Abstract
Approximately 50% of coronary artery bypass grafts using the autologous saphenous vein fail within 10 years due to intimal thickening. This study examined whether a gene therapy approach that selectively kills Wnt/β-catenin/T cell factor (TCF) activated vascular smooth muscle cells (VSMCs) using dominant-negative N-cadherin (dn-N-cadherin) reduced intimal thickening. Cultured human VSMCs infected with an adenovirus (Ad) encoding dn-N-cadherin via the TCF promoter (Ad-TOP-dn-N-cadherin) specifically expressed dn-N-cadherin in response to activation of the Wnt/β-catenin/TCF pathway. Infection with Ad-TOP-dn-N-cadherin significantly increased VSMC apoptosis (3 ± 0.2% versus 9 ± 0.7%; p < 0.05, n = 6) and significantly inhibited VSMC migration by 83 ± 15% (p < 0.05, n = 6), but did not affect VSMC proliferation (p > 0.05, n = 5). In an ex vivo human saphenous vein organ culture model, luminal delivery of Ad-TOP-dn-N-cadherin significantly increased VSMC apoptosis after 7 days of culture (4 ± 1.4% versus 9 ± 1.6%; p < 0.01, n = 6) and suppressed intimal thickening by 75 ± 7% (p < 0.05, n = 5), without a detrimental effect on endothelial cell coverage. In vivo, Ad-TOP-dn-N-cadherin significantly reduced intimal thickening at day 21 (n = 10) in comparison to the Ad-β-galactosidase (Ad-β-gal) control virus (n = 12, p < 0.05) in the mouse carotid artery ligation model. In summary, we have developed a novel approach to selectively reduce intimal thickening, which may be beneficial in reducing late vein graft failure.
Keywords: vein graft failure, apoptosis, vascular smooth muscle cell, gene therapy, cadherin, apoptosis, intimal thickening
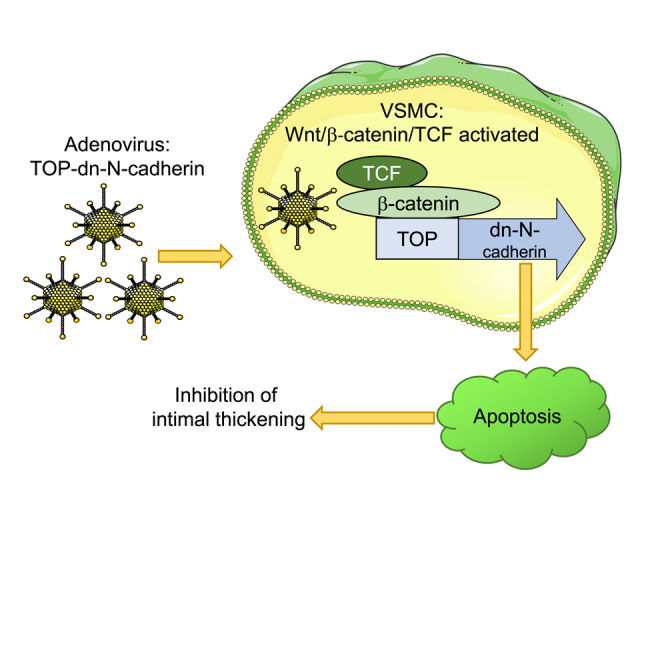
Graphical Abstract
Introduction
Coronary artery bypass grafting (CABG) remains the mainstay treatment for coronary artery disease to bypass diseased coronary arteries and resume normal blood flow to the heart. The saphenous vein is the most widely used bypass conduit; however, late vein graft failure due to intimal thickening and superimposed atherosclerosis remains a significant clinical problem and financial burden for the healthcare system.1, 2 Intimal thickening reduces vein graft patency and is characterized by the migration of vascular smooth muscle cells (VSMCs) from the media to the intima, where they proliferate and secrete extracellular matrix. This ultimately leads to failure of the graft and recurrence of symptoms. Consequently, new therapies to reduce intimal thickening are desirable.
