Abstract
Background
Due to interruption of cardiovascular autonomic control unstable blood pressure (BP) is common in individuals with spinal cord injury (SCI) above the sixth thoracic vertebral level. The impact of unstable BP on cerebral blood flow (CBF) is not well appreciated, but symptoms associated with altered cerebral perfusion are reported, which can negatively impact daily life activities.
Methods
We measured seated BP and CBF in participants with SCI and able-bodied (AB) controls on three laboratory visits to determine the inter-day reliability (intraclass correlation coefficient: ICC). BP was assessed at the finger using photoplethysmography and at the brachial artery with manual sphygmomanometry. CBF velocities (CBFv) were assessed at the middle cerebral artery using transcranial Doppler (TCD) ultrasound.
Results
Data were collected in 15 participants with chronic SCI (C3-T4) and 10 AB controls, the groups did not differ for age, height, weight or BMI; however, brachial BP (P < 0.001), finger BP (P < 0.01) and CBFv (P < 0.05) were significantly lower in the SCI group compared to the controls. The inter-day ICC for brachial BP ranged from 0.51 to 0.79, whereas the ICC for finger BP was not as high (0.17 to 0.47). The inter-day ICC for CBFv ranged from 0.45 to 0.96, indicating fair to substantial reliability.
Conclusions
These data indicate good inter-day reliability of brachial BP and TCD recording of CBFv; however, the assessment of finger BP appears to be somewhat less reliable. In addition, these data confirm reduced resting CBFv in association with hypotension in individuals with SCI compared to matched controls with low BP.
Keywords: Tetraplegia, Paraplegia, Cerebral blood flow, Blood pressure, Reliability
Introduction
Unstable blood pressure (BP) is commonly reported in individuals with spinal cord injury (SCI), which has been attributed to disruption of supraspinal autonomic cardiovascular control.1–4 Specifically, individuals with SCI above the sixth thoracic vertebral level are often chronically hypotensive5,6 with an increased prevalence of orthostatic hypotension (OH)7,8 and these individuals also may suffer large life-threatening increases in BP during a bout of autonomic dysreflexia (AD).1–3 As such, wide fluctuations in daily BP are likely, and day-to-day variation in the clinical assessment of BP may render diagnosis and treatment ineffective, particularly if internal and external conditions are not kept consistent. For instance, we reported dissociation between the prevalence of hypotension (≈40%), and the diagnosis and treatment (<1%), and found that the diagnosis of hypertension was 46%; whereas diagnosis of AD was 5%, regardless of the level of lesion in veterans with SCI.1,9 Because clinical assessment is dependent on the day-to-day reproducibility of the measurement tool, as well as an understanding of the metrics of reliability, it is important that day-to-day reliability of BP assessments be documented in the SCI population.
The impact of disrupted sympathetic nervous system control of the cerebral vasculature is not well appreciated in the SCI population because many individuals with SCI do not report symptoms of altered cerebral blood flow (CBF), which may result from unstable BP. However, increases in BP that are associated with AD can cause severe headaches and dizziness episodes which adversely impact daily activities10 and in extreme cases can result in cerebral hemorrhage and death.11–14 In the general population persistent hypotension may result in cerebral hypoperfusion,15,16 and it was recently reported that chronic cerebral hypoperfusion, secondary to diabetes, contributed to accelerated cognitive decline and neuronal cell death.17 Further, an increased incidence of hemorrhagic and ischemic stroke was noted in the Taiwanese SCI population (n=2806) compared to propensity-score matched controls (n = 28,060), which the authors speculate may relate to fluctuations in BP.18 Transcranial Doppler ultrasound (TCD) technology has been used to non-invasively assess CBF velocity (CBFv) at the middle cerebral artery (MCA)19–24 and the posterior cerebral arteries 23,24 in the SCI population. From TCD recordings of the MCA, reduced resting CBFv has been documented in persons with SCI 22,25 and altered static and dynamic autoregulation compared to controls is reported.26 However, there is conflicting results as other laboratories have documented similar MCA CBFv and functional cerebral autoregulation compared to controls.20,24 Because unstable BP may adversely impact cerebral perfusion, the day-to-day reliability of TCD recordings of CBFv should be determined in the SCI population. In addition to gaining an appreciation of the day-to-day reliability of BP and CBFv assessments in the SCI population, documenting the degree of variation inherent in these physiological signals will validate these measurement tools for use in determining the efficacy of treatment strategies aimed at improving cardiovascular and cerebrovascular stability.
The primary study objective was to determine the inter-day reliability metrics of brachial and finger BP and TCD recordings of CBFv assessments in individuals with SCI and able-bodied (AB) controls. A secondary objective was to compare seated resting CBFv between individuals with SCI and controls. We hypothesized that the day-to-day assessment of systemic and cerebral hemodynamics would be reliable in both the SCI and control groups and that, due to autonomic cardiovascular impairment resting CBFv would be significantly reduced in individuals with SCI compared to the healthy controls.
Methods
Participants
All participants (n = 25) were between the ages of 22 and 61 years with no known history of CVD, pulmonary disease, or diabetes mellitus, no acute illness or infection. Participants were not taking prescription medications for CVD and all were confirmed non-smokers. Participants with SCI (n = 15) were matched for demographic characteristics to the AB controls (n = 10)) and all participants were asked to refrain from physical activity for 12 hours and from eating for 4 hours prior to testing. The Institutional Review Board for Human Studies of the James J. Peters Veterans Affairs Medical Center granted approval for the study, and informed consent was obtained prior to initiation of the study procedures.
Study procedures
Participants visited the laboratory on 3 separate occasions and assessment of resting heart rate (HR), BP and CBFv was recorded twice in the seated position by the same study technician. Participants arrived at the laboratory approximately the same time of day on all 3 study visits and remained seated for the duration of testing. Prior to data collection, a 20-minute period of quiet rest was provided in a thermo-neutral, dimly lit room while the participants were instrumented with three ECG electrodes, the BP cuffs were fitted to the middle finger of the right hand and secured at the left brachial artery. A head harness was placed and secured to the temporal region for TCD recording of the left MCA. After instrumentation, two 5-minute assessments of simultaneous beat-to-beat systemic and cerebral hemodynamics were recorded, which were initiated at 0 and 15 minutes after the 20 minutes of quiet rest. Manual BP was assessed once during each 5-minute data recording period.
