Abstract
Objective
Brachial plexus injuries are usually severe and involve the entire brachial plexus, sometimes occurring with root avulsions. Imaging and electrodiagnostic studies are an essential part of the lesion evaluation; however, the results sometimes show a discrepancy. The cutaneous silent period (SP) is a spinal inhibitory reflex mediated by small-diameter A-delta nociceptive fibers. The aim of the study was to determine if cutaneous SP testing may serve as a useful aid in evaluation of brachial plexus injury and/or in the diagnosis of root avulsion.
Methods
In 19 patients with traumatic brachial plexus injury (15 males, age 18–62 years) we performed a clinical examination, CT myelography and neurophysiological testing. A needle EMG was obtained from muscles supplied by C5-T1 myotomes. Cutaneous SP was recorded after painful stimuli were delivered to the thumb (C6 dermatome), middle (C7) and little (C8) fingers while subjects maintained voluntary contraction of intrinsic hand muscles.
Results
Electrodiagnostic and imaging studies confirmed root avulsion (partial or total) maximally involving C5, C6 roots in 12 patients, whereas only in 4 of them the cutaneous SP was partially absent. In the remaining subjects, the cutaneous SP was preserved.
Conclusion
In brachial plexopathy even with plurisegmental root avulsion, the cutaneous SP was mostly preserved. This method cannot be recommended as a reliable test for diagnosis of single root avulsion; however, it can provide a quick physiological confirmation of functional afferent A-delta fibers through damaged roots and/or trunks. The clinicians may add this test to the diagnosis of spinal cord dysfunction.
Keywords: A-delta fibers, Brachial plexopathy, Cutaneous silent period, Electromyography, Root avulsion
Introduction
Brachial plexus injuries are relatively common in closed traction injuries incurred during sport activities, after falls or in motor vehicle accidents. Injuries are usually severe and involve the entire brachial plexus, sometimes along with root avulsions. Imaging and electrodiagnostic studies comprise an essential part of the evaluation of such a patient with traumatic brachial plexopathy, enabling clarification of the rehabilitation approach as well as surgical options, prognostication of outcome and formulation of postoperative management.1,2 The current imaging modality of choice is CT myelography and magnetic resonance imaging (MRI)3,4 to identify pre-ganglionic injury as indicative of nerve root avulsion. Electrodiagnostic testing can be helpful in nerve root and brachial plexus injuries to localize the site of the lesion, determine the extent, identify the predominant pathophysiology, and objectively quantify the severity of brachial plexopathies.5,6 Electrodiagnostic studies can also assist in providing prognostic information after nerve injury as well as after nerve repair.
Electromyographic (EMG) activity from voluntarily contracting hand muscles undergoes transient suppression following nociceptive fingertip stimulation. This spinal inhibitory reflex designated as the cutaneous silent period (SP) is mediated primarily by small-diameter A-delta fibers, which enter the spinal dorsal horn and suppress activity in spinal motor nuclei in neighboring myotomes.7–10 Cutaneous SP is altered in a variety of peripheral nervous system lesions.11–14 Absence of the cutaneous SP has been reported in a variety of myelopathic conditions, e.g. in syringomyelia15 or cervical spondylogenic myelopathy.16,17 However, in cervical radiculopathy, almost normal cutaneous SP has been observed.18 The CSP technique can quickly determine whether afferent impulses in A-delta fibers pass through individual cervical roots to enter the spinal cord. It is believed that the impulses that originate in the fingertips to produce the CSP ascend along their respective nerves and pass through the corresponding C6, C7 or C8 roots.19
The aim of this study is to confirm that cutaneous SP may be, in line with routine electromyographic testing, particularly useful in evaluation of brachial plexus injury, especially contributing to diagnosis of cervical root avulsion.
Methods
Nineteen patients with various type of brachial plexus injury were enrolled in this study (15 males, aged 18–62 years; 4 females, aged 24–48 years). All subjects underwent clinical neurological examination, imaging studies (CT myelography and/or MRI) and neurophysiologic testing including conduction motor and sensory studies, needle EMG and serial cutaneous SP testing. If patients had signs or symptoms of myelopathy, they were excluded from this study, since myelopathic conditions are known to abolish or alter the CSP.16,17 The study was approved by the local ethics committees, in compliance with the Declaration of Helsinki.
Clinical examination consists of measuring muscle strength (0 to 5 scale; 5 represents full power, 0 is plegic) and evaluation of sensitivity (0 anesthesia, 1 hypoesthesia, 2 normal sensitivity).