N-cadherin is a member of the cadherin family of cell-surface receptors that structurally link cells via physical interaction between their extracellular domains. We previously reported that overexpression of dominant-negative N-cadherin (dn-N-cadherin), which consists of amino acids 6–27 (the cytoplasmic and transmembrane domains), in isolated VSMCs promoted apoptosis and reduced intimal thickening ex vivo.3 We have also identified Wnt/β-catenin/T cell factor (TCF) signaling as a modulator of VSMC phenotype and contributor to intimal thickening.3, 4 In consideration of our previous findings, we have developed an adenovirus (Ad)-based vector, in which the pro-apoptotic dn-N-cadherin gene was driven by a Wnt/β-catenin/TCF-responsive promoter linked to a minimal cytomegalovirus (CMV) promoter. We hypothesized that introduction of Ad-TOP-dn-N-cadherin (Ad-TOP-dn-N-cad) would reduce VSMC accumulation and impair neointima formation in both our ex vivo human saphenous vein (HSV) organ culture model and our in vivo mouse carotid artery ligation model. This suicide gene approach, targeted at specifically killing Wnt/β-catenin/TCF-activated VSMCs and preserving non-activated VSMCs, is similar to that successfully employed by Williams et al.4 and Kwong et al.5 We report that Wnt/β-catenin/TCF-driven expression of dn-N-cadherin promoted apoptosis in β-catenin-activated VSMCs in vitro and effectively retarded neointima formation in saphenous vein organ cultures and ligated mouse carotid arteries. These data highlight the translational potential of our strategy in preventing saphenous vein graft degeneration and secondary cardiac events.
Results
Characterization of Ad-TOP-dn-N-cadherin In Vitro
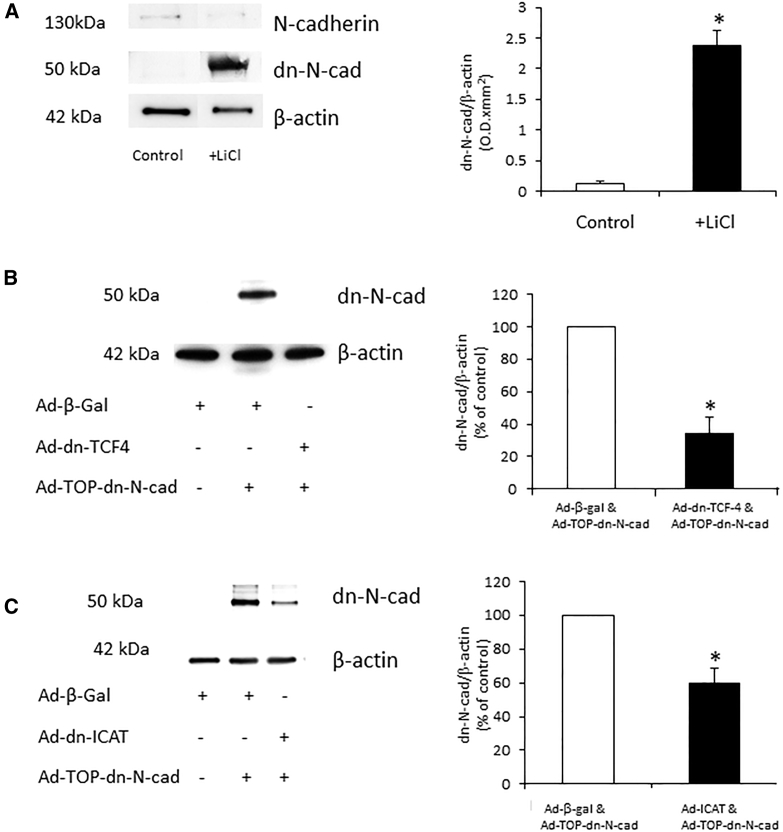
The dn-N-cadherin protein was detected by western blotting in VSMCs infected with 1,000 pfu/cell of Ad-TOP-dn-N-cad and stimulated with 10 μM lithium chloride (LiCl) (glycogen synthase kinase 3 beta [GSK-3β] inhibitor; Figure 1A). No expression of dn-N-cadherin was observed when VSMCs were infected with Ad-TOP-dn-N-cad without LiCl stimulation (Figure 1A: control) or in VSMCs infected with Ad-β-galactosidase (Ad-β-gal) (Figure 1B: control virus). Inhibition of Wnt signaling with Ad-dn-TCF-4 or Ad-inhibitor of β-catenin and TCF-4 (Ad-ICAT) significantly reduced the level of expression of dn-N-cadherin (Figures 1B and 1C, respectively).