Heart rate & blood pressure monitoring
Instrumentation included continuous monitoring of the inter-beat-interval (IBI: msec) of HR using a standard electrocardiogram (Ivy Biomedical Systems Inc., Branford, CT, USA). The ECG signal was recorded from a 3-lead configuration; lead site preparation was performed according to clinical standards and the electrodes were placed at the distal right and left clavicle and in the left lateral 5th intercostal space (V-5). Participants were in the seated upright position with the right arm supported on a table horizontal to the level of the heart for the duration of testing; beat-to-beat finger BP (mmHg) was continuously monitored at the right middle finger using photoplethysmography (Finometer ® MIDI Model-2; Finopres Medical Systems B.V., Amsterdam, The Netherlands). Although the right middle finger was used to assess finger BP in most participants, due to hand contractures and spasms, other fingers were used in a few participants; however, the digit used was kept consistent throughout the study. The finger BP cuff size was standardize throughout the study and a height calibration procedure was performed immediately (30–60 seconds) before each data collection segment. The ECG and finger BP signals were sampled at 500 Hz and were stored on a hard-drive for subsequent analysis using customized data analysis programs written with LabVIEW graphical software for instrumentation (National Instruments, Austin, TX, USA), which allowed study investigators the ability to remove any erroneous BP waveforms resulting from a spasm or due to motion artifact; however waveform reconstruction was not available on the version of finometer we used in this investigation. Brachial BP was monitored at the left brachial artery using a manual sphygmomanometer with adult BP cuff (GE Healthcare Information Technologies, Milwaukee, WI, USA). The systemic hemodynamic variables of interest included IBI, finger and brachial SBP, DBP and mean arterial pressure (MAP), which was calculated as: (SBP + DBP + DBP)/3.
Cerebral blood flow velocity monitoring
TCD ultrasound technology (Terumo Cardiovascular Systems, Tustin, CA, USA) was used to assess resting CBFv in the left MCA through the temporal window. The TCD probe was operated at a frequency of 2.0 MHz and MCA was identified by the target depth (45–55 mm), the sound and direction of flow (i.e. towards the probe), the characteristic spectral waveform (i.e. relatively faster flow compared to other cerebral vessels), and by compression of the common carotid artery which resulted in an appropriate reduction in MCA flow velocity. Once the MCA was visualized, a head-harness was used to secure probe position for subsequent testing. TCD data were collected simultaneously with the ECG and finger BP signals over the two 5-minute periods and the output included systolic flow velocity (SFV: cm/s), diastolic flow velocity (DFV: cm/s) and mean flow velocity (MFV: cm/s). Because it has been demonstrated that the diameter of the MCA does not change in response to moderate perturbation,27 it is generally well accepted that MFV reflects CBF, which has been validated and reported by several investigators.28–30 TCD signals were sampled at 500 Hz and were stored on a hard-drive for subsequent analysis using customized data acquisition and analysis programs written with LabVIEW graphical software for instrumentation.
Data analysis
Data are reported as mean ± standard deviation and were analyzed using a statistical analysis program (IBM SPSS Statistics, version 21, IBM Corp., Armonk, NY, USA). Demographic data were compared between the SCI and AB groups using unpaired t-tests. Seated resting systemic and cerebral hemodynamic data were compared with mixed factorial ANOVA models to test main and interaction effects for group (AB; SCI), intra-day (trial), and inter-day (visit). Reliability of the systemic and cerebral variables of interest was quantified using the procedures described by Weir.31 Specifically, inter-day systematic error for each dependent variable was assessed using single factor repeated measures ANOVA models with the intra-day score calculated from the mean of the two measurements collected within a given test day. From the resulting variance components in the ANOVA table, the intra-class correlation coefficient (ICC, model 2,1) and the standard error of measurement (SEME) were calculated. The SEME was calculated from the square root of the mean square error term from the repeated measures ANOVA.31 From the SEME, both the minimal detectable difference (MDD; also known as smallest detectable difference) and the coefficient of variation (CoV) were calculated. The MDD, which was calculated as: SEME*1.96*√2, where 1.96 is the Z score for a 95% level of confidence, is a useful clinical metric, reflecting the smallest degree of change necessary to exceed the typical measurement error for any particular test or procedure. As such, individual changes that are greater than or equal to the MDD can be interpreted, at the 95% level of confidence, as being sufficiently large to be considered a “real” change. The CoV was calculated as: (SEME ÷ grand mean) × 100, and reflects normalization of the SEME to the average score. To assess systematic differences (bias) between absolute and BP recordings with the brachial and finger techniques, repeated measures ANOVAs were performed to determine main and interaction effects for method (brachial vs. finger), trial (intra-day) and visit (inter-day). All analyses were conducted with α = 0.05.
Results
Study participants
There were no significant group differences for demographic characteristics of the participants (Table 1). The study population was largely male (75%), 11 were veterans (44%), and the sample was predominantly Caucasian (52%). Participants with SCI were chronically injured (range: 1–42 years), mostly cervical lesions (80%), and two-thirds were classified by the American Spinal Injury Association (ASIA) Injury Scale (AIS) as motor complete injuries (AIS A & B).
Table 1.
Subject characteristics
| Total | SCI | AB | |
|---|---|---|---|
| n = 25 | n = 15 | n = 10 | |
| Age (y) | 40 ± 12 | 43 ± 12 | 35 ± 11 |
| Age range (y) | 22–61 | 22–61 | 22–58 |
| HT (m) | 1.73 ± 0.09 | 1.76 ± 0.07 | 1.70 ± 0.11 |
| WT (kg) | 69 ± 11 | 70 ± 10 | 67 ± 12 |
| BMI (kg/m2) | 22.9 ± 3.2 | 22.8 ± 3.6 | 23.0 ± 2.7 |
| Female Sex (n) | 6 (24%) | 2 (13%) | 4 (40%) |
| Duration of SCI (y) | 13 ± 12 | ||
| Level of SCI | C3-T4 | ||
| Complete AIS A | 5 (33%) |
y = years; HT = height; m = meters; WT = weight; kg = kilograms; BMI = body mass index.
Systemic & cerebral hemodynamic data
There were no significant main or interaction effects for IBI (data not shown). The main and interaction effects for the intra-day (trial) and inter-day (visit) comparisons were not significant for manual BP; however the main effect for group was significant, suggesting that regardless of trial or visit, brachial BP was lower in the SCI compared to the AB group (Fig. 1A: 95% CI for the difference between groups: SBP: 10.6–25.1 mmHg; DBP: 4.1–16.5 mmHg; MAP: 4.7–17.2 mmHg). Main and interaction effects were not significant for the finger BP assessments; however both SBP and MAP were reduced in the SCI compared to the control group (Figure 1B). The main and interaction effects for trial and visit were not significant for CBFv; however, the group main effect was significant, indicating that regardless of trial or visit, CBFv was lower in the SCI compared to the AB group (Fig. 2: 95% CI for the difference between groups: SFV: 4.5–33.0 cm/s; DFV: 2.6–19.6 cm/s; MFV: 5.3–24.7 cm/s).
Figure 1.
