Abstract
The current treatment of schistosomiasis is based on the anti-helminthic drug praziquantel (PZQ). PZQ affects only the adult stages of schistosomes. In addition, resistance to PZQ is emerging. We suggest a drug, which could serve as a potential alternative or complement to PZQ, and as a means of treating infections at earlier, pre-granuloma stage. Derivatives of the peroxidic antimalarial drug artemisinin have been indicated as alternatives, because both plasmodia and schistosomes are blood-feeding parasites. The mechanism of action of artemisinins is related to oxidative effects of the artemisinins on intracellular reductants leading to formation of cytotoxic reactive oxygen species. We used artemisone, which has improved pharmacokinetics and anti-plasmodial activity, and reduced toxicity compared to other artemisinins in clinical use against malaria. We infected adult mice by subcutaneous injection of S. mansoni cercariae (about 200) and treated them at various times post infection by the following methods: i. artemisone suspension administered by gavage (400–450 mg/kg); ii. subcutaneous injection of a gel containing a known concentration of artemisone (115–120 mg/kg); iii. subcutaneous insertion of the drug incorporated in a solid polymer (56–60 mg/kg); iv. intraperitoneal injection of the drug solubilized in DMSO (115–120 mg/kg). Drug administration in polymers was performed to enable slow release of the artemisone that was verified in vivo and in vitro bioassays using drug-sensitive malaria parasites. We found superior strong anti-schistosome effects up to a total reduction of worm number, mainly following repetitive treatments with the drug absorbed in the polymers (73.1% and 95.9% reduction in mice treated with artemisone in gel 7 and 14, and 21, 28 and 35 days post infection, respectively). The results indicate that artemisone has a potent anti-schistosome activity. Its main importance in this context is its effectiveness in treating hosts harboring juvenile schistosomes, before egg-deposition and induction of deleterious immune responses.
Keywords: Schistosoma, Treatment, Slow release, Artemisone
Graphical abstract

Highlights
-
•
Praziquantel is mainly active against adult worms, unlike artemisone.
-
•
Improved treatment of all Schistosoma stages, using artemisone, is suggested.
-
•
Treatment in the mouse model consists of slowly released drug from injected polymer.
1. Introduction
Schistosomes are parasitic helminths, most important in terms of socio-economic and public health in tropical and subtropical areas. Schistosomiasis is associated with skin allergies, intestinal, liver and urinary pathologies. Chronic disease may also lead to cancer (Oh and Weiderpass, 2014). In addition, there are often systemic symptoms, such as retarded growth, slowing of cognitive development and the effect of continuing low-level blood loss (Bergquist et al., 2004). Schistosomiasis affects about 250 million people worldwide (WHO, 2015–2016).
Current treatment is based on the anti-helminthic drug praziquantel (PZQ) (Trainor-Moss and Mutapi, 2015). Unfortunately, following its massive use, there are reports of induction of resistance and reduced susceptibility to praziquantel in field isolates (Melman et al., 2009, Pinto-Almeida et al., 2015, Crellen et al., 2016). Artemisinin derivatives (Araujo et al., 1991, del Villar et al., 2012, Saeed et al., 2016; Wikipedia, 2016) and artemisinin analogs-synthetic peroxides e.g. ozonides and trioxolanes (Keiser et al., 2012; Xiao et al., 2012) have been proposed as alternative or complementary drugs against schistosomes. Artemisinins are widely used against malaria wherein their mechanism of action is generally accepted to involve activation by heme or non-heme iron to generate cytotoxic carbon radicals that alkylate vital intraparastic molecules (Klonis et al., 2013). However, based on a consideration of the extensive literature on C-radicals and their evident inability to act as alkylating agents, coupled with the low propensity of artemisinins to react with iron in the first place, the thesis is not without contradictions (Haynes et al., 2013). Alternatively, artemisinins may accept electrons from reduced flavin cofactors within flavin disulfide reductases such as glutathione reductase (GR), thioredoxin reductase (TrxR) and others that maintain redox homeostasis in the malaria parasite (Haynes et al., 2012). In Schistosoma species, therefore, as in the case of plasmodia, action of artemisinins may be due either to heme initiated formation of free radicals (Araújo et al., 2008, Muangphrom et al., 2016) or to abrogation of redox homeostasis by the artemisinins interacting with reduced flavin cofactors of flavin disulfide reductases. Notably within S. mansoni, the multifunctional disulfide reductase thioredoxin glutathione reductase (TGR) functionally replaces TrxR and GR of plasmodia and therefore TGR is a potentially important drug target (Alger and Williams, 2002, Kuntz et al., 2007) and is likely to be so for artemisinins. Be that as it may, whilst praziquantel affects adult schistosomes, artemisinins affect both larval and mature stages of the parasites (Shuhua et al., 2000, Fenwick et al., 2006). However, because of the complexity of the parasite, most artemisinins would have to be administered by repetitive injections, although artesunate and artemether that are water soluble and lipid soluble, respectively, could possibly be used orally (Barradell and Fitton, 1995, Bunnag et al., 1996).
In this work, we have conducted a preliminary assessment of the in vivo activity of the clinically used artemisinin derivative artemether compared with the newer aminoartemisinins artemiside and artemisone, both of which are shown to be potently active against the apicomplexan parasite Plasmodium falciparum (pf) in vitro and in vivo. Artemisone has improved pharmacokinetics and anti-plasmodial activity, and reduced toxicity compared to the artemisinin derivatives clinically used against malaria (Haynes et al., 2006, Guiguemde et al., 2014). We also include the newer N-sulfonyl aza-artemisinin derivative CKW03, that previously has also been shown to be potently active against Pf and like other N-sulfonyl azaartemisinin derivatives, has notable thermal stability (Haynes et al., 2007). Finally, we have examined the effect of controlled artemisone release from biodegradable gels on S. mansoni in mice, with the aim of improving treatment by exposing the parasites for a longer period of time to a drug concentration sufficient to eliminate the pathogen, but at dose levels non-toxic to the host.
2. Materials and methods
2.1. Artemisinin derivatives
Artemiside and CKW03 were prepared as previously described (Haynes et al., 2007, Guo et al., 2012). Artemisone was prepared as previously described (Haynes et al., 2006) or kindly donated by Cipla Mumbai, India. Artemether was a gift from the Kunming Pharmaceutical Corp. Compound structures are shown in Table 1.
Table 1.