Routine electrodiagnostic equipment was used in all experiments (Medelec Synergy, Oxford Instruments; Surrey, England). Motor nerve action potentials (CMAP) were recorded after stimulation of the median nerve, radial nerve and ulnar nerve and recorded from the abductor pollicis brevis (APB), extensor indicis proprius (EIP) and abductor digiti minimi (ADM), respectively. Sensory nerve action potentials (SNAPs) were obtained from the median nerve (index finger), from the ulnar nerve (fifth finger) and radial nerve using routine technique with antidromic electrical stimulation at the wrist and forearm. Needle electromyography (EMG) was obtained mostly from 14 muscles supplied by C5-T1 myotomes with clinical and electrodiagnostic evidence of cervical root avulsion (supraspinatus, infraspinatus, deltoid, biceps, triceps, latissimus dorsi, serratus anterior, trapesius, pectoralis, flexor carpi ulnaris, flexor digitorum superficialis, extensor digitorum communis, abductor digiti minimi, opponens pollicis). All patients underwent this diagnostic protocol to be prepared for possibly neurosurgical operation of root reconstruction.
The cutaneous SP was obtained by applying painful high-intensity electrical stimulation to the thumb (C6 dermatome), middle finger (C7), and little finger (C8) as previously described.11,18–21 Sensory thresholds following electrical stimulation with constant current square waves of 0.5 ms duration and a stimulation rate of 0.5–0.7 Hz were established for digit I, digit III and digit V in each subject. Subjects were then asked to perform thumb abduction with attempted 25% of their maximum force. The amount of muscle force was estimated by comparing to 100% (full power) previously displayed on a screen of EMG; however, muscle force has been shown to not critically influence cutaneous SP parameters in a range of 10 to 50% of maximum force.22,23 Rectified surface EMG recordings were obtained from the thenar muscles, amplified, and filtered (30 to 10000 Hz). Visual and auditory feedback of the EMG signal was provided in order to enhance control of muscle activity. Cutaneous SPs were elicited by noxious stimulation, applied 20 times above the sensory threshold with a fifty sweeps of 500 ms including a 100 ms pre-stimulus delay. Cutaneous SP curve was defined by a drop of rectified averaged EMG below 80% of baseline EMG and the final return above 80% of baseline EMG, yielding cutaneous SP onset, end, and duration at appropriate latencies. The cutaneous SP curves were recorded, rectified and averaged. The cutaneous SP onset and endpoint for each tracing were marked and their latencies were measured based on the calibration grid. The cutaneous SP duration was calculated as the difference between the endpoint and onset latencies.
The diagnosis of C6 avulsion was made if a patient had altered sensation involving the thumb, weakness in muscles supplied by C6, and EMG that showed fibrillation potentials limited to muscles supplied by the C6 root (shoulder girdle muscles, pronator teres or flexor carpi radialis). A diagnosis of C7 avulsion was made if symptoms included altered sensation involving the middle or index fingers, weakness in muscles supplied by C7, and EMG showing fibrillation potentials in muscles supplied by C7 (triceps, pronator teres or flexor carpi radialis). A C8 avulsion was diagnosed if altered sensory symptoms involved the little finger, weakness in muscles supplied by C8 (intrinsic hand muscles or forearm digital flexors) and EMG showing fibrillation potentials in muscles sharing C8 innervation.
All patients underwent CT myelography. Complete root avulsion was radiologically proved when root cyst was present (Figures 1 and 2). Partial (incomplete) root avulsion was confirmed by absence of mostly ventral part of motor fibers in axial and sagital CT myelography scans.
Figure 1.
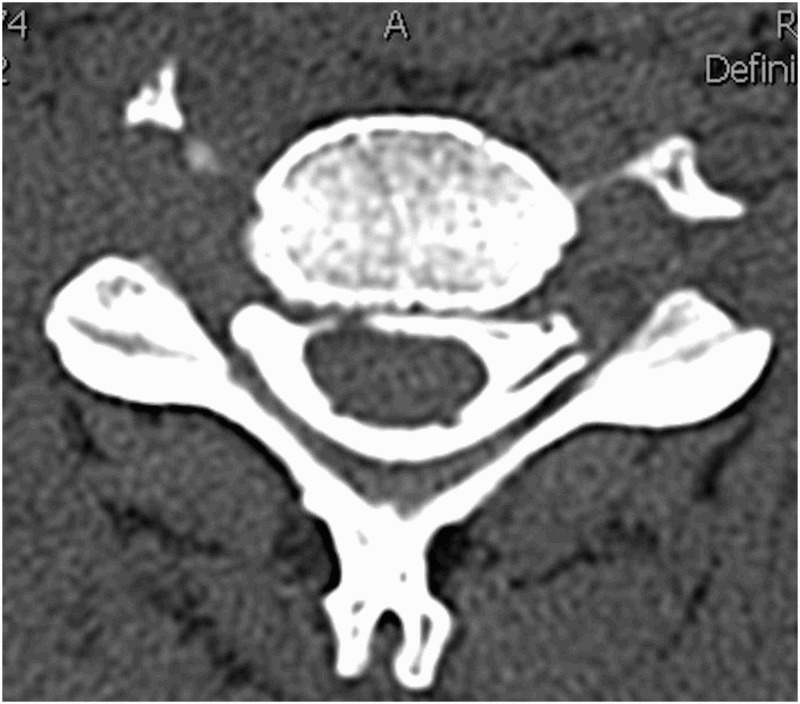
Nerve root avulsion. Axial images of CT myelogram demonstrating intact ventral and dorsal nerve roots on the left side and absence of nerve roots on the right side.
