Abstract
The fidelity of synaptic transmission depends on the integrity of the protein machinery at the synapse. Unfolded synaptic proteins undergo refolding or degradation in order to maintain synaptic proteostasis and preserve synaptic function, and buildup of unfolded/toxic proteins leads to neuronal dysfunction. Many molecular chaperones contribute to proteostasis, but one in particular, cysteine string protein (CSPα), is critical for proteostasis at the synapse. In this study we report that exported vesicles from neurons contain CSPα. Extracellular vesicles (EV’s) have been implicated in a wide range of functions. However, the functional significance of neural EV’s remains to be established. Here we demonstrate that co-expression of CSPα with the disease-associated proteins, polyglutamine expanded protein 72Q huntingtinex°n1 or superoxide dismutase-1 (SOD-1G93A) leads to the cellular export of both 72Q huntingtinex°n1 and SOD-1G93A via EV’s. In contrast, the inactive CSPαHPD-AAA mutant does not facilitate elimination of misfolded proteins. Furthermore, CSPα-mediated export of 72Q huntingtinex°n1 is reduced by the polyphenol, resveratrol. Our results indicate that by assisting local lysosome/proteasome processes, CSPα-mediated removal of toxic proteins via EVs plays a central role in synaptic proteostasis and CSPα thus represents a potential therapeutic target for neurodegenerative diseases.
Introduction
Synaptic proteostasis is the foundation of functional synaptic networks. Neurons have numerous presynaptic terminals that operate with high frequency at a great distance from the cell body and there are significant demands on the protein folding capacity at the synapse. Imbalances between the protein folding load and folding capacity result in accumulation of misfolded, nonfunctional proteins and protein aggregates that ultimately trigger synaptic loss. Cysteine string protein (CSPα, DnaJC5) is a synaptic vesicle associated co-chaperone, enriched in synapses, that forms a complex with Hsp70 family members on synaptic vesicles to facilitate presynaptic protein folding1, 2. Many Hsp70-interacting proteins, such as CSPα, contain a ~70 amino acid -J domain. Within the J domain of CSPα, the HPD motif is required for the interaction and activation of the Hsp70 family member3. Other mutations in CSPα, L115R and L116∆, cause the neurodegenerative disorder, adult neuronal ceroid lipofuscinosis (ANCL) emphasizing the critical role for CSPα in maintaining synapse function4–6. Moreover, deletion of CSPα leads to neurodegeneration in multiple experimental models. CSPα knock out mice exhibit fulminant neurodegeneration and have a reduced lifespan with no mice surviving beyond 3 months. In contrast, CSPα+/− mice are for the most part phenotypically normal, suggesting that there is a surplus of the co-chaperone under normal physiological conditions7–9. Loss-of-function CSPα Drosophila mutants demonstrate uncoordinated movements, temperature-sensitive paralysis and early lethality10. Furthermore, in C elegans, CSPα null mutants show neurodegeneration and reduced lifespan11. Clearly, dysruption or absence of CSPα has undesired consequences on the stability of synapses; nevertheless, questions remain regarding the mechanisms underlying CSPα-neuroprotection.
Some J domain-containing co-chaperones undergo secretion; DnaJB11 (Erdj3) is reported to be released from HEK-293 cells through the canonical ER/Golgi secretory pathway and DnaJB1 (Hsp40) is released from Neuro2A cells via extracellular vesicles (EV’s)12, 13. DnaJB1 is a cytosolic co-chaperone that is upregulated during the heat shock response14, 15 and is implicated in protection of the nervous system from damage14. Microvesicles and exosomes are EV’s that shuttle specific cargo such as proteins, lipid and RNA between cells16. They are released by all cell types and mediate the transfer of hydrophobic and cytosolic proteins, exerting profound effects on recipient cells and influencing proteostasis. Microvesicles are large EV’s, ~100 nm-1 μm in diameter, formed by the outward budding of the plasma membrane. Exosomes are smaller vesicles, ~40–130 nm in diameter, formed by the inward budding of the endosomal membrane to generate intraluminal vesicles that accumulate in multivesicular bodies (MVB’s). MVB’s fuse with either the plasma membrane to release exosomes or with lysosomes for degradation of the vesicles and protein contents17. Exosome-exported DnaJB1 contributes to the protein folding capacity of recipient cells (eg. reducing polyglutamine-mediated aggregation in neuroblastoma cells)13. In contrast, DnaJB11 is an ER protein that is upregulated during the unfolded protein response18 and is responsible for folding and degradation of a number of ER client proteins18–22. DnaJB11 is implicated in neural development23 as well as Gaucher’s disease22. Several chaperones, like DnaJB11, are secreted into the extracellular space where they bind misfolded proteins24 and contribute to extracellular folding capacity (eg. reducing the effect of toxic prion protein on neuroblastoma cells)12. For example, clustrin is implicated in Alzheimer’s disease and prevents aggregation of extracellular misfolded proteins in an Hsc70 and ATP-independent manner24. However, a comprehensive list of extracellular chaperones surrounding synapses has not been established. In addition to enhancing recipient cell or extracellular folding capacity, it is likely that Hsp70-interacting proteins stabilize client protein delivery through the secretory pathway or during EV-transit to recipient cells.
Distinct export routes for misfolded proteins also exist. Accumulation of misfolded-toxic proteins between cells is a common pathogenic pathway24. For example, a major hallmark of Alzheimer’s disease is the formation of extracellular plaques that contain aggregated, amyloid β peptide25. In contrast, cell-to-cell transmission of misfolded-toxic proteins such as prions26, 27, beta-amyloid peptides28, amyloid precursor protein fragments29, tau30, 31 superoxide dismutase 132–34 TDP-4335 and α-synuclein36–38 is EV-mediated. Many questions remain regarding how chaperones target and process exported misfolded proteins.
The full nature of the events governing presynaptic protein homeostasis is not understood. One possible scenario for CSPα’s neuroprotective activity is targeting of misfolded client proteins by CSPα for export, thereby reducing the protein-folding load in neurons. To begin to investigate this possibility we first sought to determine if CSPα is secreted from neurons. We report that CSPα is indeed exported from catecholaminergic-derived mouse (CAD) cells within extracellular vesicles (EV’s). We show that CSPα is released from mouse brain tissue as well as human glioblastoma cells and is found in human CSF. The human mutations CSPαL115R and CSPα∆116, and the CSPαHPD-AAA mutant that does not activate Hsp70, also undergo EV-mediated export. We also find that CSPα but not CSPαHPD-AAA, promotes cellular export of two distinct misfolded disease-causing proteins, the polyglutamine expanded protein 72Q huntingtinexon1 and superoxide dismutase-1 (SOD-1G93A). In contrast, the export of other EV components, eg. Gαs, clathrin heavy chain, flotillin-1, Hsp90 and actin, do not show CSPα-mediated secretion. This work showing CSPα-mediated export of 72Q huntingtinexon1 and SOD-1G93A is consistent with Fontaine and colleagues who, during the course of this study, reported that CSPα facilitated extracellular release of TDP-43, α-synuclein and microtubule-associated protein tau from HEK-293 cells39. Surprisingly, we find that the endogenous chaperones, DnaJB1, DnaJB11 and DnaJA1, are co-secreted with CSPα, giving rise to the possibility that several J domain-containing proteins are involved in export. Our study directly demonstrates that CSPα disposes of the misfolded-disease-causing proteins SOD-1G93A and 72Q huntingtinexon1 by promoting their export. Given that CSPα expression is required for the survival of synapses in vivo, we suggest that this CSPα-mediated export is critical for the maintenance of synapses.