Recovered worms and their percent reduction in mice treated by artemisinin derivatives.
| Compound | Structure | Recovered worms and percent reduction |
||
|---|---|---|---|---|
| Total | Male | Female | ||
| Control | – | 44.0 | 23.9 | 20.1 |
| Artemiside | 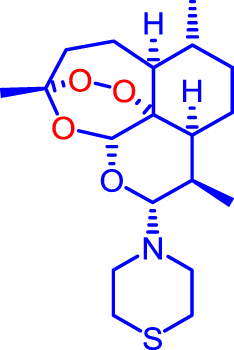 |
4.6 89.5% |
2.6 94.1% |
2.0 95.5% |
| Artemisone | 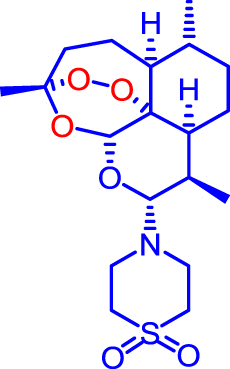 |
3.0 93.2% |
2.0 95.5% |
1.0 97.7% |
| CKW03 | 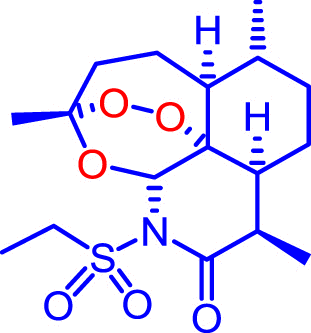 |
9.2 79.1% |
5.4 87.5% |
3.8 91.4% |
| Artemether | 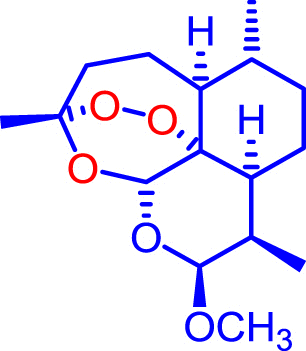 |
5.4 87.7% |
3.2 92.7% |
2.2 95.0% |
Infected mice were treated at day 21 post infection (PI) by gavage. The mice were dissected at day 49 PI and schistosomes counted. n = 5 in experimental groups and 10 in the control group. P < 0.01 in all experimental groups.
2.2. Artemisone preparations
Artemisone in polyricinoleic acid (RA)-sebacic acid (SA) gel was produced as previously described: poly (ester anhydride) was prepared by the insertion of RA into poly SA (Shikanov et al., 2004) with modifications. Briefly, 70 g of RA and 30 g of poly SA (PSA) were mixed by raising the temperature to 160 °C for 24 h under nitrogen atmosphere to yield RA-SA carboxylic acid terminated oligoesters. These oligoesters were activated by acetic anhydride and polymerized for 4 h at 140 °C under vacuum (10–15 mbar) to yield poly(RA-SA)70:30 pasty injectable polymer. Different amounts of artemisone powder were mixed in the pasty polymer and used for injection.
Block polymer PCL-MPEG was synthesized according to a previously published procedure (Bubel et al., 2013). Blockcopolymers of PCL-MPEG were fabricated by different ratios of PCL: MPEG. To create a homogeneous mixtures of PCL-b-MPEG and artemisone, different ratios of both compounds were dissolved in small amounts of tetrahydrofuran (THF; p.a., >99.9%). After all particles were dissolved, the solvent was completely evaporated. Using a heat press at 65 °C, the mixture was pressed into a polytetrafluoroethylene matrix (∼8 × 4x2 mm) and then cooled down to room temperature under a second press at about 20 °C. The polymers were sterilized by brief (5 s) washing in 70% ethanol and exposure to UV for 45 min.
Artemisone suspension for gavage was prepared by suspending artemisone (32 mg) in 7% Tween 80 (1 mL) and 3% alcohol.
2.3. Parasites
2.3.1. Schistosoma mansoni
A Liberian strain was used in the Swiss Tropical Institute (Table 1). All other experiments were performed using the Puerto Rican isolate obtained from NIH. The life cycle of S. mansoni was maintained in NMRI and ICR mice and Biomphalaria glabrata snails. The snails were raised and kept at 26 °C in aerated aquaria. Mice were routinely infected by subcutaneous injection of about 200 cercariae each. Seven to 8 weeks post-infection, shistosome eggs were extracted from the granuloma-containing livers. Snails were infected individually by exposure to 7–8 miracidia each. Cercariae were obtained from infected snails, starting at 4 weeks post infection.
2.3.2. Plasmodium berghei ANKA (PbA)
PbA strain (MRA-311, CDC, Atlanta, GA, USA) was maintained in vivo by serial transfer of parasitized erythrocytes from infected to naive mice.
2.3.3. P. falciparum NF54-luc
Parasites that stably and constitutively express luciferase were cultivated at 5% hematocrit in RPMI 1640 medium, 0.5% Albumax II (Invitrogen, Carlsbad, California, United States), 0.25% sodium bicarbonate, and 0.1 mg/mL gentamicin. Parasites were incubated at 37 °C in an atmosphere of 5% oxygen, 5% carbon dioxide, and 90% nitrogen. Parasites were cultured in media containing 4 nM WR99210 to select for stable luciferase expression. Parasite viability assays were performed either by measuring their luciferase activity (see Bioassay below).
2.4. Mice
Mice were maintained in environmentally controlled conditions (temperature, ∼23 °C; 50% humidity, free access to a standard diet and water, a 12/12-h automatically timed light/dark cycle. Mice were acclimatized for several days prior to infections.
Female NMRI mice were purchased from Charles River (Sulzfeld, Germany) and Harlan Laboratories (Blackthorn, United Kingdom). These mice were treated by gavage in the Swiss Tropical Institute. Male ICR mice were purchased from Harlan Laboratories (Rehovot, Israel). These mice were used for the schistosome infections in Tel-Aviv University (Tel Aviv University ethical committee number 01-13-076). The mice were infected a few days later and treated as indicated at various intervals following infection. Male C57BL/6 mice were purchased from Harlan Laboratories (Jerusalem, Israel) and used for plasmodial infections in the Hebrew University. The animal study protocol was approved by the Hebrew University Institutional Animal Care and Use Committee (protocol No. MD-12-13183-5; Golenser's accreditation No. 12180) and the procedures followed were in accordance with institutional guidelines.
2.5. Treatment
Treatment of mice was conducted by intraperitoneal or subcutaneous injections, and gavage or subcutaneous insertions of a solid polymer or a gel containing artemisone.