Figure 2.
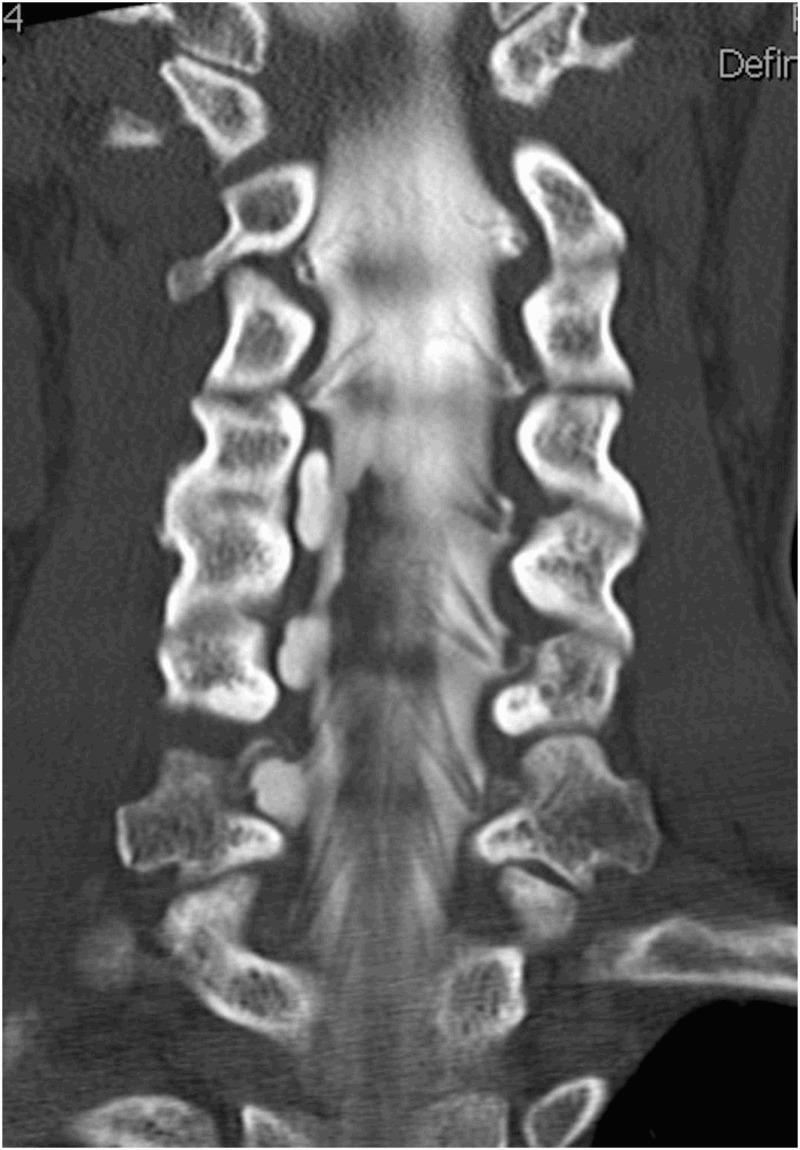
Nerve root avulsion. Coronal reconstruction of CT myelogram demonstrates normal left-sided nerve roots at multiple levels with absent nerve roots on the right C5–C7 level with root cyst
Results
Demographic, clinical and neurophysiological data are summarized in Tables 1 and 2. All patients suffered from unilateral plexus injury. Sixteen of them had been in motor vehicle accidents and 3 had sport injuries.
Table 1.