Results
CSPα is secreted from neurons
CSPα is a well-established component of the presynaptic proteostasis network7, 10, 40. To begin to address whether CSPα’s neuroprotective activity involves its export, we sought to establish if CSPα is released from neurons like DnaJB1 13 and DnaJB11 12. To this end, myc-tagged CSPα was expressed in CAD cells, the culture media was replaced with fresh media 6 hrs after transfection and at 48 hrs, the medium was collected, centrifuged (1,500Xg) to remove dead cells and the presence of exported CSPα determined. Figure 1 shows three exported species of CSPα, consistent with the unpalmitoylated (26 kDa), palmitoylated (35 kDa) and dimeric (70 kDa) cellular forms41, 42 and an additional 18 kDa N terminal CSPα fragment. Actin, is a known exosome protein and is present in the conditioned media43. Exported CSPα is approximately 11 times higher in media from CSPα transfected CAD cells compared with vector transfected control cells, demonstrating that increasing cellular CSPα levels increases its secretion (Fig. 1B). N terminal HA- or myc- and C terminal FLAG- tagged CSPα are all released from cells (Fig. 1C), indicating that epitope tags do not influence CSPα’s export, although the C terminal FLAG tag reduced immunodetection by the CSPα polyclonal antibody. In addition to CAD cells, transiently expressed FLAG-tagged-CSPα but not Flag-NECAP or Flag-DENND3 was released from HEK-293 cells (Fig. 1D). Next we wanted to know if extracellular CSPα is a feature of transient expression, or if CSPα secretion is a more widely occurring event. To this end, we evaluated seven human CSF subjects who had no clinical evidence of CNS pathology for CSPα. All seven CSF samples evaluated were positive for CSPα, although the level of CSPα (in 50 μl) varied among subject samples (Fig. 1E and data not shown). We also evaluated CSPα levels in conditioned media from human brain tumors of 11 distinct origins44, 45. Out of 11 brain tumor initiating cells examined, 9 were found to secrete CSPα. CSPα of a distinct molecular weight was identified in conditioned media from two separate human brain tumors (Fig. 1E, lower panel, lane 2 and data not shown). Together, these results confirm that export of CSPα occurs physiologically, pathologically as well as in cell culture.
Figure 1.
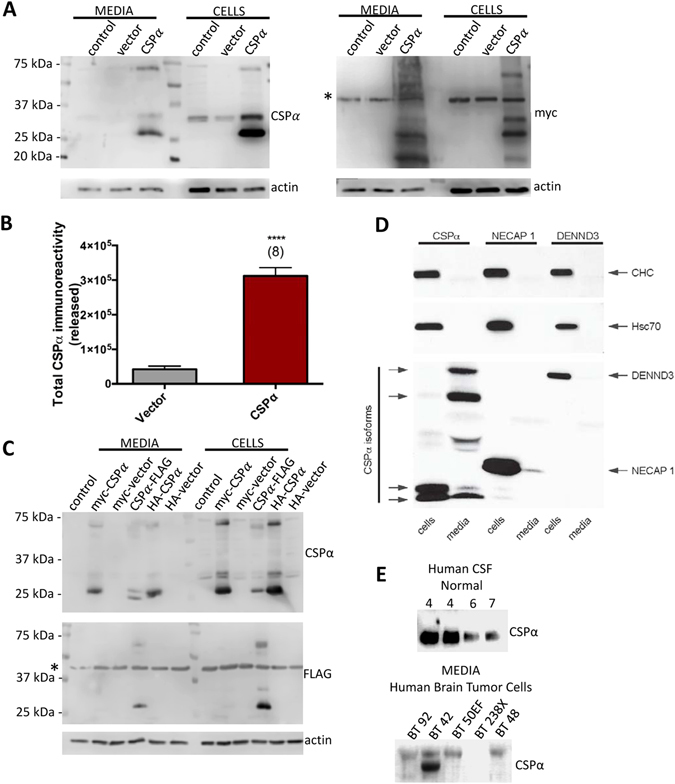
Extracellular release of CSPα. (A) Representative immunoblot of media and cell lysates from untransfected, vector and CSPα transfected cells. CAD cells transfected with 3.75 μg of cDNA encoding myc-tagged WT CSPα or vector control were lysed 48 hrs post-transfection and corresponding cell lysates (15 µg) and 10X concentrated media (40 µl) were evaluated by immunoblot with the indicated antibodies. *Indicates a non-specific band. (B) Quantification of immunoreative CSPα from vector and CSPα transfected cells (****P < 0.0001) as determined by anti-myc monoclonal. These results are representative of 8 independent experiments. (C) Immunoblot of lysates and media from CAD cells transfected with myc-, HA-, or FLAG-tagged CSPα and lysed 48 hrs post-transfection (D) Immunoblot analysis of cell lysates and culture media from HEK-293 cells transfected with Flag-CSPα, Flag-NECAP 1 or Flag-DENND3, as indicated. The bottom transfer is blotted with antibody against Flag. The top two transfers are blotted with antibodies recognizing endogenous clathrin-heavy chain (CHC) or Hsc70, as indicated. (E) Immunoblot of human cerebrospinal fluid (50 µl) and media from human brain tumor cell lines (75 μl).
CSPα is released in EV’s
Since conditioned medium contains secreted proteins and different types of extracellular vesicles; including exosomes and microvesicles (Fig. 2A), we next established the nature of exported CSPα. CSPα is one of the most highly palmitoylated neural proteins46 and we reasoned it could be secreted in exosomes that are derived from lipid rafts. Following the established ExoQuick precipitation technique (System Biosciences Inc), we isolated EV’s enriched in exosomes from cell culture media that had been centrifuged to remove cell debris. Western blot analysis reveals that CSPα is present in EV’s enriched for exosomes together with the exosomal marker flotillin 1 (Fig. 2B). Actin and Hsc70/Hsp70, known exosomal proteins43, are also present in these CSPα-containing EV’s (Fig. 2C). No large apoptotic bodies were present in the EV preparation. The observed EV size distribution in our preparations varied, with the most frequent size of EV’s being 129+/−2.2 nm, however larger EV’s were also present and the mean size of EV’s was 175.3+/−3.5 nm (Fig. 2D).
Figure 2.
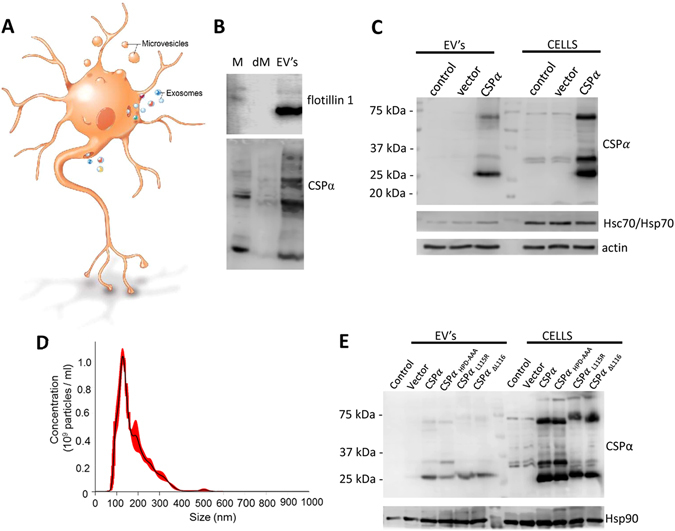
CSPα is released via EV’s. (A) Working model of extracellular vesicle release from neurons; illustration by Roula Drossis. Neural export of larger microvesicles and smaller exosomes are shown. (B) Immunoblot of media (M), media depleted of EV’s (dM) and the EV’s enriched for exosomes from CSPα transfected CAD cells. (C) Immunoblot of EV’s and corresponding cell lysates. (D) Size distribution of EV’s isolated from CSPα-transfected CAD cells. Red error bars indicate +/−1 standard error of the mean. (E) Immunoblot of EV’s and CAD cell lysates (15 µg) collected 48 hrs after transfection of 3.7 μg of vector, myc-tagged CSPα, or 5 μg of myc-tagged CSPαL115R, CSPαL116∆, CSPαHPD-AAA. Western blots are representative of 5 independent experiments.