Gavage was conducted by oral administration of 0.4 mL containing the test drugs at 400–450 mg/kg in Tween 7% and 3% ethanol in DDW. NMRI female mice, 5 week old, ∼22 g at infection with 80 cercariae, were treated at day 21 post infection (PI) by gavage of 400 mg/kg drug suspension (Table 1). ICR male mice, 7–8 weeks old, ∼33 g at infection, were treated by gavage of 450 mg/kg at days 18 and 25, and at day 21, 29 and 35 PI. Mice were dissected at day 49 post infection (Fig. 1). Mice treated by gavage once on day 42, or twice on day 42 and 49 post infection were dissected on day 61 post infection and schistosomes counted (Fig. 2).Similar ICR mice were infected and treated 20 days PI by artemisone (2mg/mouse, 60 mg/kg) in solid polymers that were inserted subcutaneously (SC) into the abdomen of mice anesthetized by Ketamine/Xylazine injection. The mice were dissected at day 49 PI and schistosomes counted (Fig. 3).
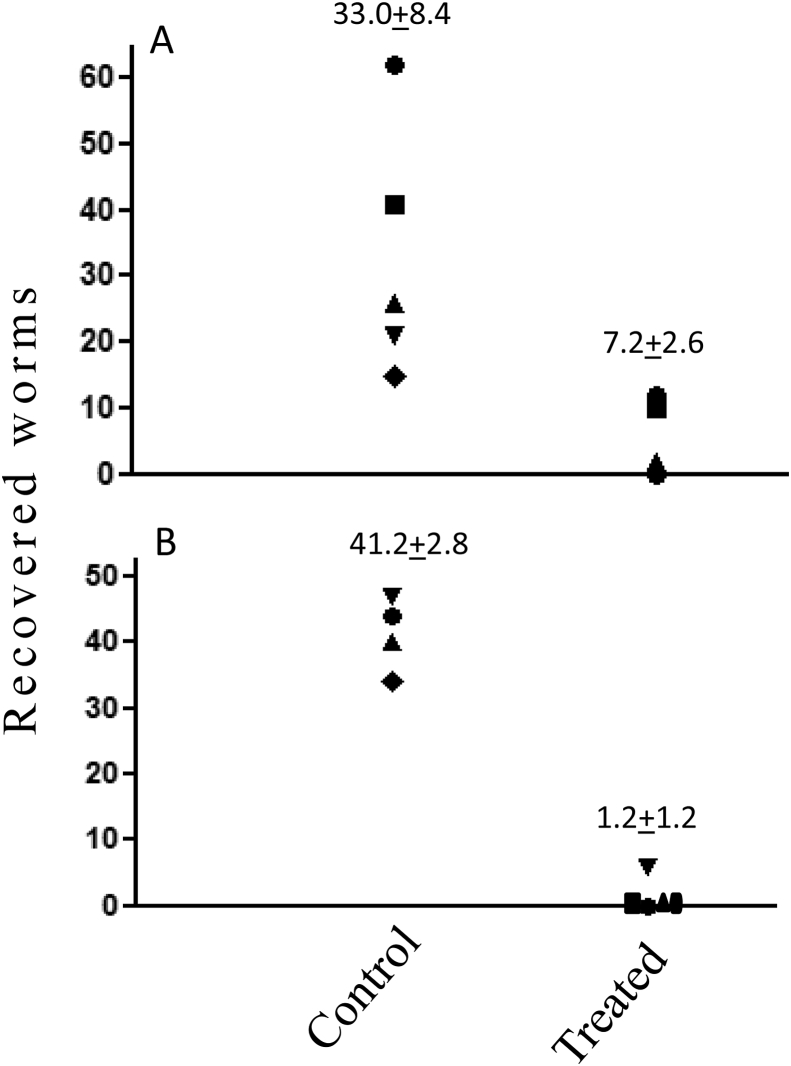
Fig. 1.
The effect of artemisone administered by gavage on S. mansoni infections. Infected mice were treated by gavage at days 18 and 25 (A), and at day 21, 29 and 35 (B) post infection (PI). Each point represents one mouse. Mice were dissected at day 49 PI and schistosomes counted. n = 5 in the control groups and 6 in the experimental groups. Difference was significant between the control group and the two artemisone treated groups group (<0.01) but was more pronounced in mice that were treated thrice compared to those that were treated twice (97.1 % vs. 70.4% reduction, respectively).
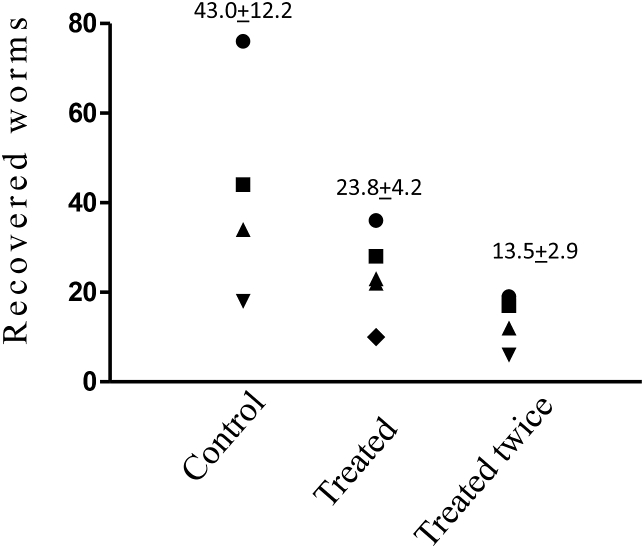
Fig. 2.
The effect of artemisone administered by late gavage treatment. Infected mice treated by gavage once on day 42, or twice on day 42 and 49 post infection (PI) were dissected on day 61 PI and schistosomes counted. n = 4 or 5 (each point represents one mouse). Difference was significant only between the control group and the group that was treated twice (68.5% reduction, p < 0.05).