Demographic, clinical and imaging data
| Clinical Findings |
Needle EMG | Imaging studies | |||||
|---|---|---|---|---|---|---|---|
| No. | Age | Sex | Side | Motor lesion | Sensory lesion | Abnormal spontaneous activity | CT-myelography |
| 1 | 18 | M | R | C5 | no lesion | C5 | ventral C5 |
| 2 | 24 | F | L | C5, C6, partial C7 | C5, C6 | C5, C6, partial C7,8 | ventral C5 |
| 3 | 62 | M | R | C5, C6 | C4, C5, C6 | C5, C6 | C5, C6 |
| 4 | 19 | M | L | C5, C6, C7, partial C8 | C5, C6 | C5, C6 | C5, C6 |
| 5 | 62 | M | R | C5, C6 | C5, C6 | C5, C6, C7 | C5 |
| 6 | 19 | M | R | C5, C6 | C5, C6 | C5, C6, C7 | no avulsion |
| 7 | 52 | M | L | C5, C6 | C5 | C5, C6 | no avulsion |
| 8 | 39 | F | R | C5, C6, C7, partial C8 | C5, C6, C7 | C5, C6 | C4, C5, C6, partial C7 |
| 9 | 43 | F | R | C5 | C5 | C5 | no avulsion |
| 10 | 39 | M | R | C5, C6, partial C7 | C4, C5, C6, C7 | C5, C6, C7, C8 | C6, C7 |
| 11 | 24 | M | R | C5, C6, C7, partial C8 | C3, C4, C5, C6, C7 | C5, C6, C7 | C8 |
| 12 | 44 | M | L | C6, C7, partial C5, C8 | C5, C6, C7, C8 | C5, C6, C7, C8 | no avulsion |
| 13 | 50 | M | R | C5, C6, C7, partial C8 | C5, C6, C7, C8 | C5, C6, C7, C8 | no avulsion |
| 14 | 42 | M | R | C5, C6, partial C7 | C5, C6 | C5, C6, C7, C8 | C5, C6, C7 |
| 15 | 39 | M | L | C5, C6, partial C7 | C5, C6 | C5, C6 | ventral C5, C6 |
| 16 | 48 | F | L | C5 | C5 | C5 | no avulsion |
| 17 | 26 | M | L | C5, C6, C7, partial C8 | C6, C7, C8 | C5, C6, C7, C8 | C6, C7, C8 |
| 18 | 28 | M | L | C5 | C5 | C5 | no avulsion |
| 19 | 49 | M | L | C5, C6, C7, C8 | C5, C6, C7, C8 | C5, C6, C7, C8 | C5, C6, C7 |
Table 2.
Cutaneous SP findings after painful stimulation of the first, third and fifth digits
| CSP 1st |
CSP 3rd |
CSP 5th |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | onset | end | duration | onset | end | duration | onset | end | duration |
| 1 | 73.0 | 104.0 | 31.0 | 71.5 | 93.0 | 21.5 | 70.0 | 88.0 | 18.0 |
| 2 | 44.5 | 139.0 | 94.5 | 59.5 | 134.5 | 75.0 | 70.0 | 146.5 | 76.5 |
| 3 | 79.5 | 138.5 | 59.0 | 80.0 | 125.5 | 45.5 | 89.0 | 127.5 | 38.5 |
| 4 | 94.0 | 125.0 | 31.0 | 74.0 | 94.5 | 20.5 | 86.5 | 130.0 | 43.5 |
| 5 | 83.5 | 166.0 | 82.5 | 100.5 | 145.5 | 45.0 | 83.5 | 136.0 | 52.5 |
| 6 | 46.5 | 133.0 | 86.5 | 47.0 | 137.0 | 90.0 | 48.5 | 127.0 | 78.5 |
| 7 | 85.5 | 167.0 | 81.5 | 94.5 | 145.0 | 50.5 | 99.0 | 133.5 | 34.5 |
| 8 | 77.5 | 126.0 | 48.5 | absent | absent | absent | absent | absent | absent |
| 9 | 67.5 | 127.0 | 59.5 | 61.5 | 138.0 | 76.5 | 65.5 | 136.5 | 71.0 |
| 10 | absent | absent | absent | 82.5 | 133.0 | 50.5 | 73.5 | 117.5 | 44.0 |
| 11 | absent | absent | absent | 81.5 | 130.0 | 48.5 | 68.0 | 128.0 | 60 |
| 12 | 55.0 | 124.0 | 69.0 | 57.5 | 125.5 | 68.0 | 71.0 | 123.0 | 52.0 |
| 13 | absent | absent | absent | 68.0 | 130.5 | 62.5 | 74.0 | 135.0 | 61.0 |
| 14 | 82.0 | 146.5 | 64.5 | 73.0 | 132.5 | 59.5 | 77.5 | 128.5 | 51.0 |
| 15 | 63.5 | 105.5 | 42.0 | 79.0 | 113.5 | 34.5 | 76.0 | 111.0 | 35.0 |
| 16 | 61.0 | 120.5 | 59.5 | 67.0 | 116.5 | 49.5 | 69.5 | 122.5 | 53.0 |
| 17 | 119.5 | 137.5 | 18.0 | 99.5 | 119.5 | 20.0 | 91.0 | 102.5 | 11.5 |
| 18 | 46.0 | 131.0 | 85.0 | 56.5 | 126.5 | 70.0 | 58.5 | 119.5 | 61.0 |
| 19 | absent | absent | absent | 141.0 | 163.0 | 22.0 | absent | absent | absent |
Mean values, in ms. Recording electrodes are placed on m.APB
Electromyographic needle examination revealed abnormal spontaneous activity in the form of positive sharp waves and fibrillations with no motor units in largely C5 and C6 innervated muscles. Electrodiagnostic and imaging studies confirmed root avulsion corresponding to C5 and C6 roots in 11 patients. One subject suffered from single C8 root avulsion (Table 1). CT myelography showed no root avulsion in 7 subjects.