We wanted to know if mutated CSPα is also secreted. Figure 2E shows that like WT CSPα, myc-tagged CSPαL115R, CSPαL116∆ and a CSPαHPD-AAA mutant that does not activate Hsp70 family members, are all released extracellularly. The pathogenesis of L115R and L116∆ CSPα mutants that cause ANCL4–6 is not established, however CSPαL115R and CSPαL116∆ are predisposed to oligomerize and have reduced palmitoylation42, 47, 48. To examine whether these mutant CSPα’s were exported or retained by neurons, myc-tagged CSPαL115R and CSPαL116∆ and loss-of-function CSPαHPD-AAA were transfected into CAD cells and EV’s enriched for exosomes were analyzed 48 hrs later. Consistent with previous reports42, 47, 48, the 35 kDa palmitoylated CSPα species is significantly reduced in CSPαL115R, CSPαL116∆ expressing cells compared to cells expressing CSPαHPD-AAA and WT CSPα and expression of CSPαL115R and CSPαL116∆ results in formation of high molecular weight aggregates. These results demonstrate that like CSPα, CSPα mutants are present in EV’s enriched for exosomes. Secretion of CSPαHPD-AAA indicates that export is independent of molecular interactions with Hsc70, whereas secretion of CSPαL115R and CSPαL116∆ suggests that export is independent of palmitoylation. Hsp90, an established exosomal protein43, is present in all EV’s (Fig. 2E).
Co-secretion with CSPα
Since the human mutations CSPαL115R and CSPαL116∆ lead to neurodegeneration but were secreted like WT CSPα, we wondered if WT and mutant CSPα differed in their time course of release. Figure 3 shows that CSPαL115R has a significantly slower time course than CSPα at 24 hrs (P < 0.05) and that CSPα, CSPαL115R and CSPαL116∆ release is abundant 48 hrs after transfection. The presence of FBS (5% exosome-free) did not alter CSPα secretion (Fig. 3B). Surprisingly, endogenous DnaJB1, DnaJB11 and DnaJA1 were found to be co-secreted with CSPα (Fig. 3B). The presence of DnaJB1, DnaJB11 and DnaJA1 in EV’s was confirmed by LC-MS/MS (data not shown). It is possible that these J domain containing chaperones are co-packaged as EV cargo, packaged into separate vesicles or alternatively, that they are independently secreted and subsequently associate with EV’s extracellularly (i.e. attached to the EV surface). CSPα and DnaJB11 secretion does not change in the presence of serum while DnaJB1 and DnaJA1 release is reduced (Fig. 3B), highlighting differences among the secreted J proteins. Flotillin 1, Hsp90 and actin, known exosomal components, are shown. We then examined CSPα-containing EV’s for presynaptic proteins that have been proposed to be CSPα targets40, 42, 49–66. As previously reported, SNAP25 expression is reduced63, 64, 67 while BKα potassium channel levels are elevated56 in synaptosomes from CSPα KO mice compared to WT mice. BKα potassium channel, SNAP25, dynamin, synaptotagmin and syntaxin are not abundant in EV’s from CAD cells (Fig. 3C). The putative CSPα client proteins Gαs, clathrin heavy chain and Rab5 were observed to be exported in the absence or presence of CSPα or CSPα mutants.
Figure 3.
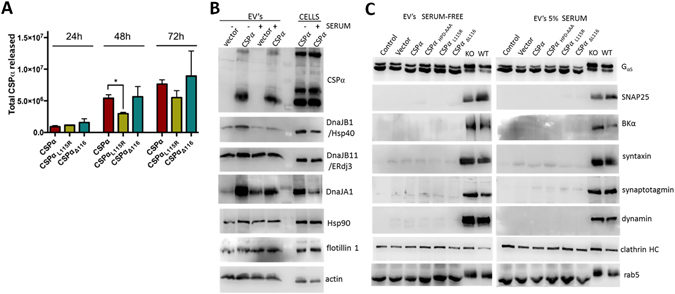
DnaJB11 and DnaJB1 are co-secreted with CSPα. (A) Quantification of the time course of WT and mutant CSPα release (media); (*P < 0.05). (B) Immunoblot of EV’s released from vector or CSPα transfected cells probed with the indicated antibodies. 6 hrs after transfection the media was replaced with either serum free media or media containing 5% serum (exosome-free) and 48 hrs after transfection media was collected and exosomes prepared. Cell lysates are shown on the left hand lanes. (C) Immunoblot of EV’s isolated from control (untransfected), vector (3.75 μg), myc-tagged CSPα (3.75 μg), CSPαHPD-AAA (5 µg), CSPαL115R (5 µg), or CSPαL116∆ (5 µg) transfected cells probed with the indicated antibodies. Crude synaptosomes from CSPα knock out and WT mice are shown in the right lanes. Western blots are representative of 5 independent experiments.
CSPα promotes/chaperones export of disease-causing proteins
We next asked if neuronal proteins associated with cellular toxicity were exported with CSPα in EV’s. To this end we introduced the polyglutamine expanded protein 72Q huntingtinexon1 or superoxide dismutase-1 (SOD-1G93A) with WT or mutant CSPα. Trinucleotide (CAG) repeat expansions of the huntingtin gene causes Huntington’s disease68. The length of the polyglutamine expansion is linked to the age of onset of Huntington’s disease and the aggregation propensity of the huntingtin protein68. Aggregates of polyglutamine expanded huntingtin have been found within genetically normal tissue grafted into patients with progressing HD, revealing cell-to-cell transit of huntingtin aggregates in vivo 69. Mutations in the gene encoding superoxide dismutase-1 (SOD-1), a copper-binding enzyme that functions as an antioxidant, lead to the motor neuron disease, amyotrophic lateral sclerosis (ALS)70. Misfolding, aggregation and cell-to-cell spread of mutant SOD-1 is mediated by both free floating aggregates as well as exosomes34, 71. Figure 4 shows that CSPα promotes cellular export of both polyglutamine expanded protein 72Q huntingtinexon1 and superoxide dismutase-1 (SOD-1G93A). CSPαL115R and CSPαL116∆ also effectively support the export of both 72Q huntingtinexon1 and SOD-1G93A. Integrin-linked kinase is not exported from cells while Gαs, clathrin heavy chain, actin and native SOD-1 (lower band) are exported in EV’s in the absence and presence of CSPα and CSPα mutants. It is not known if export of these proteins is regulated by other DnaJ’s. Although export of native huntingtin from CAD cells is not observed, both WT and mutant SOD-1 are exported and export is increased by CSPα. A small increase in the cellular release of actin, clathrin heavy chain and Gαs is observed following transfection (vector), therefore 72Q huntingtinexon1 in EV’s and SOD-1G93A in media was quantified relative to actin (Fig. 4E and F).