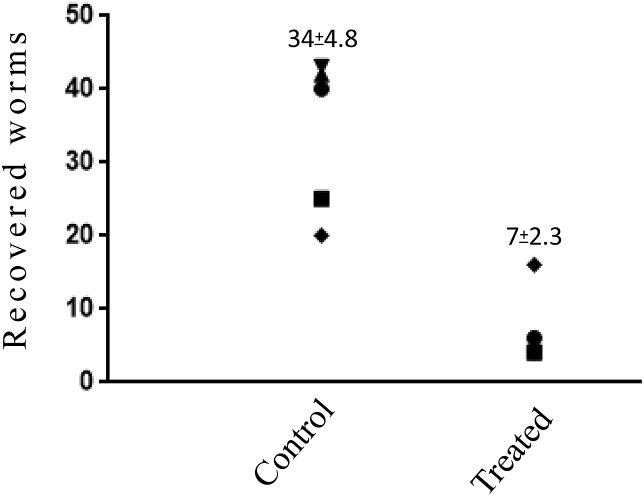
Fig. 3.
The effect of artemisone administered in solid polymers. Infected mice were treated by artemisone in solid polymers 20 days PI. Each point represents one mouse. n = 5 in all groups. Mice were dissected at day 49 PI and schistosomes counted. Schistosome number in the treated group was reduced by 79.4% (p < 0.01).
Artemisone was injected SC and IP at days 18 and 25 PI by in DMSO to similar mice, 4mg/40microL/mouse (120 mg/kg). Mice were dissected at day 49 post infection and schistosomes counted (data not shown).
Artemisone in gel (0.1 or 0.2 mL) was injected SC in the abdomen to infected ICR male mice, when 7–8 weeks old, ∼33 g, at various days post infection with schistosomes. Mice were dissected at day 49–51 PI and schistosomes counted.
Male C57BL/6 mice (7–8 weeks old, ∼25 g) were used for plasmodial infections. The animals were infected by IP injection of 50,000 erythrocytes parasitized by PbA and were treated by SC injections of artemisone in gel.
2.6. Assesment of results
Plasmodial parasitemias in mice were estimated by microscopic determination of the percent of parasitized erythrocytes in 5000 erythrocytes in Giemsa stained blood smears obtained from the mouse tail vein.
Schistosoma numbers were determined by counting worms from dissected and squashed livers, and mesenteric veins of dissected intestines of the mice, mostly 49–51 days PI unless otherwise stated. Granuloma density in the liver was assessed by press-flattening the livers between two glass plates and observation under a dissecting microscope.
P. falciparum bioassay for in vitro release of artemisone from gels was conducted as follows: artemisone released from the gel samples was quantified in a bioassay based on two-day cultures of drug sensitive P. falciparum that stably expresses a luciferase gene (see above). Gel samples containing two mg artemisone were sterilized by UV exposure and transferred to 1 mL RPMI 1640 medium in 24 well, Nunc disposable sterile plates that were incubated at 37 °C. Once a day the medium was collected and frozen until use. The polymers were washed twice in 2 mL medium, 1 mL fresh medium was added and the plates were returned to the incubator. The collected supernatants in different dilutions were examined for P. falciparum growth inhibition in triplicates, in Nunc flat bottom 96 well plates (Nunc™ MicroWell™ 96-Well Optical-Bottom Plates with Polymer Base). Free artemisone standards were added for comparison. Luciferase activity was measured in parasitized erythrocytes after removing 100 μL of the medium, following addition of 100 μL Bright-GloH luciferase reagent (Promega) in a Fluoroskan FL luminometer (Thermo). Percent inhibition of the luciferase counts reflecting anti-plasmodial activity by supernatants from experimental gels compared to control (blank) gels, is presented.
2.7. Statistics
Experiments were repeated at least once. Each group contained at least five mice. Shown are representative experiments. Experimental groups were compared to control groups using Student's t-test.
3. Results
3.1. The effect of various artemisinin derivatives administered by gavage on S. mansoni
Mice were treated on day 21 post infection (PI) by gavage. The mice were dissected at day 49 PI and schistosomes counted; N = 5 in experimental groups and 10 in the control group (Table 1). The effect was significant (P < 0.01) in all experimental groups. There was no difference in the effect on females or males. Artemiside, artemether and artemisone were equally effective. The N-ethanesulfonyl-11-azaartemisinin derivative CKW03 was somewhat less effective.
3.2. The effect of artemisone administered by gavage on S. mansoni infection
Mice were treated by gavage on different days post infection (PI). Fig. 1 depicts the results of treatment 18 and 25 (A), or 21, 29 and 35 (B) days PI. Mice were dissected on day 49 PI and schistosomes counted. Artemisone delivered by gavage significantly reduced the number of adult worms. Differences were significant between the treated and the control groups (p < 0.05) but more pronounced in mice that were treated thrice, compared to the ones that were treated twice (97.1% vs. 70.4% reduction, respectively). Fig. 2 depicts the results of late treatment, 42, or 42 and 49 days PI. Mice were dissected on day 61 PI and schistosomes counted. Artemisone administered by gavage at a late stage significantly reduced the number of adult worms only after an additional treatment (68.5% inhibition, p < 0.05). However, the method of treatment by gavage is not optimal because of the large amounts of drug needed for treatment.
3.3. The effect of artemisone administered in a solid polymer on S. mansoni infection
Mice were treated by artemisone (2mg/mouse, 60 mg/kg) in a solid polymer that was inserted S.C., on day 20 post infection. Each point represents one mouse. N = 5 in all groups. Mice were dissected on day 49 post infection and schistosomes counted (Fig. 3).
One treatment of this relatively low concentration of artemisone significantly reduced schistosome load (79.4%, p < 0.01). This surgical procedure would not be recommended for repeated treatments; therefore we shifted to drug injections, which correlate better with improved compliance.
3.4. The effect of artemisone administered in gel on S. mansoni infection
Infected mice were treated by artemisone in gel (0.2 mL) that was administered S.C., on various days PI. Mice were dissected on day 49–51 PI and schistosomes counted. This procedure was applied in day 62 in the late treatment groups for enabling expression of drug effect (Table 2).
Table 2.
Recovered worms and percent reduction in mice treated by artemisone and praziquantel administered in gel
| Day of treatment PI |
Artemisone in gel/mouse |
Control count |
Experimental count |
Percent inhibition |
|---|---|---|---|---|
| 7, 14a | 4 mg | 18.6 | 5.0 | 73.1* |
| 21, 28 | 4 mg | 40.2 | 8.5 | 81.6* |
| 21, 28, 35 | 4 mg | 39.5 | 1.6 | 95.9* |
| 21 | 4 mg | 40.2 | 24.2 | 39.8 |
| 34 | 4 mg | 61.4 | 11.0 | 82.1* |
| 42, 49 |
4 mg |
30.2 |
2.3 |
92.4* |
| Praziquantel in gel/mouse |
||||
| 7, 14a | 4 mg | 30.2 | 25.0 | 17.3 |
| 42, 49 | 4 mg | 30.2 | 0.2 | 99.3* |
Infected mice were treated at various days PI. Mice were dissected on day 49–51 PI and schistosomes counted. n = 5 in all groups. *Significant vs. control (p < 0.01).