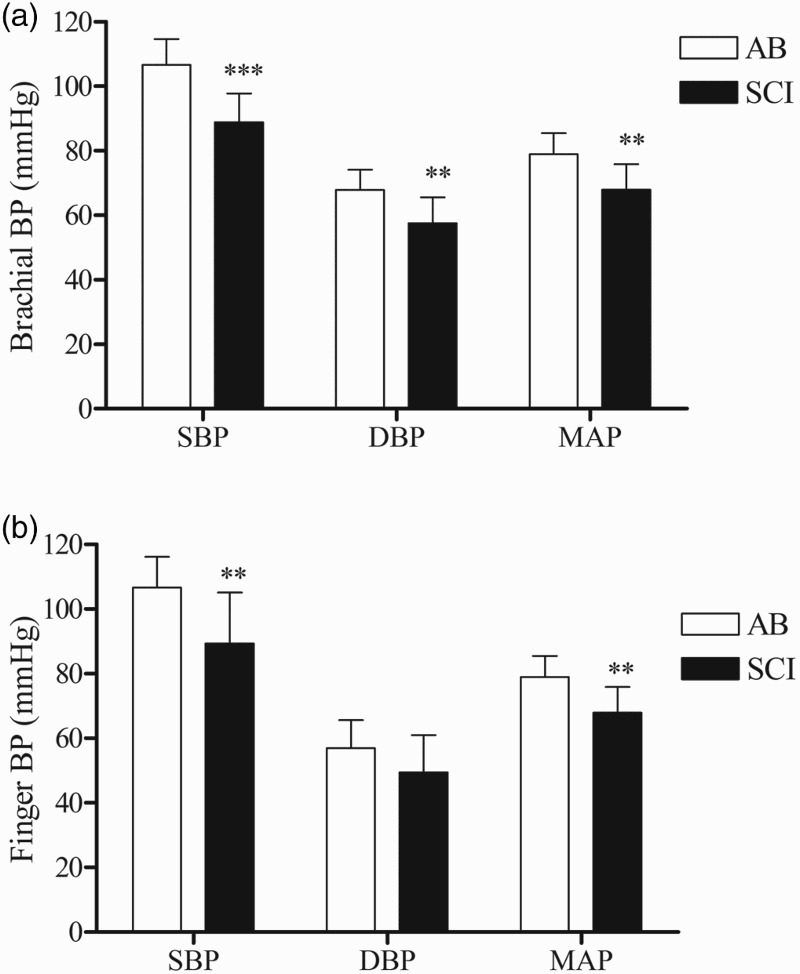
Depicts the mean ± SD of [A] brachial and [B] finger SBP, DBP and MAP for the AB (white bars) and SCI (black bars) groups. The main effect for group was significant; *** P < 0.001; ** P < 0.01 versus the AB group.
Figure 2.
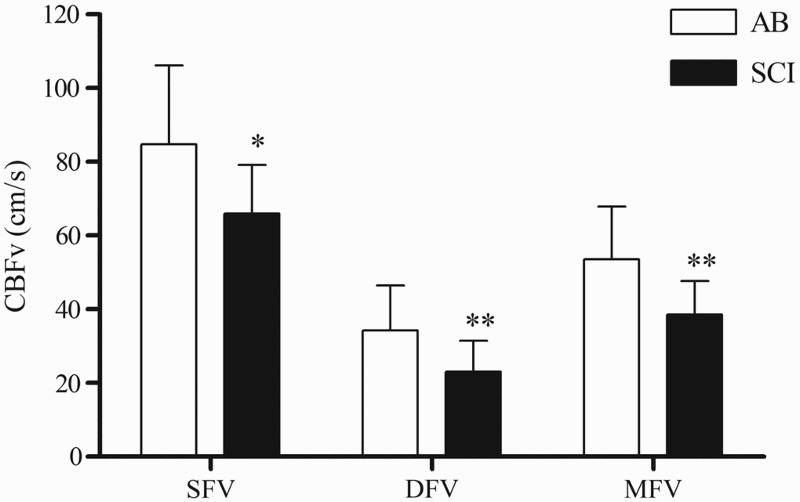
Depicts the mean ± SD of the SFV, DFV and MFV for the AB (white bars) and SCI (black bars) groups. The main effect for group was significant; * P < 0.05; ** P < 0.01 versus the AB group.
Test-retest reliability
The ICC data from the ANOVA models for each of the variables of interest is presented for both the SCI and AB groups together (Table 2A), the SCI group alone (Table 2B) and the AB group alone (Table 2C). First, for all dependent variables, there were no systematic differences in mean responses across trials (all intra-day: P ≥ 0.05). The inter-day ICC values for the IBI assessment ranged from 0.45 to 0.86, indicating “fair” to “substantial” day-to-day reliability.32 In general, the inter-day ICC values were higher for brachial BP than for finger BP assessments, indicating increased within-subject day-to-day variability with photoplethysmography at the finger; this trend was similar in AB and the SCI groups for SBP (Fig. 3) and DBP (Fig. 4). Point estimates for ICC values of brachial BP ranged from 0.51 to 0.79, which fall in the categories of “fair” to “substantial” reliability using the scheme proposed by Shrout32; the SEME values ranged from 3.8 to 7.2 mmHg and the CoV ranged from 4.2 to 11.7 %. ICC values for finger BP were less reliable and ranged from 0.17 to 0.47, indicating “slight” to “fair” reliability32; the SEME values ranged from 9.9 to 16.1 mmHg and the CoV values ranged from 12.1 to 23.8 %. The ICC calculations for the CBFv metrics ranged from 0.45 to 0.96 indicating “fair” to “substantial” reliability.32 The SEME for the CBFv metrics ranged 2.0 to 22.4 cm/s and CoV ranged from 10.6 to 22.4 %. Although between-subject variability appeared to be increased in the AB group compared to the SCI group (Fig. 5) test-retest reliability for CBFv was good in both groups (Table 2).
Table 2A.
Inter-day reliability: total group (n = 25)
|
95% CI |
||||||||
|---|---|---|---|---|---|---|---|---|
| F trials | P-value | SEM | CoV (%) | MDD | ICC | Upper | Lower | |
| IBI (msec) | 1.67 | 0.20 | 65.29 | 7.65 | 180.95 | 0.78 | 0.90 | 0.56 |
| Brachial | ||||||||
| SBP (mmHg) | 0.25 | 0.78 | 6.19 | 6.46 | 17.16 | 0.79 | 0.90 | 0.57 |
| DBP (mmHg) | 0.01 | 0.99 | 5.73 | 9.29 | 15.87 | 0.68 | 0.85 | 0.41 |
| MAP (mmHg) | 0.33 | 0.73 | 4.24 | 8.01 | 11.76 | 0.96 | 0.99 | 0.85 |
| Finger | ||||||||
| SBP (mmHg) | 1.36 | 0.27 | 14.65 | 15.22 | 40.61 | 0.46 | 0.71 | 0.15 |
| DBP (mmHg) | 0.94 | 0.40 | 11.95 | 22.77 | 33.11 | 0.34 | 0.63 | 0.06 |
| MAP (mmHg) | 0.42 | 0.66 | 12.32 | 18.24 | 34.14 | 0.47 | 0.72 | 0.16 |
| Middle Cerebral | ||||||||
| SFV (cm/sec) | 2.45 | 0.10 | 8.21 | 11.25 | 22.76 | 0.83 | 0.92 | 0.65 |
| DFV (cm/sec) | 0.30 | 0.74 | 4.74 | 17.30 | 13.13 | 0.85 | 0.93 | 0.68 |
| MFV (cm/sec) | 1.37 | 0.26 | 5.75 | 13.00 | 15.94 | 0.84 | 0.93 | 0.66 |
Table 2B.