Figure 1.
dn-N-cadherin Protein Expression Was Regulated by Activation and Inhibition of the Wnt/TCF Pathway in Isolated VSMCs
(A) Representative western blot for full-length N-cadherin, dn-N-cadherin, and β-actin (loading control) in VSMCs infected with Ad-TOP-dn-N-cadherin and stimulated with 10 μM LiCl to activate Wnt signaling. * indicates a significant difference from control, paired Student’s t test, n = 3. (B and C) VSMCs infected with Ad-TOP-dn-N-cadherin and Ad-dn-TCF-4 (B) or Ad-ICAT (C) to inhibit Wnt signaling in the presence of LiCl. Bar charts show densitometry. * indicates a significant difference from Ad-β-gal control, paired Student’s t test, n = 3 (B) and n = 4 (C). Error bars represent SEM.
Ad-TOP-dn-N-cadherin Significantly Induced VSMC Apoptosis
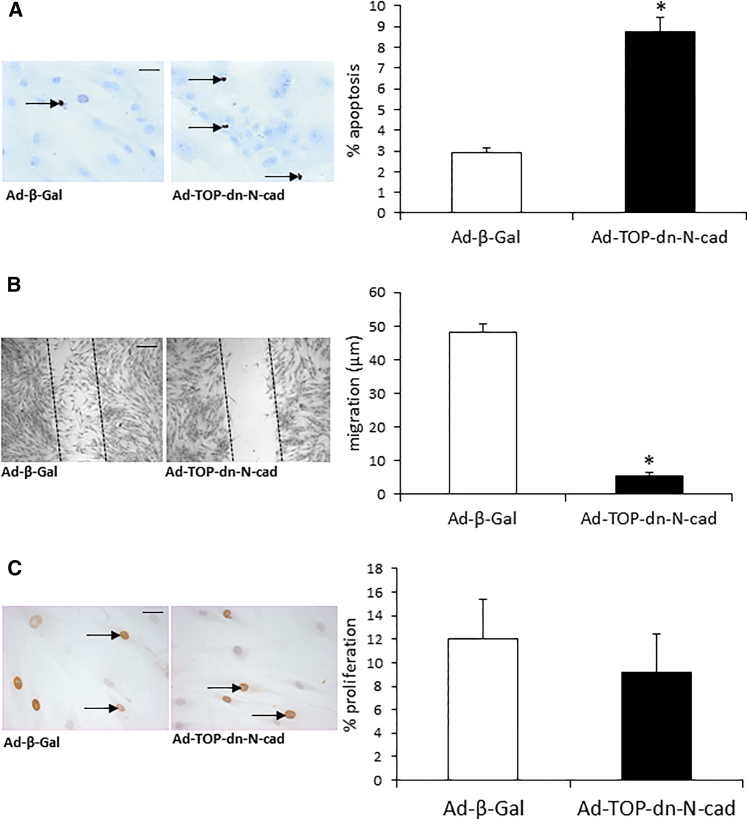
Ad-TOP-dn-N-cadherin significantly induced apoptosis in VSMCs. In situ end labeling (ISEL) showed that apoptosis occurred in approximately 9% of VSMCs infected with Ad-TOP-dn-N-cadherin in comparison to 3% with the Ad-β-gal control virus (Figure 2A). Similar results were also detected by CC-3 immunocytochemistry, with increased apoptosis in VSMCs infected with Ad-TOP-dn-N-cadherin compared to VSMCs infected with the Ad-β-gal control virus (7.9 ± 1 versus 6.1 ± 1%, n = 3).
Figure 2.
Regulated dn-N-cadherin Expression Induced Apoptosis and Retarded VSMC Migration but Did Not Affect Proliferation
(A) Representative images of in situ end labeling to detect apoptotic cells after infection with Ad-β-Gal or Ad-TOP-dn-N-cad. Arrows indicate apoptotic cells. Chart shows percentage of apoptotic VSMCs. (B) Representative images of scratch-would assay 24 hr after wounding in VSMC infected with Ad-β-Gal or Ad-TOP-dn-N-cad. Chart shows migration from the wound edge. (C) Representative images of BrdU incorporation within 24 hr in VSMCs infected with Ad-β-Gal or Ad-TOP-dn-N-cadherin. Arrows indicate some BrdU-positive cells. Chart shows percentage of proliferation (BrdU positive). * indicates a significant difference from Ad-β-Gal control. n = 5 (A and C) and n = 6 (B), p < 0.05, paired Student’s t test. Scale bars, 20 μm (A and C) and 50 μm (B). Error bars represent SEM.