Figure 4.
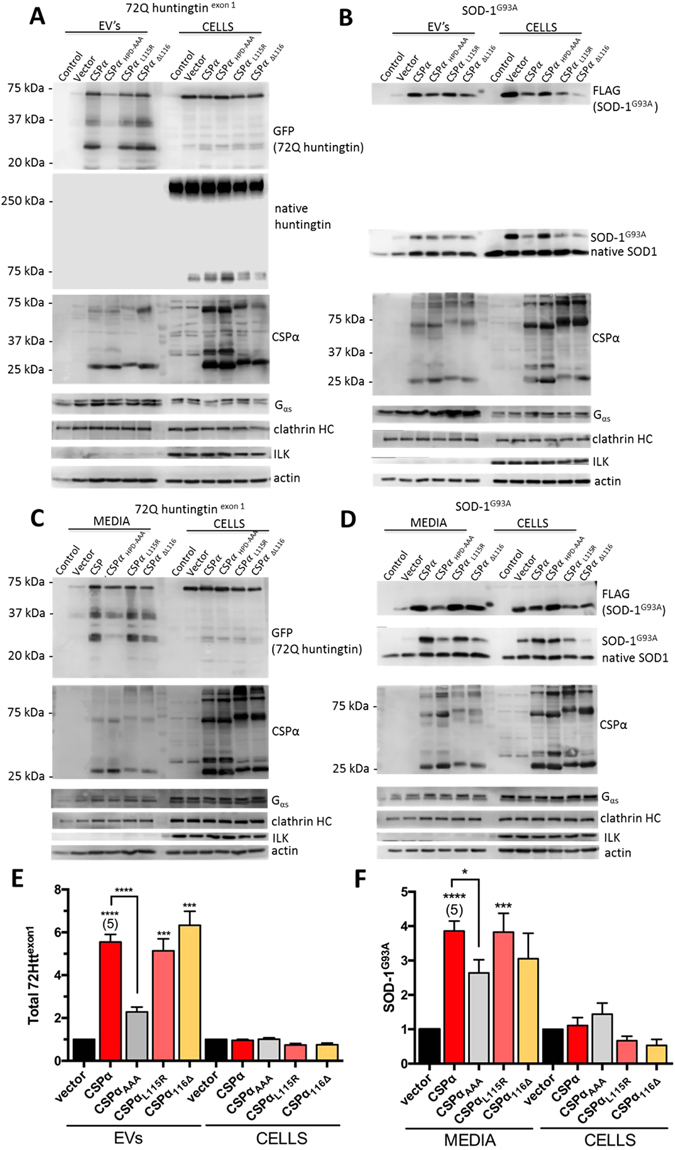
CSPα promotes 72Q huntingtinexon1 and superoxide dismutase-1 (SOD-1G93A) export. Immunoblot of EV’s and corresponding lysates (15 µg) from cells transfected with GFP-tagged 72Q huntingtinexon1 (A) or FLAG-tagged SOD-1G93A. For native huntingtin immunoblot 25 µg of cell lystates were evaluated. (B) and vector, myc-tagged CSPα, CSPαHPD-AAA, CSPαL115R, or CSPαL116∆ and probed with the indicated antibodies. 2.5 µg of the indicated cDNA was transfected. (C,D) Immunoblot of media and corresponding lysates (15 µg). (E,F) Quantification of GFP-tagged 72Q huntingtinexon1 in EV’s and FLAG-tagged SOD-1G93A in media, normalized to actin (****P < 0.0001; ***P < 0.001, *P < 0.05).
Some differences between 72Q huntingtinexon1 and SOD-1G93A secretion were observed. EV-exported-72Q huntingtinexon1 underwent degradation such that 2 prominent degradation products were found in EV’s (Fig. 4A), however no SOD-1G93A degradation was detected. Secreted 72Q huntingtinexon1 co-purified with EV’s, while secreted SOD-1G93A was both free floating and EV-associated, consistent with previous reports33. Finally, 72Q huntingtinexon1 release was 60% lower (P < 0.0001) in the presence of loss-of-function CSPαHPD-AAA compared to WT CSPα while SOD-1G93A release, was 40% lower (P < 0.05) (Fig. 4E and F). Taken together, these results demonstrate that CSPα is exported and facilitates EV export of distinct toxic misfolded proteins. This export is dependent on the HPD motif of CSPα that is required for recruitment and activation of Hsc70 by CSPα. In contrast, the export of CSPα itself is not influenced by mutations in the HPD motif.
CSPα is secreted from brain
We next evaluated whether endogenous CSPα is released from mouse brain in addition to CAD cells. Brains were obtained at 23–25 days from CSPα knock out mice when they show partial paralysis and loss of neuromuscular control7. Mouse brains were sliced, washed in PBS, incubated in media for 2 hrs and EV’s then isolated from the supernatant that had been filtered (30µm), centrifuged (2,000Xg) to remove debris and isolated by ExoQuick precipitation. Figure 5A shows that freshly removed murine brain tissue from WT mice exports EV’s containing CSPα. As expected, no CSPα release is observed from CSPα knock out mice. CSPα is present in EV’s prepared by either ultracentrifugation or ExoQuick methods. Given the smaller size of CSPα knock out relative to WT mice7, we evaluated release from both whole and hemi WT brains. We asked whether or not DnaJA1, DnaJB1, and DnaJB11 were found in EV’s from mouse brain. Figure 5B shows that DnaJA1, DnaJB1, and DnaJB11 are indeed exported from both WT and CSPα knock out mouse brains. 1.5 µg of crude synaptosomes from WT mouse is shown in the right lane. No change in levels of DnaJA1, DnaJB1, DnaJB11 is found in synaptosomes from CSPα knock out mice (Fig. 5C and D). Taken together, these results indicate that CSPα and other J proteins are released from mammalian neurons and purify with EV’s.
Figure 5.
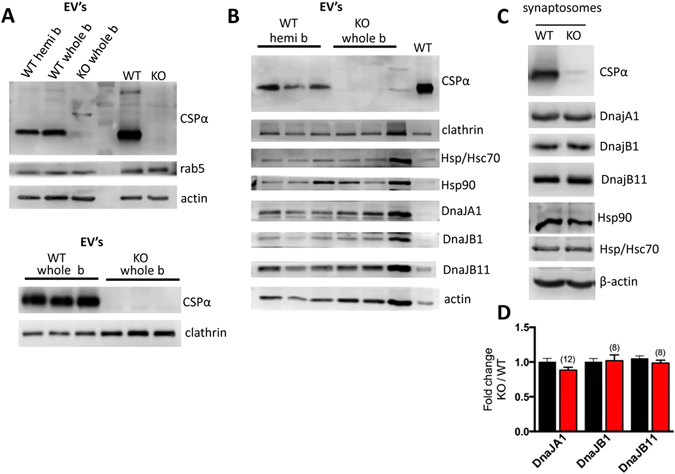
CSPα is present in EV’s isolated from brain tissues (A) Immunoblot of EV’s released from WT and CSPα knock out mouse brain probed with the indicated antibodies; upper panel EV’s prepared by ExoQuick and lower panel EV’s prepared by differential ultracentrifugation. Release from hemi and whole WT brains is shown for comparison. Synaptosomes (1.5 µg) from WT mice are shown in the right lane. (B) Immunoblot of EV’s released from WT (hemi) and CSPα knock out mouse brain (whole) probed with the indicated antibodies. (C and D) Western analysis and quantification of synaptosomes (30 µg) at 25 days probed with the indicated antibodies. β-actin is shown as loading control.