Artemisone is significant vs. praziquantel.
The effect on schistosome recovery varied with the timing and number of drug injections. One treatment at an early stage, 21 days PI, did not significantly reduce schistosome number but profoundly reduced induction of granulomas in the liver of the treated mice, in contrast with their abundance in the control mice. A relatively late single treatment on day 34 PI reduced also the schistosome number by 82%. Two treatments, at an early stage (on days 7 and 14 or 21 and 28) PI also reduced schistosome number by about 73 and 82%, respectively. The effect was further increased to 96% reduction due to an additional treatment at day 35. There was no significant effect when using increased amounts of artemisone in quantities above 4 mg in identical gels, probably due to artemisone crystallization in the gel (data not shown). Identical doses of artemisone and praziquantel were tested in days 7 and 14, and days 42 and 49 PI. While the praziquantel did not affect parasite development at the early stage PI in comparison with the significant activity of the artemisone, it was highly active, similarly to artemisone after administration at the late stage.
3.5. The effect of artemisone in DMSO on S. mansoni infection
Infected mice were treated at day 18 and 25 days PI by I.P., and S.C. injections of artemisone in DMSO, 4mg/40μl/mouse (∼120 mg/kg). Mice were dissected at day 49 post infection and schistosomes counted. The experiments included 5 mice in each group. There was no alleviating effect of the S.C. treatment (18.6 ± 3.4 schistosomes in the control group vs. 14.8 ± 4.2 in the treated group). The mice that were treated by I.P. injections suffered from gross toxic effects and were culled.
3.6. P. falciparum bioassay of in vitro artemisone release from gels
Gels containing artemisone were incubated in medium and their dilutions were examined in P. falciparum cultures. Supernatants from media incubated with blank gels had no effect on P. falciparum development. In contrast, considerable amounts of artemisone were released in vitro, spanning at least 7 days (Table 3). For example, a dilution of 15,625 of supernatant collected on day 3 killed most of the parasites (meaning that the amount of released artemisone was above 16 μg on that day according to the assay of free artemisone (concentrations of 0.1–10 ng/mL were examined and the ED50 was about one ng/ml). The ED50 of free artemisone was estimated by identical methods and was about 1 ng/mL.
Table 3.
Percent inhibition of Plasmodium falciparum development by artemisone released in vitro from gels.
Considerable amounts of active artemisone were released in vitro, spanning at least 7 days. For example, a final dilution of 1/15,625 of supernatant collected on day 3 inhibited 68% of parasite development (meaning that the amount of artemisone that was released in days 3 and 5 was about 16 and 5 μg, respectively).
The ED50 of free artemisone was estimated by identical methods and was about one ng/ml.
Significant in comparison with supernatants of blank gels.
3.7. Artemisone bioassay in Plasmodium berghei ANKA (PbA) infection
Mice were treated 3 days before infection with PbA by injections of artemisone in gel, 2mg/0.2mL/mouse (∼80 mg/kg). N = 3 in the experimental group and 5 in the control group. All control mice treated with empty gel died within eight days PI of cerebral malaria while the experimental mice treated by artemisone in the gel died 16–18 days PI, of anemic malaria (Fig. 4).
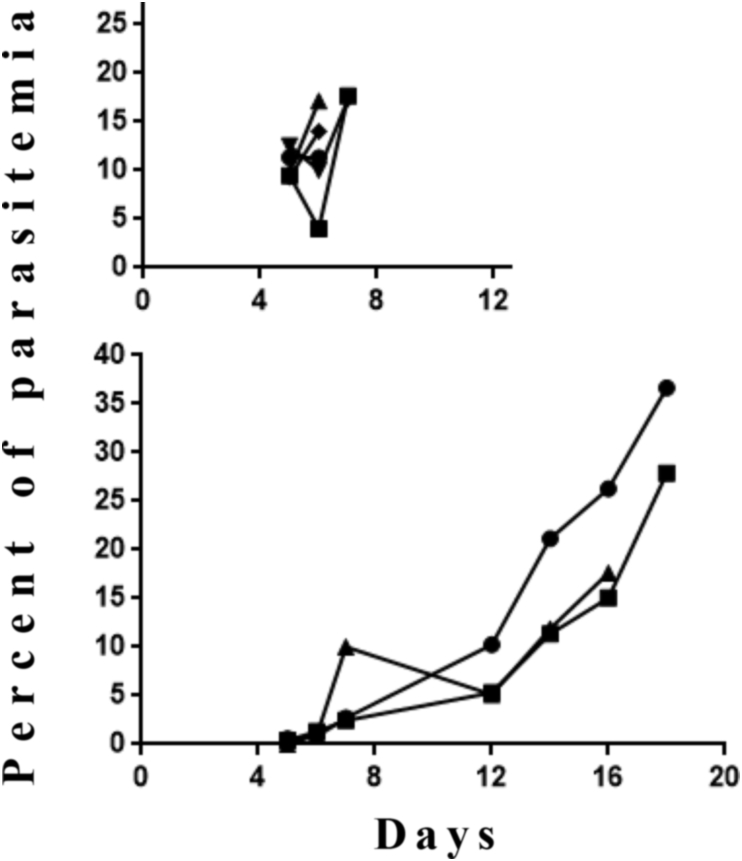
Fig. 4.
Artemisone bioassay in mice infected by P. berghei ANKA (PbA). Mice were treated by artemisone in gel, 2mg/0.2ml/mouse (∼80 mg/kg), 3 days before infection with PbA. Each line represents one mouse. n = 3 in the experimental group and 5 in the control group. Difference was significant between the control and the experimental group (p < 0.01).
4. Discussion
Current treatment of schistosomiasis is based on the anti-helminthic drug praziquantel (PZQ). However, following its massive use, there are already reports of PZQ reduced sensitivity (references are cited in the Introduction). Artemisinin and its current clinical derivatives collectively referred to as artemisinins, have been proposed as alternative or complementary drugs. Current reports reflect a significant but not total reduction of worm load after repeated (at least four doses) of intra-gastric treatment with high amounts of an artemisinin (Saeed et al., 2016).