Inter-day reliability: SCI only (n = 15)
|
95% CI |
||||||||
|---|---|---|---|---|---|---|---|---|
| F trials | P-value | SEM | CoV | MDD | ICC | Upper | Lower | |
| IBI (msec) | 0.82 | 0.45 | 61.81 | 7.19 | 171.29 | 0.86 | 0.95 | 0.63 |
| Brachial | ||||||||
| SBP (mmHg) | 0.23 | 0.80 | 7.22 | 8.13 | 20.00 | 0.56 | 0.83 | 0.15 |
| DBP (mmHg) | 0.16 | 0.85 | 6.75 | 11.72 | 18.70 | 0.53 | 0.82 | 0.12 |
| MAP (mmHg) | 0.13 | 0.88 | 6.90 | 10.15 | 19.12 | 0.51 | 0.81 | 0.10 |
| Finger | ||||||||
| SBP (mmHg) | 0.72 | 0.49 | 16.11 | 18.02 | 44.63 | 0.39 | 0.74 | 0.03 |
| DBP (mmHg) | 1.62 | 0.22 | 11.78 | 23.81 | 32.64 | 0.37 | 0.73 | 0.02 |
| MAP (mmHg) | 1.07 | 0.36 | 12.65 | 19.72 | 35.07 | 0.44 | 0.77 | 0.05 |
| Middle Cerebral | ||||||||
| SFV (cm/sec) | 1.37 | 0.27 | 8.10 | 12.28 | 22.46 | 0.69 | 0.89 | 0.31 |
| DFV (cm/sec) | 0.03 | 0.97 | 5.17 | 22.39 | 14.33 | 0.71 | 0.90 | 0.34 |
| MFV (cm/sec) | 0.73 | 0.49 | 6.02 | 16.95 | 16.69 | 0.67 | 0.88 | 0.27 |
Table 2C.
Inter-day reliability: AB only (n = 10)
|
95% CI |
||||||||
|---|---|---|---|---|---|---|---|---|
| F trials | P-value | SEM | CoV | MDD | ICC | Upper | Lower | |
| IBI (msec) | 0.78 | 0.47 | 73.49 | 8.70 | 203.66 | 0.44 | 0.83 | 0.00 |
| Brachial | ||||||||
| SBP (mmHg) | 0.63 | 0.55 | 4.43 | 4.16 | 12.29 | 0.75 | 0.94 | 0.28 |
| DBP (mmHg) | 1.13 | 0.35 | 3.76 | 5.54 | 10.41 | 0.71 | 0.92 | 0.20 |
| MAP (mmHg) | 0.30 | 0.74 | 3.77 | 4.78 | 10.46 | 0.74 | 0.94 | 0.26 |
| Finger | ||||||||
| SBP (mmHg) | 0.77 | 0.48 | 13.00 | 12.08 | 36.03 | 0.17 | 0.68 | 0.09 |
| DBP (mmHg) | 2.53 | 0.11 | 10.99 | 19.29 | 30.46 | 0.20 | 0.71 | 0.06 |
| MAP (mmHg) | 1.73 | 0.21 | 9.90 | 13.44 | 27.45 | 0.38 | 0.81 | 0.00 |
| Middle Cerebral | ||||||||
| SFV (cm/sec) | 0.36 | 0.70 | 20.70 | 11.60 | 57.37 | 0.45 | 0.84 | 0.01 |
| DFV (cm/sec) | 2.39 | 0.12 | 2.00 | 12.46 | 5.54 | 0.96 | 0.99 | 0.84 |
| MFV (cm/sec) | 0.32 | 0.73 | 4.28 | 10.59 | 11.86 | 0.92 | 0.98 | 0.70 |
95% CI = 95% confidence interval; IBI = inter-beat-interval; SBP = systolic blood pressure; DBP = diastolic blood pressure; MAP = mean arterial pressure; SFV = systolic flow velocity; DFV = diastolic flow velocity; MFV = mean flow velocity.
Figure 3.
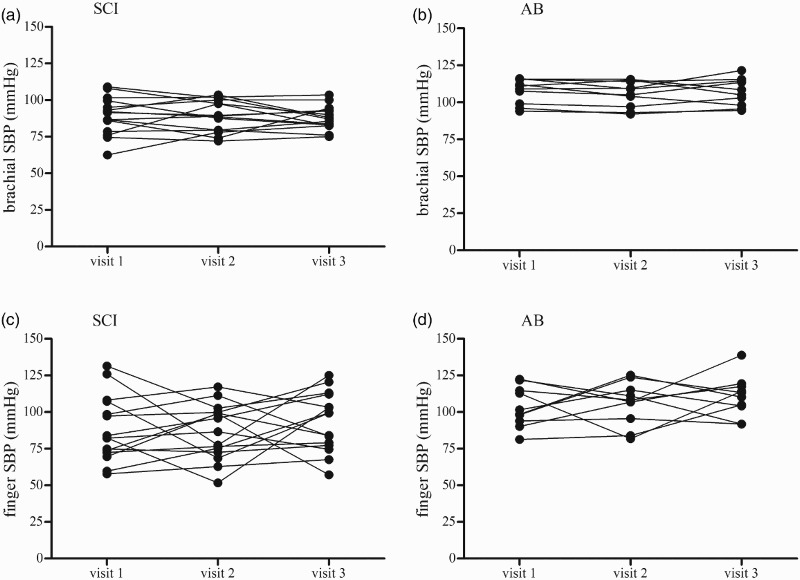
Depicts inter-day (visit) variability in SBP at the brachial artery in the SCI (3A) and AB (3B) participants and at the finger in the SCI (3C) and AB (3D) participants. Data reflect the mean of two measurements taken 15 minutes apart on each study visit.
Figure 4.
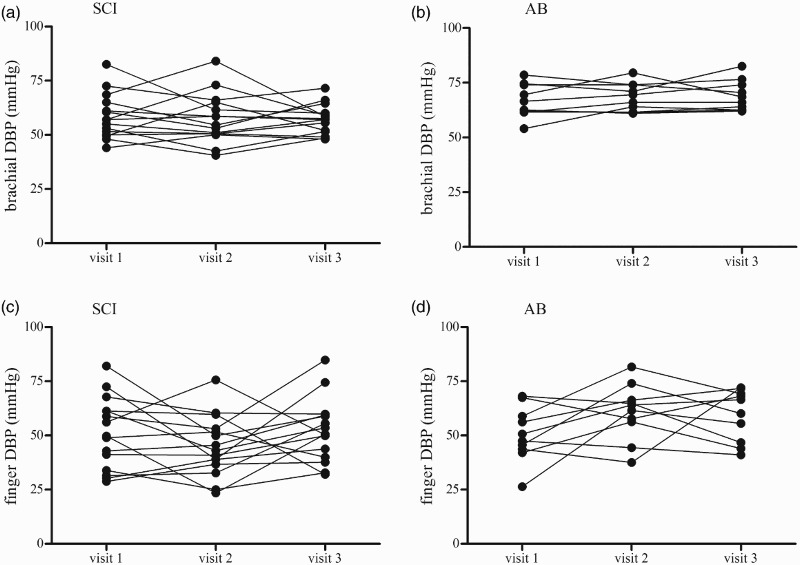
Depicts inter-day (visit) variability in DBP at the brachial artery in the SCI (4A) and AB (4B) participants and at the finger in the SCI (4C) and AB (4D) participants. Data reflect the mean of two measurements taken 15 minutes apart on each study visit.
