained from other sources (i.e. not prescribed by a healthcare provider), and thus not recorded within the dataset.
Conclusion
The use of multiple medications is a common standard of care in management of complications following SCI. The complexity of treatment regimens following SCI places patients at increased risk for DRPs including adverse drug events. The current study confirmed this by demonstrating individuals with SCI are more likely to be prescribed five or medications, to be taking more high-risk medications and have a higher incidence of DRPs. The higher rate of polypharmacy and DRPs can impact rehabilitation goals and community integration following neurologic injury. Therefore, polypharmacy needs to be recognized and discusssed by both healthcare providers as well as the patients they serve.
Acknowledgments
Darrin Cecil had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Each of the authors acknowledges no conflicts of interest.
Disclaimer statements
Contributors There were no additional contributors to this research.
Funding This work was supported in part through a grant from the National Institutes of Health, National Center on Minority Health and Health Disparities (RC-4MD005760; PI; PK).
Conflicts of interest None.
Ethics This research was approved by the University of Kentucky's Office of Research Integrity's Institutional Review Board.
References
- 1.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 1994;43(6):749–56. [DOI] [PubMed] [Google Scholar]
- 2.Kroll T, Neri MT, Ho PS. Secondary conditions in spinal cord injury: results from a prospective survey. Disabil Rehabil 2007;29(15):1229–37. [DOI] [PubMed] [Google Scholar]
- 3.Jensen MP, Kuehn CM, Amtmann D, Cardenas DD. Symptom burden in persons with spinal cord injury. Arch Phys Med Rehabil 2007;88(5):638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiodo AE, Scelza WM, Kirshblum SC, Wuermser LA, Ho CH, Priebe MM. Spinal cord injury medicine. 5. Long-term medical issues and health maintenance. Arch Phys Med Rehabil 2007;883 Suppl 1:S76–83. [DOI] [PubMed] [Google Scholar]
- 5.Noonan VK, Kopec JA, Zhang H, Dvorak MF. Impact of associated conditions resulting from spinal cord injury on health status and quality of life in people with traumatic central cord syndrome. Arch Phys Med Rehabil 2008;89(6):1074–82. [DOI] [PubMed] [Google Scholar]
- 6.Bourgeois FT, Shannon MW, Valim C, Mandl KD. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf 2010;19(9):901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard RL, Avery AJ, Slavenburg S, Royal S, Pipe G, Lucassen P et al. . Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 2007;63(2):136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viktil KK, Blix HS, Moger TA, Reikvam A. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol 2007;63(2):187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohout RK, Saunders LL, Krause JS. The relationship between prescription medication use and ability to ambulate distances after spinal cord injury. Arch Phys Med Rehabil 2011;92(8):1246–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause JS, Zhai Y, Saunders LL, Carter RE. Risk of mortality after spinal cord injury: an 8-year prospective study. Arch Phys Med Rehabil 2009;90(10):1708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krause JS, Saunders LL. Risk of mortality and life expectancy after spinal cord injury: the role of health behaviors and participation. Top Spinal Cord Inj Rehabil 2010;16(2):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrera-Chacon JM, Mendez-Suarez JL, Jauregui-Abrisqueta ML, Palazon R, Barbara-Bataller E, Garcia-Obrero I. Oxycodone improves pain control and quality of life in anticonvulsant-pretreated spinal cord-injured patients with neuropathic pain. Spinal Cord 2011;49(1):36–42. [DOI] [PubMed] [Google Scholar]
- 13.Mestre H, Alkon T, Salazar S, Ibarra A. Spinal cord injury sequelae alter drug pharmacokinetics: an overview. Spinal Cord 2011;49(9):955–60. [DOI] [PubMed] [Google Scholar]
- 14.Segal JL, Brunnemann SR, Gordon SK, Eltorai IM. Amikacin pharmacokinetics in patients with spinal cord injury. Pharmacotherapy 1988;8(2):79–81. [DOI] [PubMed] [Google Scholar]
- 15.Segal JL, Brunnemann SR, Gray DR. Gentamicin bioavailability and single-dose pharmacokinetics in spinal cord injury. Drug Intell Clin Pharm 1988;22(6):461–5. [DOI] [PubMed] [Google Scholar]
- 16.Gore M, Brix Finnerup N, Sadosky A, Tai KS, Cappelleri JC, Mardekian J et al. . Pain-related pharmacotherapy, healthcare resource use and costs in spinal cord injury patients prescribed pregabalin. Spinal Cord 2013;51(2):126–33. [DOI] [PubMed] [Google Scholar]
- 17.Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M. Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department: a prospective survey. Drugs Aging 2009;26(6):475–82. [DOI] [PubMed] [Google Scholar]
- 18.Pedros C, Quintana B, Rebolledo M, Porta N, Vallano A, Arnau JM. Prevalence, risk factors and main features of adverse drug reactions leading to hospital admission. Eur J Clin Pharmacol 2014;70(3):361–7. [DOI] [PubMed] [Google Scholar]
- 19.Rouleau P, Guertin PA. Traumatic and nontraumatic spinal-cord-injured patients in Quebec, Canada. Part 3: pharmacological characteristics. Spinal Cord 2011;49(2):186–95. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen T, Johansson S, Kennerfalk A, Wallander MA, Svardsudd K. Prescription drug use, diagnoses, and healthcare utilization among the elderly. Ann Pharmacother 2001;35(9):1004–9. [DOI] [PubMed] [Google Scholar]
- 21.Linjakumpu T, Hartikainen S, Klaukka T, Veijola J, Kivela SL, Isoaho R. Use of medications and polypharmacy are increasing among the elderly. J Clin Epidemiol 2002;55(8):809–17. [DOI] [PubMed] [Google Scholar]
- 22.Vranken JH. Elucidation of pathophysiology and treatment of neuropathic pain. Cent Nerv Syst Agents Med Chem 2012;12(4):304–14. [DOI] [PubMed] [Google Scholar]
- 23.Hwang M, Chlan KM, Vogel LC, Zebracki K. Substance use in young adults with pediatric-onset spinal cord injury. Spinal Cord 2012;50(7):497–501. [DOI] [PubMed] [Google Scholar]
- 24.January AM, Zebracki K, Chlan KM, Vogel LC. Mental health and risk of secondary medical complications in adults with pediatric-onset spinal cord injury. Top Spinal Cord Inj Rehabil 2014;20(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders LL, Krause JS. Psychological factors affecting alcohol use after spinal cord injury. Spinal Cord 2011;49(5):637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]