Resveratrol inhibits export of 72Q huntingtinexon1
Finally we asked whether resveratrol treatment alters the CSPα promotion of disease-causing protein export. Resveratrol, a polyphenol with neurprotective, cardioprotective and anti-inflammatory properties, has been previously shown to ameliorate the reduced viability in C. elegans CSPα mutants11. Here we found that 50 µM resveratrol reduced 72Q huntingtinexon1 export by 70% (Fig. 6), indicating that the CSPα-mediated export of 72Q huntingtinexon1 is resveratrol-sensitive.
Figure 6.
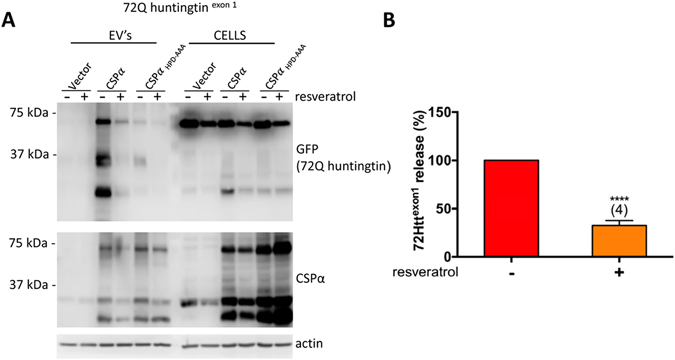
Resveratrol inhibits export of 72Q huntingtinexon1 (A) Immunoblot of EV’s and corresponding lysates (15 µg) from cells transfected with vector, CSPα or CSPαHPD-AAA and GFP-tagged 72Q huntingtin. 2.5 µg of the indicated cDNA was transfected. Where indicated, cells were treated with 50 μM resveratrol 6 hrs after transfection. β-actin is shown as loading control. (B) Quantification of CSPα mediated export of GFP-tagged 72Q huntingtinexon1 in EV’s in the presence and absence of resveratrol (****p < 0.0001).
Discussion
In this study we directly demonstrate that the neuroprotective, synaptic chaperone CSPα is secreted via EV’s and has a key role in the export of two misfolded, aggregation-prone proteins, polyglutamine expanded protein 72Q huntingtinexon1 and SOD-1 with a glycine to alanine amino acid substitution at position 93. Protection against the buildup of misfolded proteins that threaten synaptic function is in-line with CSPα’s well-documented neuroprotective function7, 10. In addition to ANCL, the rare neurodegenerative disease caused by L115R and ∆L116 mutations4–6, CSPα dysfunction has been implicated in a number of other neurodegenerative disorders including Alzheimer’s, Parkinson’s and Huntington’s disease however mechanistic links have not been determined40, 42, 55, 67, 72, 73. Our results clearly link CSPα to the motor neuron disorder ALS and polyglutamine expansion disorders like Huntington’s disease and are congruent with a recent study by Fontaine and colleagues who reported that release of TDP-43, α-synuclein and microtubule-associated protein tau from HEK-293 cells is enhanced by CSPα39. Together, these studies suggest the CSPα pathway is a common mechanism that promotes/chaperones export of misfolded proteins, that said, the fate of the misfolded proteins that are exported by CSPα remains to be established. The CSPα-mediated export of 72Q huntingtinexon1 is resveratrol-sensitive. Effective clearance of the misfolded cargo by recipient cells would represent a novel quality control pathway, on the other hand, delivery of misfolded proteins to recipient cells and subsequent propagated protein misfolding is consistent with the pathogenic prion-like spreading reported for disease-causing proteins such as, beta-amyloid, tau, TDP-43, α-synuclein, huntingtin and SOD-1. This intersection–i.e. off-site disposal vs propagated misfolding of CSPα-exported protein–is significant, and further experimentation is required to better understand these processes.
The endogenous co-secretion of DnaJA1, DnaJB1, DnaJB11 with CSPα from both CAD cells and mouse brain tissue, indicates that cellular release of “J domain”-containing proteins is more common than previously appreciated. There are many possible roles for secreted “J domain”-containing chaperones, which likely target distinct client proteins including: regulation of the assembly of the EV proteins (eg. tetraspanins, clathrin), selection of EV cargo for export, ensuring delivery of conformationally intact and functional hydrophobic and cytosolic proteins between cells, increasing the folding capacity of recipient cells via cell-to-cell transit of molecular chaperones, selection of aggregate-prone toxic proteins for elimination by export and off-site degradation, regulation of trafficking of EV’s to recipient cells and regulation of protein conformation in the extracellular space. Such processes could involve additional regulatory proteins. For example, a CSPα-interacting protein74, SGT (small glutamine-rich tetratricopeptide repeat containing protein) is reported to regulate whether or not misfolded proteins are targeted to late endosomes/MVBs75. These possible roles are based on the assumption that the secreted J proteins are indeed functional and don’t themselves represent protein trash dedicated for export. It is clear that mutated J proteins can be exported, yet it remains to be determined if exported J proteins can promote Hsp70 ATPase activity. Several J domain-containing proteins have neuroprotective activity15 and some, when mutated, cause neurodegenerative disease76, 77. For example, DnaJB6 and DnaJB2 are implicated in protection from Huntington’s disease78 and ALS79 respectively. Some J domain-containing proteins have well characterized intracellular roles; for example Sec63 (DnaJC23), is involved in protein translocation across the ER membrane80 and auxilin (DnaJC6), is important in uncoating clathrin coated vesicles during endocytosis81, 82. The fulminant neurodegeneration observed in CSPα knock-out models clearly indicates that other J proteins do not compensate in its absence.
There is currently great interest in EV’s as vehicles for intercellular communication as well as shuttles for the spread of misfolded proteins that cause neurodegenerative disorders and tumor cell metastasis. We suggest that the maintenance of optimally functional synapses involves chaperone mediated-export of dysfunctional proteins. CSPα-mediated neuroprotection is activity- dependent8, 83 and interestingly, release of exosomes from differentiated neurons has been reported to be regulated by synaptic activity84. An activity-dependent build-up of misfolded proteins may be common to the pathogenic cascade of distinct neurodegenerative diseases. Therefore, understanding export pathways is important, as it may allow for the development of therapeutics aimed at treating the progression of neurodegenerative disease.
Materials and Methods
Expression of chaperones, SOD-1G93A and 72Q huntingtinexon1 in CAD cells
Maintenance of CAD (catecholaminergic derived CNS cells) was described before56. For expression in CAD cells, cDNAs encoding for CSPα and CSPα mutants were expressed in the plasmid myc-pCMV, HA-pCMV, pcDNA3.1-FLAG and pcDNA3.1-GFP. All mutations were confirmed by DNA sequence analysis. Constructs encoding Myc-tagged CSPα, Flag-tagged CSPα, HA-tagged Myc-tagged CSPα, Myc-tagged CSPαL115R, Myc-tagged CSPαΔL116, Myc-tagged CSPαHPD-AAA, GFP-72Q huntingtinexon1 and SOD-1G93A-FLAG were transiently transfected with Lipofectamine 3000 (Invitrogen) in Opti-MEM™ medium (Invitrogen). Media was changed to serum-free media 6 hrs post-transfection, or where indicated 5% exosome free FBS (Systems Biosciences). Where indicated, 50 µM resveratrol treatment (Sigma) started 6 hrs post-transfection.