Figure 5.
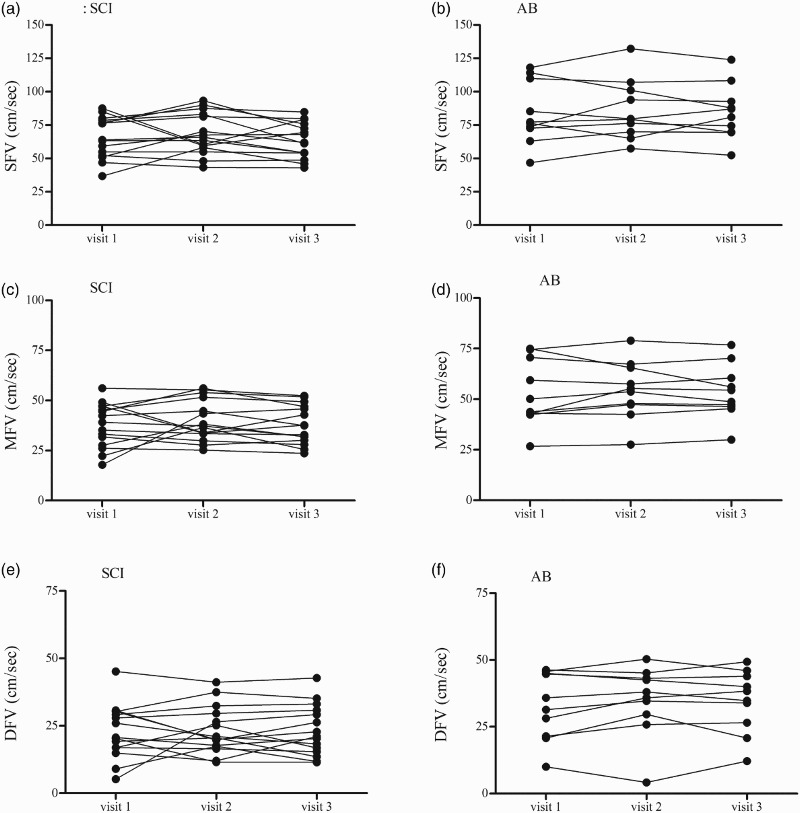
Depicts inter-day (visit) variability in SFV, MFV and DFV in the SCI (5A, 5C, 5E) and AB (5B, 5D, 5F) participants.
Minimal detectable difference
For the BP assessments, the MDD values were markedly lower (i.e. better) with the manual sphygmomanometer based estimates than the finger photoplethysmography estimates. Specifically, for brachial BP the MDD values ranged from 10.4 to 20.0 mmHg while MDD for finger BP ranged from 27.4 to 44.6 mmHg. The MDD for the CBFv metrics ranged from 5.5 to 57.4 cm/s. It must be appreciated that the large MDD value for CBFv reflects proportionally increased SFV in AB group, and not poorer reliability of assessment, because the SEME (CoV) was 11.6% for SFV, which was in line with the other CoV values for the CBFv metrics.
Bias
The ANOVA analyses examining systematic differences (bias) between the brachial and finger BP recordings showed no significant interaction effects. The main effect for method (brachial vs. finger) was not significant for SBP (brachial mean ± SD = 95.9 ± 12.3 mmHg, finger = 96.3 ± 16.0, P = 0.91) or MAP (brachial = 72.3 ± 9.1, finger = 67.0 ± 12.9, P = 0.09); however, the main effect for method was significant for DBP (brachial = 61.7 ± 8.9, finger = 52.5 ± 10.9, P < 0.001, effect size = 0.93).
Discussion
These data suggest that: (1) the inter-day assessment brachial BP appears to be more reliable than finger BP in both SCI and AB groups; (2) inter-day assessment of CBFv was reliable in both the SCI and AB groups; (3) significantly reduced indices of BP and CBFv in individuals with SCI compared to AB controls and 4) markedly lower DBP values recorded with the finger than brachial recording. Therefore, these readily accessible clinical tools may be used to assess the effects of clinical intervention aimed at increasing systemic BP and CBF in individuals with SCI with the goal of improving cognitive function and QOL.
Test-retest reliability of any physiological signal should be confirmed prior to widespread clinical application and to validate use for hypothesis testing in research protocols. Obtaining a reliable brachial BP signal over the course of three days in individuals with high level SCI is of clinical importance because autonomic cardiovascular impairment often results in wide fluctuations in daily BP.3,4,33 To our knowledge the inter-day reliability of seated resting BP has not been reported in the SCI population; however, a recent report documented substantial inter-day reliability for SBP (ICC = 0.79) and DBP (ICC = 0.92) in response to a sit-up test in 8 individuals with chronic SCI.34 Although our ICC data for brachial BP were lower than reported by Currie et al.,34 values were comparable for SBP (ICC = 0.56) and DBP (ICC = 0.53) in hypotensive individuals with SCI while at seated rest. It must be appreciated that during testing there was no subjective (symptoms) or objective ( + 20/10 mmHg) evidence of AD or OH (–20/10 mmHg), which may have contributed to the reliability of brachial BP documented. We acknowledge that many individuals with high thoracic and cervical SCI may have frequent bouts of AD, which contributes to the wide fluctuations in daily BP previously noted.3,35 Further, because we did not assess supine BP we could not determine if the hypotension documented in our participants with SCI met the definition of OH36; however, given the increased prevalence of OH in the SCI population,7,8,33 it is likely that many of our participants with SCI met criteria.