Ad-TOP-dn-N-cadherin Significantly Reduced VSMC Migration but Had No Effect on VSMC Proliferation
We assessed the effect of Ad-TOP-dn-N-cadherin infection on VSMC migration using the scratch-wound assay. Ad-TOP-dn-N-cadherin significantly reduced VSMC migration in comparison to Ad-β-gal at 24 hr (Figure 2B). We next determined the effect of Ad-TOP-dn-N-cadherin on VSMC proliferation. Bromodeoxyuridine (BrdU) immunocytochemistry showed that there was no significant difference in proliferation between Ad-TOP-dn-N-cadherin and Ad-β-gal (Figure 2C).
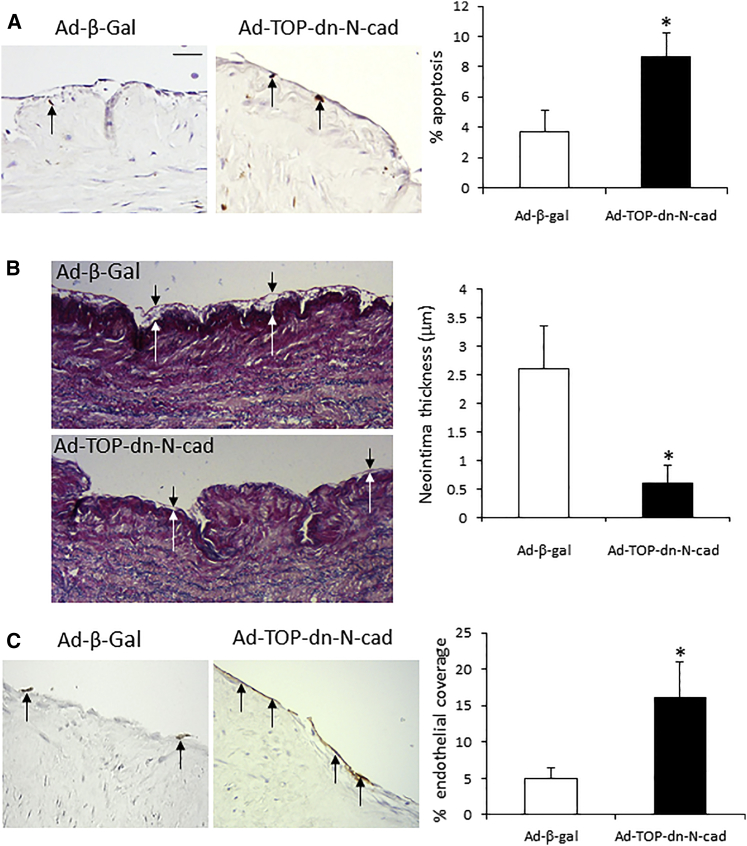
Ad-TOP-dn-N-cadherin Significantly Reduced Neointima Formation in HSV Organ Cultures
Expression of β-galactosidase was detected at both 3 and 7 days after infection in the majority of the cells on the surface of the vein segments and was undetectable in the uninfected vein segments (Figures S3A–S3D). ISEL of transverse tissue sections demonstrated that infection of HSV segments with Ad-TOP-dn-N-cadherin significantly increased intimal cell apoptosis at day 7 in comparison to the Ad-β-gal control virus (Figure 3A). Moreover, infection with Ad-TOP-dn-N-cadherin significantly reduced intimal thickening by day 7, as visualized by elastic van Gieson (EVG) staining (Figure 3B). QBend-10 immunohistochemistry showed a significant increase in endothelial cell (EC) coverage after infection with Ad-TOP-dn-N-cadherin by day 7 in comparison to Ad-β-gal control virus (Figure 3C). Ad-TOP-dn-N-cadherin infection did not influence VSMC proliferation or migration or medial apoptosis in the organ culture model (Table 1; Figure S2).
Figure 3.
Overexpression of Ad-TOP-dn-N-cadherin in Human Saphenous Vein Organ Cultures Significantly Induced VSMC Apoptosis, Suppressed Intimal Thickening, and Enhanced Endothelial Coverage
HSV was infected with Ad-β-gal or Ad-TOP-dn-N-cad and cultured for 7 days. (A) Representative images show apoptotic intimal cells detected by in situ end labeling (brown, arrows indicate some positive cells). Chart shows percentage of apoptotic cells. (B) Representative images show EVG staining, white arrows indicate intima:medial boundary, and black arrows indicate intimal surface. Chart shows neointima thickness. (C) Representative images of EC coverage detected by QBend-10 immunohistochemistry (brown, arrows indicate some of the endothelial cells). * indicates a significant difference from Ad-β-gal control. n = 6 (A), n = 5 (B), and n = 7 (C), p < 0.05, paired Student’s t test. Scale bar, 20 μm; applies to all panels. Error bars represent SEM.