PZQ is effective mainly against mature schistosomes (as could be also seen in our experiments) while artemisinins are also effective against early developmental stages (when compared to PZQ at the second to the fifth week PI (Utzinger et al., 2007). Therefore, improved results are expected to derive from a combination therapy using PZQ and an artemisinin. Experiments in mice reveal an additive effect of artemisinin and PZQ in S. mansoni infection (Botros et al., 2010) and in hamsters infected with S. japonicum (Utzinger et al., 2001). However, treatment of infected human patients with the combinations in endemic areas did not improve the performance of PZQ probably because certain worm stages were not exposed simultaneously to the two drugs (Praticò et al., 2014, Saeed et al., 2016).
Artemisone proved superior to other artemisinin derivatives, especially in terms of its greatly enhanced efficacy against P. falciparum, its metabolic profile that precludes formation of dihydroartemisinin, its relative stability and reduced toxicity (Schmuck et al., 2003, Haynes et al., 2006, Haynes et al., 2007b, Nagelschmitz et al., 2008, Obaldia et al., 2009, Waknine-Grinberg et al., 2010). Given thes advantages)and considering the similarity of mechanism of action in plasmodia and schistosomes (discussed in the Introduction), coupled with our current preliminary results (Table 1), artemisone was selected for further experiments in mice infected with S. mansoni. A similar effect on female and male reduction that was detected in this experiment hints to an identical mechanism of action in both genders. However, it is possible that an injury induced in the female may affect the male and vice versa, due to the obligatory relationship between the genders (Lu et al., 2016). Such a sequential effect would be eventually reflected in an equal mortality rate of both genders. We sought to improve the results by using a sustained drug release system. In general, sustained release may limit the amount of the drug in question to a non-toxic level whilst maintaining its efficacy during an extended period. This is highly relevant for artemisinins that at certain levels are neurotoxic (Wesche et al., 1994, Schmuck and Haynes, 2000, Schmuck et al., 2002, Campos et al., 2008). Sustained release also allows for extended treatment of the changing stages of schistosomes. As a control for the sustained mode of the treatment we used subcutaneous (SC), intraperitoneal (IP) injections and gavage. Two methods of sustained release of the drug were applied, namely release from a solid polymer that necessitates insertion using a relatively straightforward surgical procedure, and injectable gel.
Artemisone administered by gavage at a relatively early stage, namely on day 21 or on days 18 and 25 PI, significantly reduced the number of adult worms and was most pronounced in mice that were treated thrice (97.1%). Late gavage at day 42 PI had no effect, but an additional treatment one week later induced 68.5% adult worm elimination. However, the method of treatment by gavage is not optimal because of the large amounts of drug needed for treatment probably due to low degree of absorption. Consequently, we used artemisone incorporated into polymers.
One treatment 20 days post-infection (PI) using a relatively low concentration of artemisone (60 mg/kg) released from a solid polymer significantly reduced the Schistosoma load (79.4%). This result confirms the curative potential of artemisone at a relatively early stage of infection. However, insertion of a solid polymer that is associated with a surgical procedure might not be recommended for repeated treatments. Therefore, we evaluated the effect of injections of artemisone within a soft biodegradable gel that would be more convenient for repetitive treatments. The applicability and safety of this polymer has been described by Shikanov and Domb (2006). The effect on the number of recovered schistosomes was dependent on the number of treatments and the timing of the injection PI. One injection, 34 days PI, was more effective than 21 days PI (82.1% vs. 39.1% inhibition respectively). However, even the single treatment at 21 days PI prevented induction of granulomas in the liver of the treated mice, in contrast with their abundant formation in the control mice. This is a critical finding because granulomas are the main cause and indication of Schistosoma pathology (Hams et al., 2013).
IP injections of 4mg/40 μL DMSO induce toxic effects. Control repeated SC injections of 4mg/40 μL DMSO had no effect on the maturation of the schistosomes. The half-life of artemisone is about 3 h (Haynes et al., 2006, Nagelschmitz et al., 2008) so the amount retained in the plasma of injected mice would not be detectable three days after injection of this dose of artemisone. In contrast, we show that artemisone is released from the gels in vitro during at least seven days, and retain in vivo efficacy for at least three days by using an in vivo bioassay of mice infected with drug sensitive Plasmodium berghei ANKA. The effect of the released drug also was examined in mice in a prophylactic manner, in which the artemisone containing gel was injected three days before plasmodial infection. While all mice that were injected with empty gels died of cerebral malaria within seven days PI, the mice treated with the prophylactic gel survived during a much longer period (6.2 vs. 17.3 days, respectively).
Overall, we demonstrate that a sustained release procedure for artemisone induced both elimination of schistosomes and reduction of hepatic pathology greatly in excess of that achieved by per os delivery. The results justify further consideration of treatment of schistosomiasis with artemisinins, as well as using these in with combination with a partner drug in sustained release systems. The main advantage of artemisinins over praziquantel is their efficacy against early developmental stages of the parasite, which in many cases are the cause of acute schistosomiasis syndromes.
Conflicts of interests
The authors declare that they have no conflicts of interests.
Acknowledgements
We thank Cipla for the kind donation of artemisone and the DFG for financial support. RKH acknowledges support from the South African Medical Research Council (MRC) with funds from the National Treasury under its Economic Competitiveness and Support Package. The South African National Research Foundation is thanked for financial support to RKH.