The cutaneous SP onset latency, endpoint latency and duration are summarized in Table 2. Cutaneous SPs after stimulation of D1, D3 and D3 were recorded in almost the normal range in 4 patients with C5 root lesion, in 4 patients with sensory and motor lesions of C5 and C6 roots, in 3 patients with sensory and motor lesions of C5 and C6 roots and partial motor lesion of C7 root, and one patient with sensitive lesion of C4–C6 roots and motor lesion of C5 and C6 had normal cutaneous SPs. See example at Figure 3.
Figure 3.
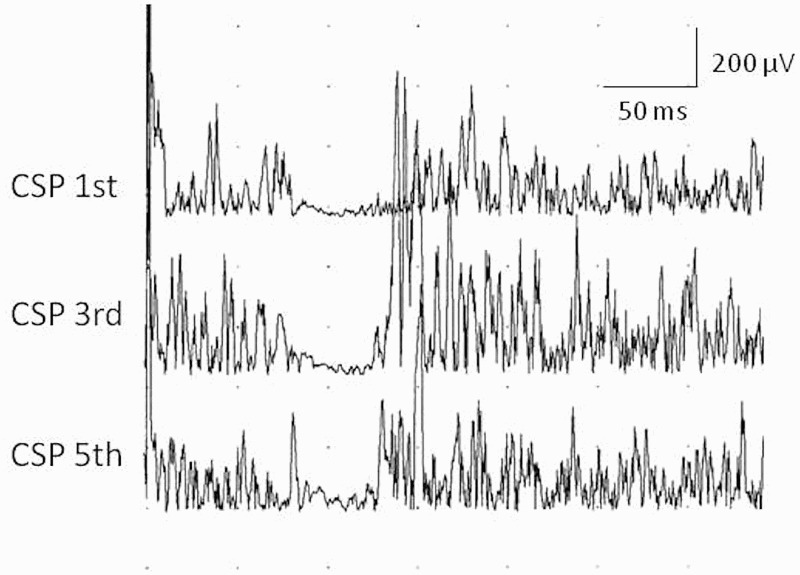
Example of well preseved cutaneous silent period in abductor pollicis brevis muscle following noxious stimulation of the first, the third and the fifth fingers in patient No. 3 with root avulsion of C5 and C6.
Cutaneous SPs after stimulation of D1 were absent in 4 subjects with sensory lesion of C6 dermatome (thumb). In these patients CT myelography showed discrepant results: no observed root avulsion (Case 13), root avulsion only of C8 (Case 11) and root avulsions of C6 and C7 (Case 10, 19). Cutaneous SP after stimulation of D3 and D5 was absent in C7 and C8 dermatomes in one subject with root avulsion of C4–C7 (Case 8). The cutaneous SP was absent after stimulation of D1 and D5 in one subject with avulsion of C4–C7 roots.
No clear correlation between CT myelography findings and cutaneous SP recordings were observed.
Discussion
The main finding of this study is that brachial plexopathy is not overall associated with an absence of the cutaneous SP in corresponding dermatome with root avulsion, especially in a single root avulsion. Absence or alteration of cutaneous SPs are present only in cases with plurisegmental complete sensory and motor deficits including C5, C6 and C7 roots.
Brachial plexus injuries represent devastating injuries with unpredictable outcomes in terms of residual neuropathic pain24 and/or motor functions. The management of brachial plexus injury has considerably improved when new diagnostic modalities, microsurgical techniques such as nerve repair, grafting, and transfer were studied in animal studies25,26 and were introduced into clinical practice.27 The main diagnostic tools to assess brachial plexus injuries are CT myelography and MRI;5,28 however, electrodiagnostic studies are of immense help prior to brachial plexus surgery.1,2 Presence of pseudomeningocele on CT myelography indicates a root avulsion injury3 but false-positive findings have been found in patients with intact roots, and false-negative results have been reported during surgery.