The poor inter-day reliability of the finger BP assessment in both the SCI and AB groups was a surprise and deviates from prior results in various populations.37–41 Because finger BP assessment was less reliable than brachial BP in both the SCI and AB groups, we believe that this finding does not relate to the degree of autonomic cardiovascular impairment. However, cold hand and finger temperatures, particularly in participants with SCI, may have contributed to peripheral vasoconstriction and variation in finger blood flow among the study visits and low ICC values. Furthermore, although we calibrated the finger photoplethysmographer prior to all data collection segments, the physiocal was turned off during the actual data collection to avoid interference in the waveform, which may have had an erroneous influence on finger BP. We appreciate that poor test-retest reliability of the finger BP assessments is troublesome because we recognize that this is a commonly used research tool with significant clinical testing implications. Of note, the finometer we used was an older version (Model-2 © 2007 FMS) that did not have the capabilities of the return-to-flow calibration or waveform reconstruction, which are available on the newer models and may significantly improve test-retest reliability of the finger BP assessment.42–45
Our data suggest that the inter-day assessment of CBFv was reliable in both the SCI and AB groups. A previous report describing the day-to-day ICC for MFV indicate poor reliability in the AB controls (ICC = 0.33) but substantial reliability in participants with SCI (ICC = 0.99).22 Although our data indicate more modest reliability for CBFv in participants with SCI (ICC range = 0.67–0.71), the findings reported by Wilson et al. are limited by a small sample of only 3 individuals with tetraplegia tested on 2 study visits.22 While the inter-day assessment of CBFv seems equally reliable in both groups, an interesting observation from these data was that the between-subject variation in CBFv appeared to be increased in the AB compared to the SCI group. It should be noted that increased between-subject variability will inflate (improve) the ICC, all else being equal.31 That said, we believe that the relatively homogeneous and lower CBFv in those with SCI likely reflects hypotension secondary to impaired autonomic cardiovascular control, which is well described in individuals with high thoracic and cervical lesions.3,24,26,46
Another important methodological consideration is the MDD, which can help clinicians distinguish between a true effect in an individual patient and measurement error. That is, the MDD is not an estimate of a “meaningful” change in terms of clinical importance. Instead, this estimate reflects the magnitude of change that one would have to see in an individual patient in this population, using these methods, for one to be confident that a “real” change occurred in that patient, at a given level of confidence. As a default, the 95% level of confidence was chosen for our MDD calculations, and the resulting values suggest that when assessing potential changes in a given individual, large changes in a measurement must occur to conclude a change is “real” (e.g. ≈19 mmHg for MAP and ≈16 cm/s for MFV). This is somewhat discouraging since our previous data on the BP and CBFv effects of midodrine hydrochloride (10 mg) during head-up tilt in hypotensive individuals with SCI suggest an average increase in MAP of 12 mmHg, but only an average increase in MFV of 0.6 cm/s.21 However, it should be appreciated that these are mean data following a single dose of midodrine and individual responses varied (MAP: –9 to + 35 mmHg; MFV: –6 to + 11 cm/s).21 As such, mean changes may be statistically significant at a group level but are harder to detect in individuals using a stringent level of confidence. We should note that a less stringent level of confidence for MDD may be more appropriate when making inferences about individual responses (e.g. simply 1.5 to 2 times the SEME; the 95% level of confidence equals 2.77 times the SEME), as previously suggested.47
We found significantly reduced CBFv in hypotensive participants with SCI compared AB controls with low BP. Findings of reduced resting CBFv have been reported in individuals with SCI,22,25,48 and recently, Sahota et al. showed that impaired autoregulatory control of CBF relates to the level and completeness of injury to the autonomic nervous system in persons with higher levels of SCI.26 Although several laboratories have reported that CBFv did not differ between individuals with SCI and AB controls, resting MFV was 16% reduced in both SCI cohorts compared to that of the controls.22,49 Our data support the notion that not only is MFV reduced in persons with SCI compared to AB controls, but SFV and DFV are also significantly lower. These findings indicate persistent cerebral hypoperfusion throughout the entire cardiac cycle in hypotensive individuals with chronic SCI, which may contribute to the increased incidence of cerebrovascular disease50 and ischemic stroke,18 as well as cognitive deficits,23,51,52 and impairment in health related QOL.10
Study limitations
The participants with SCI were hypotensive, which we believe contributed to the significantly reduced CBFv compared to the AB controls. Therefore findings of reduced CBFv cannot be extrapolated to individuals with SCI who are not hypotensive. In addition, because the level of SCI was restricted to individuals with some degree of disruption in supraspinal sympathetic cardiovascular control (i.e. above the sixth thoracic vertebrae) application of the findings to individuals with more intact cardiovascular autonomic control may not be appropriate. It must be appreciated that the “gold-standard” of BP assessment (i.e. intra-arterial) was not used to test the reliability or validity of these non-invasive measures of BP, which should be considered in a future research initiative. The finger photoplethysmographer used in this investigation (Finometer ® MIDI Model-2) did not include the return-to-flow procedure to calibrate finger BP to brachial artery pressure, which may have contributed to the low ICC values reported for finger BP. Although it is well appreciated that CBFv, as measured by TCD ultrasound in the MCA, reflects CBF because arterial diameter remains fairly constant, the assessment of change is preferred to absolute values. However, these data were collected in the seated resting condition; as such, change was not assessed and therefore the data should be interpreted with caution. Finally, we cannot make any assertions regarding the contribution of frequent AD and/or OH to our finding of poor test-retest reliability of the finger BP assessments or of the reduced CBFv because we did not assess subjective reporting on the frequency, duration or severity of these conditions in our participants with SCI.
Conclusions
The findings suggest reliable day-to-day assessment of brachial BP and TCD ultrasound assessment of CBFv in hypotensive individuals with SCI and AB controls with low BP. However, in our laboratory, finger photoplethysmography did not provide a reliable inter-day assessment of BP in either the SCI or AB cohorts. The data also suggest that resting CBFv is reduced in hypotensive individuals with SCI compared to demographically-matched AB controls with low BP. Future initiatives should be aimed at documenting CBFv in individuals with SCI that are not hypotensive and with variable levels and completeness of injury to establish the influence of hypotension versus cardiovascular autonomic dysfunction on cerebral blood flow in this population.
Disclaimer statements
Conflict of interest We certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated.
Funding This research was supported by the Veterans Affairs Rehabilitation Research and Development Service (Grant # A6161W).