References
- Alger H.M., Williams D.L. The disulfide redox system of Schistosoma mansoni and the importance of a multifunctional enzyme, thioredoxin glutathione reductase. Mol. Biochem. Parasitol. 2002;121:129–139. doi: 10.1016/s0166-6851(02)00031-2. [DOI] [PubMed] [Google Scholar]
- Araújo J.Q., Carneiro J.W., de Araujo M.T., Leite F.H., Taranto A.G. Interaction between artemisinin and heme. A Density Functional Theory study of structures and interaction energies. Bioorg. Med. Chem. 2008;16:5021–5029. doi: 10.1016/j.bmc.2008.03.033. [DOI] [PubMed] [Google Scholar]
- Araujo N., Kohn A., Katz N. Activity of the artemether in experimental schistosomiasis mansoni. Mem. Inst. Oswaldo Cruz. 1991;86:185–188. doi: 10.1590/s0074-02761991000600042. [DOI] [PubMed] [Google Scholar]
- Barradell L.B., Fitton A. Artesunate. A review of its pharmacology and therapeutic efficacy in the treatment of malaria. Drugs. 1995;50:714–741. doi: 10.2165/00003495-199550040-00009. [DOI] [PubMed] [Google Scholar]
- Bergquist R., Utzinger J., Chollet J., Shu-Hua X., Weiss N.A., Tanner M. Triggering of high-level resistance against S. mansoni reinfection by artemether in the mouse model. Am. J. Trop. Med. Hyg. 2004;71(2004):774–777. [PubMed] [Google Scholar]
- Botros S.S., Hammam O., Mahmoud M., Bergquist R. Praziquantel efficacy in mice infected with PZQ non-susceptible S. mansoni isolate treated with artemether: parasitological, biochemical and immunohistochemical assessment. APMIS. 2010;118:692–702. doi: 10.1111/j.1600-0463.2010.02645.x. [DOI] [PubMed] [Google Scholar]
- Bubel K., Zhang Y., Assem Y., Agarwal S., Greiner A. Tenside-free biodegradable polymer nanofiber nonwovens by “green electrospinning. Macromolecules. 2013;46:7034–7042. [Google Scholar]
- Bunnag D., Kanda T., Karbwang J., Thimasarn K., Pungpak S., Harinasuta T. Artemether or artesunate followed by mefloquine as a possible treatment for multidrug resistant falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 1996;90:415–417. doi: 10.1016/s0035-9203(96)90529-5. [DOI] [PubMed] [Google Scholar]
- Campos S., de la Cerda P., Rivera A. Fatal artesunate toxicity in a child. J. Pediatr. Infect. Dis. Soc. 2008;3:69–75. [Google Scholar]
- Crellen T., Walker M., Lamberton P.H., Kabatereine N.B., Tukahebwa E.M., Cotton J.A., Webster J.P. Reduced efficacy of praziquantel against Schistosoma mansoni is associated with multiple rounds of mass drug administration. Clin. Infect. 2016;63:1151–1159. doi: 10.1093/cid/ciw506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Villar L.P., Burguillo F.J., Lo' pez-Aban J., Muro A. Systematic review and meta-analysis of artemisinin based therapies for the treatment and prevention of schistosomiasis. PLoS One. 2012;7(9):e45867. doi: 10.1371/journal.pone.0045867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick A., Rollinson D., Southgate V. Implementation of human schistosomiasis control: challenges and prospects. Adv. Parasitol. 2006;61:567–622. doi: 10.1016/S0065-308X(05)61013-5. [DOI] [PubMed] [Google Scholar]
- Guiguemde W.A., Hunt N.H., Guo J., Marciano A., Haynes R.K., Clark J., Guy R.K., Golenser J. Treatment of murine cerebral malaria by artemisone in combination with conventional antimalarial drugs: antiplasmodial effects and immune responses. Antimicrob. Agents Chemother. 2014;58:4745–4754. doi: 10.1128/AAC.01553-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Guiguemde A.W., Bentura-Marciano A., Clark J., Haynes R.K., Chan W.C., Wong H.-N., Hunt N.H., Guy R.K., Golenser J. Synthesis of artemiside and its effects in combination with conventional drugs against severe murine malaria. Antimicrob. Agents Chemother. 2012;56:163–173. doi: 10.1128/AAC.05006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hams E., Aviello G., Fallon P.G. The Schistosoma granuloma: friend or foe? Front. Immunol. 2013;4(article 89):1–8. doi: 10.3389/fimmu.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes R.K., Chan H.-.W., Lung C.-M., Ng N.-C., Wong H.-W., Shek L.-Y., Williams I.D., Gomes M.F., Cartwright A. Artesunate and dihydroartemisinin (DHA): unusual decomposition products formed under mild conditions and questions on the fitness of DHA as an antimalarial drug. ChemMedChem. 2007;2:1448–1463. doi: 10.1002/cmdc.200700064. [DOI] [PubMed] [Google Scholar]
- Haynes R.K., Cheu K.-W., Chan H.-W., Wong H.-N., Li K.Y., Tang M.M.-K., Chen M.-J., Guo Z.-F., Guo Z.-H., Sinniah K., Witte A.B., Coghi P., Monti D. Interactions between artemisinins and other antimalarial drugs in relation to the co-factor model – a unifying proposal for drug cction. ChemMed¬Chem. 2012;7:2204–2226. doi: 10.1002/cmdc.201200383. [DOI] [PubMed] [Google Scholar]
- Haynes R.K., Cheu K.-W., N'Da D.D., Coghi P., Monti D. Considerations on the mechanism of action of artemisinin antimalarials: Part 1-The 'Carbon Radical' and 'Heme' hypotheses. Infect. Disord. – Drug Targets. 2013;13:217–277. doi: 10.2174/1871526513666131129155708. [DOI] [PubMed] [Google Scholar]
- Haynes R.K., Fugmann B., Stetter J., Rieckmann K., Heilmann H.D., Chan H.W. Artemisone - a highly active antimalarial drug of the artemisinin class. Angew. Chem. Int. Engl. 2006;45:2082–2088. doi: 10.1002/anie.200503071. [DOI] [PubMed] [Google Scholar]
- Haynes R.K., Wong H.-N., Lee K.W., Lung C.-M., Shek L.-Y., Williams I.D., Croft S.L., Vivas L., Rattray L., Stewart L. Preparation of N-Sulfonyl and -Carbonyl-11-Azaartemisinins with greatly enhanced thermal stabilities: in vitro antimalarial activities. ChemMedChem. 2007;2:1464–1479. doi: 10.1002/cmdc.200700065. [DOI] [PubMed] [Google Scholar]