Electrodiagnostic tests are recommended to determine preganglionic avulsion or postganglionic rupture or nerve distension, the level of plexus involvement and the number of root avulsion.1,2,29,30 The main techniques are motor and sensory nerve conduction, needle electromyography (EMG), somatosensory and motor evoked potentials, dermatomal somatosensory evoked potentials31 and where indicated, intra-operative monitoring.32 When evaluating cervical roots and brachial plexus injuries, motor amplitudes may not add much to the diagnostic localization, however, sensory nerve conduction is of important value. Needle EMG is required to document and record the axon loss, its proximal extent, and the completeness of the lesion, especially for proximal muscles where CMAP recording is not possible. The presence of one or more root avulsion is a critical factor in surgical decision-making and it helps the neurosurgeon to identify potential donor nerves that may be suitable for use as transfers. The prognosis of surgical reconstruction is dependent on good correlation between clinical, morphological and electrophysiological findings.33,34
Cutaneomuscular reflexes are useful tests for studying sensorimotor integration of the nervous system. One of the most robust cutaneomuscular reflexes is designated as cutaneous SP. Cutaneous SP is defined as a brief pause in the voluntary EMG activity that occurs in response to a painful stimulus applied to a cutaneous nerve. It is a spinal inhibitory reflex7–10 with afferent impulses entering the spinal dorsal horn and suppressing activity in spinal motor nuclei. Afferent impulses that generate the cutaneous SP are carried primarily by smaller, slower conducting A-delta fibers.11,12,21 Conditions that interrupt this reflex pathway should be associated with absence or delay of the cutaneous SP. The cutaneous SP shows a high sensitivity for detecting spinal cord lesions, including intramedullary spinal cord lesions35 and myelopathy due to spondylosis,16,17 syringomyelia,15 whiplash injury36 or other structural abnormalities of the cervical spine that abolish or alter the cutaneous SP. Cutaneous SP is preserved in radiculopathy, probably because afferent impulses are carried by smaller, slower conducting “injury-resistant” A-delta fibers.18 These results provide important missing evidence that ensures specificity of cutaneous SP alterations in the diagnosis of cervical myelopathy. Preserved cutaneous SP in traumatic brachial plexopathy has been already demonstrated.37
This study demonstrates for the first time a correlation between the cutaneous SP and traumatic root avulsion. The nociceptive stimuli that originate in the dermatomes of the hand to produce the cutaneous SP pass through the corresponding C6, C7 or C8 roots. Surprisingly, the cutaneous SP is still present despite of a root avulsion in an appropriate dermatome and on the other hand, severe multi-segmental root avulsion is also not always necessarily associated with abolished cutaneous SP. In some of our patients with sensory and motor lesion of C6 to C8 roots proven by CT myelography, cutaneous SP was still preserved. One possible contributing factor to the preserved cutaneous SP in root avulsions is that partially conducting volleys in A-delta fibers are transmitted to an interneuronal network of the intact spinal cord. The incoming afferent signals are amplified, leading to inhibition of spinal motoneuron pools. It has been already published that small lesions in the central part of the cervical spinal cord may completely abolish the cutaneous SP.16,17,35 In theory, sensory dermatomes including nociceptive receptors are overlapping, and activating more the small thin afferent fibers. Transmission along A-delta fibers from different digits and the corresponding C6, C7 or C8 roots, which are more or less impaired, still induce the robust spinal protective reflex, defending the subject from nociceptive stimuli and potential jeopardy. This study also contributes to basic physiology of protective spinal inhibitory reflexes in traumatic conditions.
Partial incomplete root avulsion is typically ventral when motor root fibers are injured. These motor fibers are probably also more sensitive to damage than sensory fibers, especially A-delta ones.18 The diameter and the number of nerve fibers of each nerve root differs in cervical, thoracic and lumbar segments38 and the better knowledge about preserved nerve fibers continuity provides basic data for future neuroanastomosis.27 Preserved cutaneous SP proves that spinal cord circuit is functionally intact. This observation may thus have implications in reconstructive surgery as well as in neurorehabilitation with better functional restoration.
In conclusion, the cutaneous SP technique can be used to quickly determine whether afferent impulses in A-delta fibers pass through cervical roots to enter the spinal cord. However, root avulsion is not overall associated with absence of the cutaneous SP, thus this method cannot be recommended as a reliable test for diagnosis of a single root avulsion. It can only document residual continuity in severe brachial plexopathies, when continuity cannot be detected by standard electrodiagnostic or imaging studies. The cutaneous SP is a robust protective spinal reflex which is a part of a basic complex spinal reflex mechanism helping to defense the human body against the harmful stimuli. The clinicians may use this simple spinal inhibitory reflex as a physiologic aid in the diagnosis of preserved or impaired spinal cord function in different traumatic conditions.
Acknowledgments
The authors thank the Department of Radiology, Third Faculty of Medicine, Charles University, Prague, Czech Republic, for radiological documentation.
Disclaimer statements
Contributors All contributors meet author criteria.
Funding Supported by Research Projects of Charles University PRVOUK P34, Grant Project of the Czech Ministry of Health NT13693, 260168/SVV/2015.
Conflicts of interest There are no conflicts of interest.
Ethics approval Aproved by Local Research Ethics Committee.