CSF
Cerbrospinal fluid (CSF) samples were collected by lumbar puncture under general anesthesia prior to microdiskectomy surgery. The subjects were symptomatic with degenerative disc disease refractory to non-surgical management for over 12 wks but lacked evidence of CNS pathology on history and physical examination by a neurosurgeon. All the samples were collected with informed consent for participation and publication in accordance with the guidelines and regulations of the University of Calgary institutional ethics/licensing committee.
Brain Tumor Initiating Cells
Surgical samples from patients with newly diagnosed or recurrent glioblastoma were obtained from the Tumor Tissue Bank within the Arnie Charbonneau Cancer Institute, transported to the BTIC Core Facility (Calgary, Alberta) and established as described previously44, 45. This study has Institutional review board approval under the “Brain Tumor and Related Tissue Bank protocol-V2” and approved by Foothills Hospital and the Conjoint Health Research Ethics Board and all methods were performed in accordance with the relevant guidelines and regulations. All established cell lines used were validated for identity by short tandem repeat analysis performed by Calgary Laboratory Services. All BTIC lines were grown in serum free-media supplemented with EGF (20 ng/ml; Peprotech, FGF2 (20 ng/ml; R&D Systems Inc, and heparin sulfate (2 mg/ml: R&D Systems). For the collection of conditioned media (CM) individual BTIC lines were plated at one million cells/ml, after 48 hrs media was collected and centrifuged to remove debris.
CAD cell Media and EV collection
Media was collected 48 hrs after transfection unless otherwise indicated and spun at 1,500Xg for 10 min to remove cell debris. Media was concentrated 10Xs with YM 30,000, centricon filter (Millipore) at 5,000Xg for 30 min. Cells were lysed in: 40 mM Tris (pH7.4), 150 mM NaCl, 2 mM EDTA, 1 mM EGTA, 1% Triton X-100, 0.1% SDS with 1 mM Na3VO4, 0.5 mM PMSF and protease inhibitors (Sigma) at 4 °C for 1 hr. Lysates were centrifuged at 15000Xg for 5 min at 4° C and the supernatant (soluble fraction) was collected and stored at −70° C. Protein concentration of the soluble CAD cell lysate was determined using the Pierce BCA protein assay.
For isolation of EV’s, media was harvested 48 hrs after transfection, spun at 2000Xg for 10 min to remove cell debris and the supernatant was combined 5:1 with exoquick precipitation solution (SBI), incubated overnight at 4 °C, centrifuged at 7,000Xg for 30 min, washed and resuspended in PBS.
Immunoblotting
Proteins were separated by SDS-PAGE and electrotransferred from polyacrylamide gels to nitrocellulose membrane (0.2 µm pore size). Membranes were blocked in tris-buffered saline (TBS) containing 0.1% Tween 20, 1% BSA and then incubated with primary antibody overnight at 4° C. The membranes were washed and incubated with horseradish peroxidase-coupled secondary antibody for ~2 hrs at room temperature. Bound antibodies (Supp Table 1) on the membranes were detected by incubation with Pierce chemiluminescent reagent and exposure to Cdigit, LiCor (Mandel). The chemiluminescent signals were quantified using image studio digits software (Mandel).
EV size determination
Nanosight finite track length analysis was used to determine the EV size distribution (System Biosciences).
Brain Slices
All mice were maintained in accordance with an animal protocol approved by the University of Calgary and the Guidelines for Lab Animal Safety. All methods were conducted in accordance with the relevant University of Calgary guidelines and regulations. We isolated EV’s from the brains of 23–25 day old WT and littermate CSPα knock out mice by a procedure modified from ref. 85. Mouse brains were removed, separated into hemi brains and the hemi brain sliced into four pieces and incubated for 2 hrs at 37 °C in 6 ml of hibernate A. Media was filtered through a 30 µm filter, centrifuged 2,000Xg for 10 min, the supernatant combined 5:1 with exoquick precipitation solution (SBI), incubated overnight at 4 °C, centrifuged at 7,000Xg for 30 min and washed and resuspended in PBS. For preparation of EV’s by differential ultracentrifugation, media was filtered through a 30 µm filter and sequentially at centrifuged 2,000Xg for 10 min, and at 10,000Xg for 30 min at 4 °C to discard membranes and debris. The supernatant was then centrifuged at 100,000Xg for 2 hrs to pellet EV’s enriched in exosomes.
Statistical Analysis
All values are presented as the mean ± SEM and statistical significance was analyzed using a Student’s t-test. Calculations were performed using GraphPad Prism 6 software.
Electronic supplementary material
Acknowledgements
The authors would like to express their gratitude to Drs. Frank Visser, David Schriemer and Laurent Brechenmacher for technical support and Roula Drossis for the illustration. We are grateful to Dr. Andy Braun for detailed discussion during these experiments. The work was supported by a grant from the Alberta Prion Research Foundation.
Author Contributions
J.E.A.B. wrote the main manuscript text and prepared the figures. J.T.D. contributed to Figures 1–6. J.D. contributed to Figures 1 and 5. C.K. contributed to Figures 1 and 3. M.A., S.M.R., S.C., M.F., M.G. and P.S.M. contributed to Figure 1.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01115-6
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Braun JE, Wilbanks SM, Scheller RH. The cysteine string secretory vesicle protein activates Hsc70 ATPase. J Biol Chem. 1996;271(42):25989–25993. doi: 10.1074/jbc.271.42.25989. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain LH, Burgoyne RD. Activation of the ATPase activity of heat-shock proteins Hsc70/Hsp70 by cysteine-string protein. Biochem J. 1997;322(Pt 3):853–858. doi: 10.1042/bj3220853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol. Cell Biol. 2010;11(8):579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benitez BA, et al. Exome-sequencing confirms DNAJC5 mutations as cause of adult neuronal ceroid-lipofuscinosis. PLoS ONE. 2011;6(11):e26741. doi: 10.1371/journal.pone.0026741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noskova L, et al. Mutations in DNAJC5, encoding cysteine-string protein alpha, cause autosomal-dominant adult-onset neuronal ceroid lipofuscinosis. Am. J Hum. Genet. 2011;89(2):241–252. doi: 10.1016/j.ajhg.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velinov M, et al. Mutations in the Gene DNAJC5 Cause Autosomal Dominant Kufs Disease in a Proportion of Cases: Study of the Parry Family and 8 Other Families. PLoS ONE. 2012;7(1):e29729. doi: 10.1371/journal.pone.0029729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Chacon, R. et al. The synaptic vesicle protein CSP alpha prevents presynaptic degeneration. 42(2), 237 (2004). [DOI] [PubMed]
- 8.Garcia-Junco-Clemente P, et al. Cysteine string protein-alpha prevents activity-dependent degeneration in GABAergic synapses. J Neurosci. 2010;30(21):7377–7391. doi: 10.1523/JNEUROSCI.0924-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Ortega E, Ruiz R, Tabares L. CSPalpha, a Molecular Co-chaperone Essential for Short and Long-Term Synaptic Maintenance. Front Neurosci. 2017;11:39. doi: 10.3389/fnins.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zinsmaier, K. E. et al. Paralysis and early death in cysteine string protein mutants of Drosophila. 263(5149), 977 (1994). [DOI] [PubMed]