- Keiser J., Ingram K., Vargas M., Chollet J., Wang X., Dong Y., Vennerstrom J.L. In vivo activity of aryl ozonides against Schistosoma species. Antimicrob. Agents Chemother. 2012;56:1090–1092. doi: 10.1128/AAC.05371-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonis N., Creek D.J., Tilley L. Iron and heme metabolism in Plasmodium falciparum and the mechanism of action of artemisinins. Curr. Opin. Microbiol. 2013;16:722–727. doi: 10.1016/j.mib.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Kuntz A.N., Davioud-Charvet E., Sayed A.A., Califf L.L., Dessolin J. Thioredoxin glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target. PLoS Med. 2007;4(6):e206. doi: 10.1371/journal.pmed.0040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Sessler F., Holroyd N., Hahnel S., Quack T., Berriman M., Grevelding C.G. Schistosome sex matters: a deep view into gonad-specific and pairing-dependent transcriptomes reveals a complex gender interplay. Sci. Rep. 2016;6:31150. doi: 10.1038/srep31150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melman S.D., Steinauer M.L., Cunningham C., Kubatko L.S., Mwangi I.N., Wynn N.B., Mutuku M.W., Karanja D.M., Colley D.G., Black C.L., Secor W.,E., Mkoji G.M., Loker E.S. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl. Trop. Dis. 2009;3(8):e504. doi: 10.1371/journal.pntd.0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muangphrom P., Seki H., Fukushima E.O., Muranaka T. Artemisinin-based antimalarial research: application of biotechnology to the production of artemisinin, its mode of action, and the mechanism of resistance of Plasmodium parasites. J. Nat. Med. 2016;70:318–334. doi: 10.1007/s11418-016-1008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelschmitz J., Voith B., Wensing G., Roemer A., Fugmann B., Haynes R.K. First assessment in humans of the safety, tolerability, pharmacokinetics, and ex vivo pharmacodynamic antimalarial activity of the new artemisinin derivative artemisone. Antimicrob. Agents Chemother. 2008;52:3085–3091. doi: 10.1128/AAC.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaldia N., Kotecka B.M., Haynes R.K., Fugmann B., Edstein M.D., Kyle D.E., Rieckmann K.H. Evaluation of artemisone combinations in Aotus Monkeys infected with Plasmodium falciparum. Antimicrob. Agents Chemother. 2009;53:3592–3594. doi: 10.1128/AAC.00471-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J.K., Weiderpass E. Infection and cancer: global distribution and burden of diseases. Ann. Glob. Health. 2014;80:384–392. doi: 10.1016/j.aogh.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Pinto-Almeida A., Mendes T., Armada A., Belo S., Carrilho E., Viveiros M., Afonso A. The role of efflux pumps in Schistosoma mansoni praziquantel resistant phenotype. PLoS One. 2015;10(10):e0140147. doi: 10.1371/journal.pone.0140147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praticò L., Mariani B., Brunetti E., Maserati R., Bruno A., Novati S., Chichino G. Failure of repeated treatment with praziquantel and arthemeter in four patients with acute schistosomiasis. J. Travel Med. 2014;21:133–136. doi: 10.1111/jtm.12098. [DOI] [PubMed] [Google Scholar]
- Saeed M.E., Krishna S., Greten H.J., Kremsner P.J., Efferth T. Anti-schistosomal activity of artemisinin derivatives in vivo and inpatients. Pharmacol. Res. 2016;110:216–226. doi: 10.1016/j.phrs.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Schmuck G., Haynes R.K. Establishment of an in vitro screening model for neurodegeneration induced by antimalarial drugs of the artemisinin type. Neurotox. Res. 2000;2:37–49. doi: 10.1007/BF03033326. [DOI] [PubMed] [Google Scholar]
- Schmuck G., Roehrdanz E., Haynes R.K., Kahl R. Neurotoxic mode of action of artemisinin. Antimicrob. Agents. Chemother. 2002;46:821–827. doi: 10.1128/AAC.46.3.821-827.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuck G., Temerowski M., Haynes R.K., Fugmann B. Identification of non-neurotoxic artemisinin derivatives in vivo and in vitro. Res. Adv. Antimicrob. Agents Chemother. 2003;3:35–47. [Google Scholar]
- Shikanov A., Domb A.J. Poly(sebacic acid-co-ricinoleic acid) biodegradable injectable in situ gelling polymer. Biomacromolecules. 2006;7:288–296. doi: 10.1021/bm050648+. [DOI] [PubMed] [Google Scholar]
- Shikanov A., Vaisman B., Krasko M.Y., Nyska A., Domb A.J. Poly(sebacic acid-co-ricinoleic acid) biodegradable carrier for paclitaxel: in vitro release and in vivo toxicity. J. Biomed. Mater. Res. A. 2004;1:47–54. doi: 10.1002/jbm.a.20101. [DOI] [PubMed] [Google Scholar]
- Shuhua X., Chollet J., Weiss N.A., Bergquist R.N., Marcel Tanner M. Preventive effect of artemether in experimental animals infected with Schistosoma mansoni. Parasitol. Int. 2000;49:19–24. doi: 10.1016/s1383-5769(00)00028-3. [DOI] [PubMed] [Google Scholar]
- Trainor-Moss S., Mutapi F. Schistosomiasis therapeutics: whats in the pipeline? Expert Rev. Clin. Pharmacol. 2015 doi: 10.1586/17512433.2015.1102051. http://dx.doi.org/10.1586/17512433.2015.1102051 [DOI] [PubMed] [Google Scholar]
- Utzinger J., Chollet J., Jiqing Y., Jinyan M., Tanner M., Shuhua X. Effect of combined treatment with praziquantel and artemether on Schistosoma japonicum and Schistosoma mansoni in experimentally infected animals. Acta Trop. 2001;80:9–18. doi: 10.1016/s0001-706x(01)00138-3. [DOI] [PubMed] [Google Scholar]
- Utzinger J.1., Xiao S.H., Tanner M., Keiser J. Artemisinins for schistosomiasis and beyond. Curr. Opin. Investig. Drugs. 2007;8:105–116. [PubMed] [Google Scholar]
- Waknine-Grinberg J.H., Hunt N., Bentura-Marciano A., McQuillan J.A., Chan H.-W., Chan W.-C., Barenholz Y., Haynes R.K., Golenser J. Artemisone effective against murine cerebral malaria. Malar. J. 2010;9:1–15. doi: 10.1186/1475-2875-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche D.L., DeCoster M.A., Tortella F.C., Brewer T.G. Neurotoxicity of artemisinin analogs in vitro. Antimicrob. Agents Chemother. 1994;38:1813–1819. doi: 10.1128/aac.38.8.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2015-2016. Schistosomiasis – PCT Database.http://www.who.int/schistosomiasis/en [Google Scholar]
- Wikipedia . 2016. Artemisinin.https://en.wikipedia.org/wiki/Artemisinin#Artemisinin_derivatives [Google Scholar]
- Xiao S.H., Xue J., Mei J.Y., Jiao P.Y. Effectiveness of synthetic trioxolane OZ78 against Schistosoma japonicum in mice and rabbits. Parasitol. Res. 2012;110:2307–2314. doi: 10.1007/s00436-011-2765-x. [DOI] [PubMed] [Google Scholar]