References
- 1.Chanlalit C, Vipulakorn K, Jiraruttanapochai K, Mairiang E, Chowcheun P. Value of clinical findings, electrodiagnosis and magnetic resonance imaging in the diagnosis of root lesions in traumatic brachial plexus injuries. J Med Assoc Thai 2005;88(1):66–70. [PubMed] [Google Scholar]
- 2.Puri V, Chaudhry N, Jain KK, Chowdhury D, Nehru R. Brachial plexopathy: a clinical and electrophysiological study. Electromyogr Clin Neurophysiol 2004;44(4):229–35. [PubMed] [Google Scholar]
- 3.Roger B, Travers V, Laval-Jeantet M. Imaging of posttraumatic brachial plexus injury. Clin Orthop Relat Res 1988;237:57–61. [PubMed] [Google Scholar]
- 4.Ochi M, Ikuta Y, Watanabe M, Kimori K, Itoh K. The diagnostic value of MRI in traumatic brachial plexus injury. J Hand Surg Br 1994;19(1):55–9. doi: 10.1016/0266-7681(94)90050-7 [DOI] [PubMed] [Google Scholar]
- 5.Parry GJ. Electrodiagnostic studies in the evaluation of peripheral nerve and brachial plexus injuries. Neurol Clin 1992;10(4):921–34. [PubMed] [Google Scholar]
- 6.Abul-Kasim K, Backman C, Björkman A, Dahlin LB. Advanced radiological work-up as an adjunct to decision in early reconstructive surgery in brachial plexus injuries. J Brachial Plex Peripher Nerve Inj 2010;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caccia MR, McComas AJ, Upton ARM, Blogg T. Cutaneous reflexes in small muscles of the hand. J Neurol Neurosurg Psychiatry 1973;36(6):960–77. doi: 10.1136/jnnp.36.6.960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floeter MK. Cutaneous silent periods. Muscle Nerve 2003;28(4):391–401. doi: 10.1002/mus.10447 [DOI] [PubMed] [Google Scholar]
- 9.Shefner JM, Logigian EL. Relationship between stimulus strength and the cutaneous silent period. Muscle Nerve 1993;16(3):278–82. doi: 10.1002/mus.880160306 [DOI] [PubMed] [Google Scholar]
- 10.Uncini A, Kujirai T, Gluck B, Pullman S. Silent period induced by cutaneous stimulation. Electroencephalogr Clin Neurophysiol 1991;81(5):344–52. doi: 10.1016/0168-5597(91)90023-Q [DOI] [PubMed] [Google Scholar]
- 11.Leis AA, Kofler M, Ross MA. The silent period in pure sensory neuronopathy. Muscle Nerve 1992;15(12):1345–8. doi: 10.1002/mus.880151209 [DOI] [PubMed] [Google Scholar]
- 12.Svilpauskaite J, Truffert A, Vaiciene N, Magistris MR. Cutaneous silent period in carpal tunnel syndrome. Muscle Nerve 2006;33(4):487–93. doi: 10.1002/mus.20496 [DOI] [PubMed] [Google Scholar]
- 13.Aurora SK, Ahmad BK, Aurora TK. Silent period abnormalities in carpal tunnel syndrome. Muscle Nerve 1998;21(9):1213–5. doi: [DOI] [PubMed] [Google Scholar]
- 14.Han JK, Oh K, Kim BJ, Koh SB, Kim JY, Park KW, et al. Cutaneous silent period in patients with restless leg syndrome. Clin Neurophysiol 2007;118(8):1705–10. doi: 10.1016/j.clinph.2007.04.024 [DOI] [PubMed] [Google Scholar]
- 15.Stetkarova I, Kofler M, Leis AA. Cutaneous and mixed nerve silent periods in cervical syringomyelia. Clinical Neurophysiology 2001;112(1):78–85. doi: 10.1016/S1388-2457(00)00486-7 [DOI] [PubMed] [Google Scholar]
- 16.Lo YL, Tan YE, Dan YF, Leoh TH, Tan SB, Tan CT, et al. Cutaneous silent periods in the evaluation of cord compression in cervical spondylosis. J Neurol 2007;254(1):14–9. doi: 10.1007/s00415-007-0142-6 [DOI] [PubMed] [Google Scholar]
- 17.Stetkarova I, Kofler M. Cutaneous silent periods in the assessment of mild cervical spondylotic myelopathy. Spine 2009;34(1):34–42. doi: 10.1097/BRS.0b013e31818f8be3 [DOI] [PubMed] [Google Scholar]
- 18.Leis AA, Kofler M, Stetkarova I, Stokic DS. The cutaneous silent period is preserved in cervical radiculopathy: significance for the diagnosis of cervical myelopathy. Eur Spine J 2011;20(2):236–9. doi: 10.1007/s00586-010-1627-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kofler M. Functional organization of exteroceptive inhibition following nociceptive electrical fingertip stimulation in humans. Clin Neurophysiol 2003;114(6):973–80. doi: 10.1016/S1388-2457(03)00060-9 [DOI] [PubMed] [Google Scholar]