- 11.Kashyap SS, et al. Caenorhabditis elegans dnj-14, the orthologue of the DNAJC5 gene mutated in adult onset neuronal ceroid lipofuscinosis, provides a new platform for neuroprotective drug screening and identifies a SIR-2.1-independent action of resveratrol. Hum. Mol. Genet. 2014;23(22):5916–5927. doi: 10.1093/hmg/ddu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genereux JC, et al. Unfolded protein response-induced ERdj3 secretion links ER stress to extracellular proteostasis. EMBO J. 2015;34(1):4–19. doi: 10.15252/embj.201488896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi T, et al. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc. Natl. Acad. Sci. USA. 2015;112(19):E2497–E2506. doi: 10.1073/pnas.1412651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown IR. Heat shock proteins and protection of the nervous system. Ann. N. Y. Acad Sci. 2007;1113:147–158. doi: 10.1196/annals.1391.032. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Braun AP, Braun JE. Biological Roles of Neural J Proteins. Cell Mol. Life Sci. 2008;65(15):2385–2396. doi: 10.1007/s00018-008-8089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budnik V, Ruiz-Canada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat. Rev. Neurosci. 2016;17(3):160–172. doi: 10.1038/nrn.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu. Rev. Cell Dev. Biol. 2012;28:337–362. doi: 10.1146/annurev-cellbio-092910-154152. [DOI] [PubMed] [Google Scholar]
- 18.Shen Y, Hendershot LM. ERdj3, a stress-inducible endoplasmic reticulum DnaJ homologue, serves as a cofactor for BiP’s interactions with unfolded substrates. Mol. Biol. Cell. 2005;16(1):40–50. doi: 10.1091/mbc.E04-05-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buck TM, et al. The endoplasmic reticulum-associated degradation of the epithelial sodium channel requires a unique complement of molecular chaperones. Mol. Biol. Cell. 2010;21(6):1047–1058. doi: 10.1091/mbc.E09-11-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoshino T, et al. Endoplasmic reticulum chaperones inhibit the production of amyloid-beta peptides. Biochem. J. 2007;402(3):581–589. doi: 10.1042/BJ20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Y, et al. Regulated release of ERdj3 from unfolded proteins by BiP. EMBO J. 2008;27(21):2873–2882. doi: 10.1038/emboj.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan YL, et al. ERdj3 is an endoplasmic reticulum degradation factor for mutant glucocerebrosidase variants linked to Gaucher’s disease. Chem. Biol. 2014;21(8):967–976. doi: 10.1016/j.chembiol.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong YH, et al. Protogenin defines a transition stage during embryonic neurogenesis and prevents precocious neuronal differentiation. J. Neurosci. 2010;30(12):4428–4439. doi: 10.1523/JNEUROSCI.0473-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyatt AR, et al. Extracellular chaperones and proteostasis. Annu. Rev. Biochem. 2013;82:295–322. doi: 10.1146/annurev-biochem-072711-163904. [DOI] [PubMed] [Google Scholar]
- 25.Querfurth HW, LaFerla FM. Alzheimer’s disease. N. Engl. J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 26.Fevrier B, et al. Cells release prions in association with exosomes. Proc Natl Acad Sci USA. 2004;101(26):9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vella LJ, et al. Enrichment of prion protein in exosomes derived from ovine cerebral spinal fluid. Vet. Immunol. Immunopathol. 2008;124(3–4):385–393. doi: 10.1016/j.vetimm.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Rajendran L, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA. 2006;103(30):11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharples RA, et al. Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J. 2008;22(5):1469–1478. doi: 10.1096/fj.07-9357com. [DOI] [PubMed] [Google Scholar]
- 30.Polanco JC, et al. Extracellular Vesicles Isolated from the Brains of rTg4510 Mice Seed Tau Protein Aggregation in a Threshold-dependent Manner. J. Biol. Chem. 2016;291(24):12445–12466. doi: 10.1074/jbc.M115.709485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stern RA, et al. Preliminary Study of Plasma Exosomal Tau as a Potential Biomarker for Chronic Traumatic Encephalopathy. J. Alzheimers. Dis. 2016;51(4):1099–1109. doi: 10.3233/JAD-151028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomes C, et al. Evidence for secretion of Cu,Zn superoxide dismutase via exosomes from a cell model of amyotrophic lateral sclerosis. Neurosci. Lett. 2007;428(1):43–46. doi: 10.1016/j.neulet.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 33.Grad LI, et al. Exosome-dependent and independent mechanisms are involved in prion-like transmission of propagated Cu/Zn superoxide dismutase misfolding. Prion. 2014;8(5):331–335. doi: 10.4161/19336896.2014.983398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grad LI, et al. Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosome-dependent and -independent mechanisms. Proc. Natl. Acad. Sci. USA. 2014;111(9):3620–3625. doi: 10.1073/pnas.1312245111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nonaka T, et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 2013;4(1):124–134. doi: 10.1016/j.celrep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez-Erviti L, et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol. Dis. 2011;42(3):360–7. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danzer KM, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol. Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emmanouilidou E, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein oligomers are targeted to, and impair, the 26S proteasome. Neurobiol. Aging. 2010;31(6):953–968. doi: 10.1016/j.neurobiolaging.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Fontaine SN, et al. DnaJ/Hsc70 chaperone complexes control the extracellular release of neurodegenerative-associated proteins. EMBO J. 2016;35(14):1537–1549. doi: 10.15252/embj.201593489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, Y. Q. et al. Identification of CSPalpha Clients Reveals a Role in Dynamin 1 Regulation. 74(1), 136 (2012). [DOI] [PMC free article] [PubMed]
- 41.Braun, J. E. and Scheller, R. H. Cysteine string protein, a DnaJ family member, is present on diverse secretory vesicles. 34(11), 1361 (1995). [DOI] [PubMed]
- 42.Donnelier J, et al. Increased Expression of the Large Conductance, Calcium-Activated K+ (BK) Channel in Adult-Onset Neuronal Ceroid Lipofuscinosis. PLoS ONE. 2015;10(4):e0125205. doi: 10.1371/journal.pone.0125205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basso M, Bonetto V. Extracellular Vesicles and a Novel Form of Communication in the Brain. Front Neurosci. 2016;10:127. doi: 10.3389/fnins.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lun X, et al. Disulfiram when Combined with Copper Enhances the Therapeutic Effects of Temozolomide for the Treatment of Glioblastoma. Clin. Cancer Res. 2016;22(15):3860–3875. doi: 10.1158/1078-0432.CCR-15-1798. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen SA, et al. Novel MSH6 mutations in treatment-naive glioblastoma and anaplastic oligodendroglioma contribute to temozolomide resistance independently of MGMT promoter methylation. Clin. Cancer Res. 2014;20(18):4894–4903. doi: 10.1158/1078-0432.CCR-13-1856. [DOI] [PubMed] [Google Scholar]
- 46.Kang, R. et al. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. 456(7224), 904 (2008). [DOI] [PMC free article] [PubMed]
- 47.Greaves J, et al. Palmitoylation-induced aggregation of cysteine-string protein mutants that cause neuronal ceroid lipofuscinosis. J. Biol. Chem. 2012;287(44):37330–9. doi: 10.1074/jbc.M112.389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, Y. Q. and Chandra, S. S. Oligomerization of Cysteine String Protein alpha mutants causing adult neuronal ceroid lipofuscinosis. Biochim. Biophys. Acta1842(11), 2136 (2014). [DOI] [PMC free article] [PubMed]