References
- 1.Wecht JM, Zhu C, Weir JP, Yen C, Renzi C, Galea M. A prospective report on the prevalence of heart rate and blood pressure abnormalities in veterans with spinal cord injuries. J Spinal Cord Med 2013;36(5):454–62. doi: 10.1179/2045772313Y.0000000109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krassioukov A. Autonomic function following cervical spinal cord injury. Respir Physiol Neurobiol 2009;169(2):157–64. doi: 10.1016/j.resp.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 3.Krassioukov A. Autonomic dysreflexia: current evidence related to unstable arterial blood pressure control among athletes with spinal cord injury. Clin J Sport Med 2012;22(1):39–45. [DOI] [PubMed] [Google Scholar]
- 4.Hubli M, Gee CM, Krassioukov AV. Refined assessment of blood pressure instability after spinal cord injury. Am J Hypertens 2015;28(2):173–81. doi: 10.1093/ajh/hpu122 [DOI] [PubMed] [Google Scholar]
- 5.Munakata M, Kameyama J, Kanazawa M, Nunokawa T, Moriai N, Yoshinaga K. Circadian blood pressure rhythm in patients with higher and lower spinal cord injury: simultaneous evaluation of autonomic nervous activity and physical activity. J Hypertens 1997;15(12 Pt 2):1745–9. doi: 10.1097/00004872-199715120-00083 [DOI] [PubMed] [Google Scholar]
- 6.Rosado-Rivera D, Radulovic M, Handrakis JP, Cirnigliaro CM, Jensen AM, Kirshblum S,. et al Comparison of 24-hour cardiovascular and autonomic function in paraplegia, tetraplegia, and control groups: implications for cardiovascular risk. J Spinal Cord Med 2011;34(4):395–403. doi: 10.1179/2045772311Y.0000000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claydon VE, Krassioukov AV. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma 2006;23(12):1713–25. doi: 10.1089/neu.2006.23.1713 [DOI] [PubMed] [Google Scholar]
- 8.Illman A, Stiller K, Williams M. The prevalence of orthostatic hypotension during physiotherapy treatment in patients with an acute spinal cord injury. Spinal Cord 2000;38(12):741–7. doi: 10.1038/sj.sc.3101089 [DOI] [PubMed] [Google Scholar]
- 9.Zhu C, Galea M, Livote E, Signor D, Wecht JM. A retrospective chart review of heart rate and blood pressure abnormalities in veterans with spinal cord injury. J Spinal Cord Med 2013;36(5):463–75. doi: 10.1179/2045772313Y.0000000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlozzi NE, Fyffe D, Morin KG, Byrne R, Tulsky DS, Victorson D, et al Impact of blood pressure dysregulation on health-related quality of life in persons with spinal cord injury: development of a conceptual model. Arch Phys Med Rehabil 2013;94(9):1721–30. doi: 10.1016/j.apmr.2013.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita T, Hiramatsu H, Sakai N, Namba H. Cerebral hemorrhage due to posterior reversible encephalopathy syndrome associated with autonomic dysreflexia in a spinal cord injury patient. Neurol Med Chir (Tokyo) 2012;52(9):640–3. doi: 10.2176/nmc.52.640 [DOI] [PubMed] [Google Scholar]
- 12.Valles M, Benito J, Portell E, Vidal J. Cerebral hemorrhage due to autonomic dysreflexia in a spinal cord injury patient. Spinal Cord 2005;43(12):738–40. doi: 10.1038/sj.sc.3101780 [DOI] [PubMed] [Google Scholar]
- 13.Eltorai I, Kim R, Vulpe M, Kasravi H, Ho W. Fatal cerebral hemorrhage due to autonomic dysreflexia in a tetraplegic patient: case report and review. Paraplegia 1992;30(5):355–60. doi: 10.1038/sc.1992.82 [DOI] [PubMed] [Google Scholar]
- 14.Dolinak D, Balraj E. Autonomic dysreflexia and sudden death in people with traumatic spinal cord injury. Am J Forensic Med Pathol 2007;28(2):95–8. doi: 10.1097/PAF.0b013e3180600f99 [DOI] [PubMed] [Google Scholar]
- 15.Duschek S, Schandry R. Reduced brain perfusion and cognitive performance due to constitutional hypotension. Clin Auton Res 2007;17(2):69–76. doi: 10.1007/s10286-006-0379-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duschek S, Schandry R. Cognitive performance and cerebral blood flow in essential hypotension. Psychophysiology 2004;41(6):905–13. doi: 10.1111/j.1469-8986.2004.00249.x [DOI] [PubMed] [Google Scholar]
- 17.Kwon KJ, Lee EJ, Kim MK, Kim SY, Kim JN, Kim JO, et al Diabetes augments cognitive dysfunction in chronic cerebral hypoperfusion by increasing neuronal cell death: implication of cilostazol for diabetes mellitus-induced dementia. Neurobiol Dis 2015;73:12–23. doi: 10.1016/j.nbd.2014.08.034 [DOI] [PubMed] [Google Scholar]
- 18.Wu JC, Chen YC, Liu L, Chen TJ, Huang WC, Cheng H, et al Increased risk of stroke after spinal cord injury: a nationwide 4-year follow-up cohort study. Neurology 2012;78(14):1051–7. doi: 10.1212/WNL.0b013e31824e8eaa [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez F, Chang JY, Banovac K, Messina D, Martinez-Arizala A, Kelley RE. Autoregulation of cerebral blood flow in patients with orthostatic hypotension after spinal cord injury. Paraplegia 1991;29(1):1–7. doi: 10.1038/sc.1991.1 [DOI] [PubMed] [Google Scholar]
- 20.Handrakis JP, DeMeersman RE, Rosado-Rivera D, LaFountaine MF, Spungen AM, Bauman WA, et al Effect of hypotensive challenge on systemic hemodynamics and cerebral blood flow in persons with tetraplegia. Clin Auton Res 2009;19(1):39–45. doi: 10.1007/s10286-008-0496-6 [DOI] [PubMed] [Google Scholar]
- 21.Wecht JM, Rosado-Rivera D, Handrakis JP, Radulovic M, Bauman WA. Effects of midodrine hydrochloride on blood pressure and cerebral blood flow during orthostasis in persons with chronic tetraplegia. Arch Phys Med Rehabil 2010;91(9):1429–35. doi: 10.1016/j.apmr.2010.06.017 [DOI] [PubMed] [Google Scholar]
- 22.Wilson LC, Cotter JD, Fan JL, Lucas RA, Thomas KN, Ainslie PN. Cerebrovascular reactivity and dynamic autoregulation in tetraplegia. Am J Physiol Regul Integr Comp Physiol 2010;298(4):R1035–42. doi: 10.1152/ajpregu.00815.2009 [DOI] [PubMed] [Google Scholar]
- 23.Phillips AA, Warburton DE, Ainslie PN, Krassioukov AV. Regional neurovascular coupling and cognitive performance in those with low blood pressure secondary to high-level spinal cord injury: improved by alpha-1 agonist midodrine hydrochloride. J Cereb Blood Flow Metab 2014;34(5):794–801. doi: 10.1038/jcbfm.2014.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips AA, Krassioukov AV, Ainslie PN, Warburton DE. Perturbed and spontaneous regional cerebral blood flow responses to changes in blood pressure after high-level spinal cord injury: the effect of midodrine. J Appl Physiol (1985) 2014;116(6):645–53. doi: 10.1152/japplphysiol.01090.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catz A, Bluvshtein V, Korczyn AD, Pinhas I, Gelernter I, Nissel T, et al Modified cold pressor test by cold application to the foot after spinal cord injury: suggestion of hemodynamic control by the spinal cord. Am J Phys Med Rehabil 2007;86(11):875–82. doi: 10.1097/PHM.0b013e3181583caf [DOI] [PubMed] [Google Scholar]