- 20.Kofler M, Stetkarova I, Wissel J. Nociceptive EMG suppression in triceps brachii muscle in humans. Clin Neurophysiol 2004;115(5):1052–6. doi: 10.1016/j.clinph.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 21.Kofler M, Valls-Solé J, Vasko P, Bocek V, Stetkarova I. Influence of limb temperature on cutaneous silent periods. Clin Neurophysiol 2014;125(9):1826–33. doi: 10.1016/j.clinph.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 22.Kofler M, Kumru H, Stetkarova I, Schindler C, Fuhr P. Muscle force up to 50% of maximum does not affect cutaneous silent periods in thenar muscles. Clin Neurophysiol 2007;118(9):2025–30. doi: 10.1016/j.clinph.2007.06.005 [DOI] [PubMed] [Google Scholar]
- 23.Rodi Z, Springer C. Influence of muscle contraction and intensity of stimulation on the cutaneous silent period. Muscle Nerve 2011;43(3):324–8. doi: 10.1002/mus.21868 [DOI] [PubMed] [Google Scholar]
- 24.Haninec P, Kaiser R, Mencl L, Waldauf P. Usefulness of screening tools in the evaluation of long-term effectiveness of DREZ lesioning in the treatment of neuropathic pain after brachial plexus injury. BMC Neurol 2014;9(1):225. doi: 10.1186/s12883-014-0225-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chew DJ, Murrell K, Carlstedt T, Shortland PJ. Segmental spinal root avulsion in the adult rat: a model to study avulsion injury pain J. Neurotrauma 2013;30(3):160–72. doi: 10.1089/neu.2012.2481 [DOI] [PubMed] [Google Scholar]
- 26.Pintér S, Gloviczki B, Szabó A, Márton G, Nógrádi A. Increased survival and reinnervation of cervical motoneurons by riluzole after avulsion of the C7 ventral root. J Neurotrauma 2010;27(12):2273–82. doi: 10.1089/neu.2010.1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haninec P, Mencl L, Keiser R. End-to-side neurorrhaphy in brachial plexus reconstruction. J Neurosurg 2013;119(3):689–94. doi: 10.3171/2013.6.JNS122211 [DOI] [PubMed] [Google Scholar]
- 28.Gasparotti R, Lodoli G, Meoded A, Carletti F, Garozzo D, Ferraresi S. Feasibility of diffusion tensor tractography of brachial plexus injuries at 1.5 T. Invest Radiol 2013;48(2):104–12. doi: 10.1097/RLI.0b013e3182775267 [DOI] [PubMed] [Google Scholar]
- 29.Vredeveld JW, Slooff BC, Blaauw G, Richards R. Validation of an electromyography and nerve conduction study protocol for the analysis of brachial plexus lesions in 184 consecutive patients with traumatic lesions. J Clin Neuromuscul Dis 2001;2(3):123–8. doi: 10.1097/00131402-200103000-00002 [DOI] [PubMed] [Google Scholar]
- 30.O'Shea K, Feinberg JH, Wolfe SW. Imaging and electrodiagnostic work-up of acute adult brachial plexus injuries. J Hand Surg Eur Vol 2011;36(9):747–59. doi: 10.1177/1753193411422313 [DOI] [PubMed] [Google Scholar]
- 31.Dikmen PY, Oge AE. Diagnostic use of dermatomal somatosensory-evoked potentials in spinal disorders: Case series. J Spinal Cord Med 2013;36(6):672–8. doi: 10.1179/2045772313Y.0000000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberle J, Antoniadis G, Kast E, Richter HP. Evaluation of traumatic cervical nerve root injuries by intraoperative evoked potentials. Neurosurgery 2002;51(5):1182–8. discussion 1188–90. doi: 10.1097/00006123-200211000-00012 [DOI] [PubMed] [Google Scholar]
- 33.Trojaborg W. Clinical, electrophysiological, and myelographic studies of 9 patients with cervical spinal root avulsions: discrepancies between EMG and X-ray findings. Muscle Nerve 1994;17(8):913–22. doi: 10.1002/mus.880170811 [DOI] [PubMed] [Google Scholar]
- 34.Tsai PY, Chuang TY, Cheng H, Wu HM, Chang YC, Wang CP. Concordance and discrepancy between electrodiagnosis and magnetic resonance imaging in cervical root avulsion injuries. J Neurotrauma 2006;23(8):1274–81. doi: 10.1089/neu.2006.23.1274 [DOI] [PubMed] [Google Scholar]
- 35.Kofler M, Kronenberg MF, Brenneis C, Felber A, Saltuari L. Cutaneous silent periods in intramedullary spinal cord lesions. J Neurol Sci 2003;216(1):67–79. doi: 10.1016/S0022-510X(03)00211-9 [DOI] [PubMed] [Google Scholar]
- 36.Lo YL, Tan YE, Dan YF, Fook-Chong S, Boolsambatra P, Yue WM, et al. Role of spinal inhibitory mechanisms in whiplash injuries. J Neurotrauma 2007;24(6):1055–67. doi: 10.1089/neu.2006.0222 [DOI] [PubMed] [Google Scholar]
- 37.Vasko P, Bocek V, Mencl L, Stetkarova I. Cutaneous silent period in brachial plexus injury. Clinl Neurophysiol 2014;125(5)(Suppl):e42. doi: 10.1016/j.clinph.2013.12.092 [DOI] [Google Scholar]
- 38.Liu Y, Zhou X, Ma J, Ge Y, Cao X. The diameters and numer of nerve fibers in spinal nerve roots. J Spinal Cord Med 2015;38(4):532–7. doi: 10.1179/1079026814Z.000000000273 [DOI] [PMC free article] [PubMed] [Google Scholar]