- 49.Ahrendt E, et al. Cysteine String Protein Limits Expression of the Large Conductance, Calcium-Activated K(+) (BK) Channel. PLoS ONE. 2014;9(1):e86586. doi: 10.1371/journal.pone.0086586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boal, F. et al. A charged prominence in the linker domain of the cysteine-string protein Cspalpha mediates its regulated interaction with the calcium sensor synaptotagmin 9 during exocytosis. FASEB J25(1), 132 (2011). [DOI] [PubMed]
- 51.Boal, F. et al. The variable C-terminus of cysteine string proteins modulates exocytosis and protein-protein interactions. 43(51), 16212 (2004). [DOI] [PubMed]
- 52.Chamberlain LH, et al. The synaptic vesicle protein, cysteine-string protein, is associated with the plasma membrane in 3T3-L1 adipocytes and interacts with syntaxin 4. J Cell Sci. 2001;114(Pt 2):445. doi: 10.1242/jcs.114.2.445. [DOI] [PubMed] [Google Scholar]
- 53.Chen S, et al. Enhancement of presynaptic calcium current by cysteine string protein. J Physiol. 2002;538:383–9. doi: 10.1113/jphysiol.2001.013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans GJ, Morgan A. Phosphorylation-dependent interaction of the synaptic vesicle proteins cysteine string protein and synaptotagmin I. Biochem. J. 2002;364(Pt 2):343–347. doi: 10.1042/bj20020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henderson MX, et al. Neuronal ceroid lipofuscinosis with DNAJC5/CSPalpha mutation has PPT1 pathology and exhibit aberrant protein palmitoylation. Acta Neuropathol. 2016;131(4):621–637. doi: 10.1007/s00401-015-1512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kyle BD, et al. The Large Conductance, Calcium-activated K(+) (BK) Channel is regulated by Cysteine String Protein. Sci. Rep. 2013;3:2447. doi: 10.1038/srep02447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leveque, C. et al. Interaction of cysteine string proteins with the alpha1A subunit of the P/Q-type calcium channel. 273(22), 13488 (1998). [DOI] [PubMed]
- 58.Miller, L. C. et al. Molecular Detrminants of Cysteine String Protien Modulation of N-type Calcium Channels. 116(4), 2967 (2003). [DOI] [PubMed]
- 59.Nie Z, et al. Overexpression of cysteine-string proteins in Drosophila reveals interactions with syntaxin. J Neurosci. 1999;19(23):10270. doi: 10.1523/JNEUROSCI.19-23-10270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rozas, J. L. et al. Motorneurons Require Cysteine String Protein-alpha to Maintain the Readily Releasable Vesicular Pool and Synaptic Vesicle Recycling. 74(1), 151 (2012). [DOI] [PubMed]
- 61.Sakisaka T, et al. rab-alphaGDI activity is regulated by a Hsp90 chaperone complex. EMBO. 2002;21:6125–6135. doi: 10.1093/emboj/cdf603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seagar M, et al. Interactions between proteins implicated in exocytosis and voltage-gated calcium channels. Philos Trans R Soc Lond B Biol Sci. 1999;354(1381):289–97. doi: 10.1098/rstb.1999.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma M, et al. CSPalpha knockout causes neurodegeneration by impairing SNAP-25 function. EMBO J. 2012;31(4):829–841. doi: 10.1038/emboj.2011.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma M, Burre J, Sudhof TC. CSPalpha promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity. Nat Cell Biol. 2011;13(1):30–39. doi: 10.1038/ncb2131. [DOI] [PubMed] [Google Scholar]
- 65.Sharma M, Burre J, Sudhof TC. Proteasome inhibition alleviates SNARE-dependent neurodegeneration. Sci. Transl. Med. 2012;4(147):147ra113–147ra113. doi: 10.1126/scitranslmed.3004028. [DOI] [PubMed] [Google Scholar]
- 66.Wu, M. N. et al. Syntaxin 1A interacts with multiple exocytic proteins to regulate neurotransmitter release in vivo. 23, 593 (1999). [DOI] [PubMed]
- 67.Chandra, S. et al. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. 123(3), 383 (2005). [DOI] [PubMed]
- 68.Gusella JF, MacDonald ME. Molecular genetics: unmasking polyglutamine triggers in neurodegenerative disease. Nat. Rev. Neurosci. 2000;1(2):109–115. doi: 10.1038/35039051. [DOI] [PubMed] [Google Scholar]
- 69.Cicchetti F, et al. Mutant huntingtin is present in neuronal grafts in Huntington disease patients. Ann. Neurol. 2014;76(1):31–42. doi: 10.1002/ana.24174. [DOI] [PubMed] [Google Scholar]
- 70.Kaur SJ, McKeown SR, Rashid S. Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis. Gene. 2016;577(2):109–118. doi: 10.1016/j.gene.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 71.Munch C, O’Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc. Natl. Acad. Sci. USA. 2011;108(9):3548–3553. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller LC, et al. Cysteine String Protein (CSP) inhibition of N-type calcium channels is blocked by mutant huntingtin. J Biol Chem. 2003;278:53072–53081. doi: 10.1074/jbc.M306230200. [DOI] [PubMed] [Google Scholar]
- 73.Tiwari SS, et al. Evidence that the presynaptic vesicle protein CSPalpha is a key player in synaptic degeneration and protection in Alzheimer’s disease”. Mol. Brain. 2015;8(1):6. doi: 10.1186/s13041-015-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tobaben, S. et al. A trimeric protein complex functions as a synaptic chaperone machine. 31, 987 (2001). [DOI] [PubMed]
- 75.Uytterhoeven, V. et al. Hsc70-4 Deforms Membranes to Promote Synaptic Protein Turnover by Endosomal Microautophagy. 88(4), 735 (2015). [DOI] [PubMed]
- 76.Kakkar V, Prins LC, Kampinga HH. DNAJ proteins and protein aggregation diseases. Curr. Top. Med. Chem. 2012;12(22):2479–2490. doi: 10.2174/1568026611212220004. [DOI] [PubMed] [Google Scholar]
- 77.Koutras C, Braun JE. J protein mutations and resulting proteostasis collapse. Front Cell Neurosci. 2014;8:191. doi: 10.3389/fncel.2014.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kakkar V, et al. The S/T-Rich Motif in the DNAJB6 Chaperone Delays Polyglutamine Aggregation and the Onset of Disease in a Mouse Model. Mol. Cell. 2016;62(2):272–283. doi: 10.1016/j.molcel.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 79.Novoselov SS, et al. Molecular chaperone mediated late-stage neuroprotection in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. PLoS ONE. 2013;8(8):e73944. doi: 10.1371/journal.pone.0073944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vembar SS, et al. J domain co-chaperone specificity defines the role of BiP during protein translocation. J. Biol. Chem. 2010;285(29):22484–22494. doi: 10.1074/jbc.M110.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eisenberg E, Greene LE. Multiple roles of auxilin and hsc70 in clathrin-mediated endocytosis. Traffic. 2007;8(6):640–646. doi: 10.1111/j.1600-0854.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 82.Sousa R, Lafer EM. The role of molecular chaperones in clathrin mediated vesicular trafficking. Front Mol. Biosci. 2015;2:26. doi: 10.3389/fmolb.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmitz F, et al. CSPalpha-deficiency causes massive and rapid photoreceptor degeneration. Proc Natl Acad Sci USA. 2006;103(8):2926–2931. doi: 10.1073/pnas.0510060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lachenal G, et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell Neurosci. 2011;46(2):409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 85.Perez-Gonzalez R, et al. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J. Biol. Chem. 2012;287(51):43108–43115. doi: 10.1074/jbc.M112.404467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