- 26.Sahota IS, Ravensbergen HR, McGrath MS, Claydon VE. Cerebrovascular responses to orthostatic stress after spinal cord injury. J Neurotrauma 2012;29(15):2446–56. doi: 10.1089/neu.2012.2379 [DOI] [PubMed] [Google Scholar]
- 27.Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, et al Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol (1985) 2014;117(10):1084–9. doi: 10.1152/japplphysiol.00651.2014 [DOI] [PubMed] [Google Scholar]
- 28.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 1982;57(6):769–74. doi: 10.3171/jns.1982.57.6.0769 [DOI] [PubMed] [Google Scholar]
- 29.Bishop CC, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke 1986;17(5):913–5. doi: 10.1161/01.STR.17.5.913 [DOI] [PubMed] [Google Scholar]
- 30.Larsen FS, Olsen KS, Hansen BA, Paulson OB, Knudsen GM. Transcranial Doppler is valid for determination of the lower limit of cerebral blood flow autoregulation. Stroke 1994;25(10):1985–8. doi: 10.1161/01.STR.25.10.1985 [DOI] [PubMed] [Google Scholar]
- 31.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 2005;19(1):231–40. [DOI] [PubMed] [Google Scholar]
- 32.Shrout PE. Measurement reliability and agreement in psychiatry. Stat Methods Med Res 1998;7(3):301–17. doi: 10.1177/096228029800700306 [DOI] [PubMed] [Google Scholar]
- 33.Claydon VE, Steeves JD, Krassioukov A. Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord 2006;44(6):341–51. doi: 10.1038/sj.sc.3101855 [DOI] [PubMed] [Google Scholar]
- 34.Currie KD, Wong SC, Warburton DE, Krassioukov AV. Reliability of the sit-up test in individuals with spinal cord injury. J Spinal Cord Med 2015;38(4):563–6. doi: 10.1179/2045772315Y.0000000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frisbie JH. Unstable baseline blood pressure in chronic tetraplegia. Spinal Cord 2007;45(1):92–5. doi: 10.1038/sj.sc.3101920 [DOI] [PubMed] [Google Scholar]
- 36.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci 2011;161(1-2):46–8. doi: 10.1016/j.autneu.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 37.Lemson J, Hofhuizen CM, Schraa O, Settels JJ, Scheffer GJ, van der Hoeven JG. The reliability of continuous noninvasive finger blood pressure measurement in critically ill children. Anesth Analg 2009;108(3):814–21. doi: 10.1213/ane.0b013e318194f401 [DOI] [PubMed] [Google Scholar]
- 38.Hirschl MM, Binder M, Herkner H, Bur A, Brunner M, Seidler D, et al Accuracy and reliability of noninvasive continuous finger blood pressure measurement in critically ill patients. Crit Care Med 1996;24(10):1684–9. doi: 10.1097/00003246-199610000-00014 [DOI] [PubMed] [Google Scholar]
- 39.Poncelet P, Durand P, Lechantre R, Mounier-Vehier C, Fouquoire B, Petetin N, et al [Measurement of blood pressure in obese patients: reliability and value of finger measurement (Finapres)]. Arch Mal Coeur Vaiss 1992;85(8):1165–8. [PubMed] [Google Scholar]
- 40.Bos WJ, Imholz BP, van Goudoever J, Wesseling KH, van Montfrans GA. The reliability of noninvasive continuous finger blood pressure measurement in patients with both hypertension and vascular disease. Am J Hypertens 1992;5(8):529–35. doi: 10.1093/ajh/5.8.529 [DOI] [PubMed] [Google Scholar]
- 41.Kurki T, Smith NT, Head N, Dec-Silver H, Quinn A. Noninvasive continuous blood pressure measurement from the finger: optimal measurement conditions and factors affecting reliability. J Clinical Monit 1987;3(1):6–13. doi: 10.1007/BF00770876 [DOI] [PubMed] [Google Scholar]
- 42.Castiglioni P, Parati G, Omboni S, Mancia G, Imholz BP, Wesseling KH, et al Broad-band spectral analysis of 24 h continuous finger blood pressure: comparison with intra-arterial recordings. Clin Sci (Lond) 1999;97(2):129–39. [PubMed] [Google Scholar]
- 43.Westerhof BE, Guelen I, Parati G, Groppelli A, van Montfrans GA, Wieling W, et al Variable day/night bias in 24-h non-invasive finger pressure against intrabrachial artery pressure is removed by waveform filtering and level correction. J Hypertens 2002;20(10):1981–6. doi: 10.1097/00004872-200210000-00017 [DOI] [PubMed] [Google Scholar]
- 44.Gizdulich P, Imholz BP, van den Meiracker AH, Parati G, Wesseling KH. Finapres tracking of systolic pressure and baroreflex sensitivity improved by waveform filtering. J Hypertens 1996;14(2):243–50. doi: 10.1097/00004872-199602000-00014 [DOI] [PubMed] [Google Scholar]
- 45.Gizdulich P, Prentza A, Wesseling KH. Models of brachial to finger pulse wave distortion and pressure decrement. Cardiovasc Res 1997;33(3):698–705. doi: 10.1016/S0008-6363(97)00003-5 [DOI] [PubMed] [Google Scholar]
- 46.Phillips AA, Ainslie PN, Krassioukov AV, Warburton DE. Regulation of cerebral blood flow after spinal cord injury. J Neurotrauma 2013;30(18):1551–63. doi: 10.1089/neu.2013.2972 [DOI] [PubMed] [Google Scholar]
- 47.Hopkins WG. Measures of reliability in sports medicine and science. Sports Med 2000;30(1):1–15. doi: 10.2165/00007256-200030010-00001 [DOI] [PubMed] [Google Scholar]
- 48.Catz A, Itzkovich M, Tesio L, Biering-Sorensen F, Weeks C, Laramee MT, et al A multicenter international study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord 2007;45(4):275–91. [DOI] [PubMed] [Google Scholar]
- 49.Wecht JM, Rosado-Rivera D, Jegede A, Cirnigliaro CM, Jensen MA, Kirshblum S, et al Systemic and cerebral hemodynamics during cognitive testing. Clin Auton Res 2012;22(1):25–33. doi: 10.1007/s10286-011-0139-1 [DOI] [PubMed] [Google Scholar]
- 50.Groah SL, Weitzenkamp D, Sett P, Soni B, Savic G. The relationship between neurological level of injury and symptomatic cardiovascular disease risk in the aging spinal injured. Spinal Cord 2001;39(6):310–7. doi: 10.1038/sj.sc.3101162 [DOI] [PubMed] [Google Scholar]
- 51.Jegede AB, Rosado-Rivera D, Bauman WA, Cardozo CP, Sano M, Moyer JM,. et al Cognitive performance in hypotensive persons with spinal cord injury. Clin Auton Res 2010;20(1):3–9. doi: 10.1007/s10286-009-0036-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wecht JM, Bauman WA. Decentralized cardiovascular autonomic control and cognitive deficits in persons with spinal cord injury. J Spinal Cord Med 2013;36(2):74–81. doi: 10.1179/2045772312Y.0000000056 [DOI] [PMC free article] [PubMed] [Google Scholar]