Table 1.
Effect of Ad-TOP-dn-N-cad on HSV Organ Cultures
| Ad-β-gal | Ad-TOP-dn-N-cad | |
|---|---|---|
| Total number of intimal cells/mm | 10.0 ± 1.5 | 3.9 ± 1.0a |
| Intimal proliferation (%) | 18.7 ± 6.8 | 19.7 ± 3.7 |
| Medial proliferation (%) | 7.3 ± 2.1 | 7.9 ± 2.6 |
| Migration (% BrdU −ve/mm) | 0.042 ± 0.09 | 0.040 ± 0.005 |
| Medial apoptosis | 12.3 ± 5.4 | 13.3 ± 4.6 |
Segments of human saphenous vein were infected with Ad-TOP-dn-N-cad or Ad-β-gal and cultured for 7 days prior to analysis, n = 6.
Indicates significant difference (paired Student’s t test, p < 0.05).
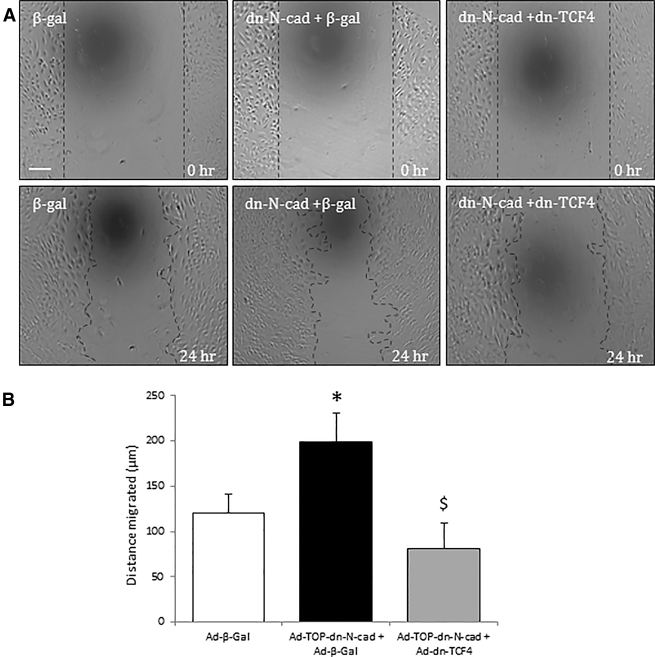
Ad-TOP-dn-N-cadherin Promoted EC Migration
We assessed the effect of Ad-TOP-dn-N-cadherin infection on EC migration using the scratch-wound assay. Ad-TOP-dn-N-cadherin significantly increased human umbilical vein endothelial cell (HUVEC) migration in comparison to Ad-β-gal at 24 hr (Figure 4). Importantly, this was suppressed by overexpression of the β-catenin signaling inhibitor dn-TCF4 (Figure 4). Moreover, Ad-TOP-dn-N-cadherin did not significantly affect apoptosis rates compared to Ad-β-gal (6 ± 1% versus 7 ± 2%).
Figure 4.
Regulated dn-N-cadherin Expression Promoted EC Migration
(A) Representative images of the wounding assay performed in HUVECs infected with Ad-β-gal or Ad-TOP-dn-N-cad, with or without Ad-β-gal or Ad-dn-TCF4. Scale bar, 100 μm; applies to all panels. (B) The distance from the wound edge after 24 hr was measured. * indicates a significant difference from Ad-β-Gal control; $ indicates a significant difference from Ad-TOP-dn-N-cad+Ad-β-Gal, n = 4, p < 0.05, ANOVA and Student Newman Keuls post hoc test. Error bars represent SEM.
Ad-TOP-dn-N-cadherin Significantly Reduced Neointima Formation after Ligation of Mouse Carotid Arteries
Expression of β-galactosidase was detected at 3 days after infection in the majority of the cells within the artery and was undetectable in the uninfected artery (Figures S3E and S3F).
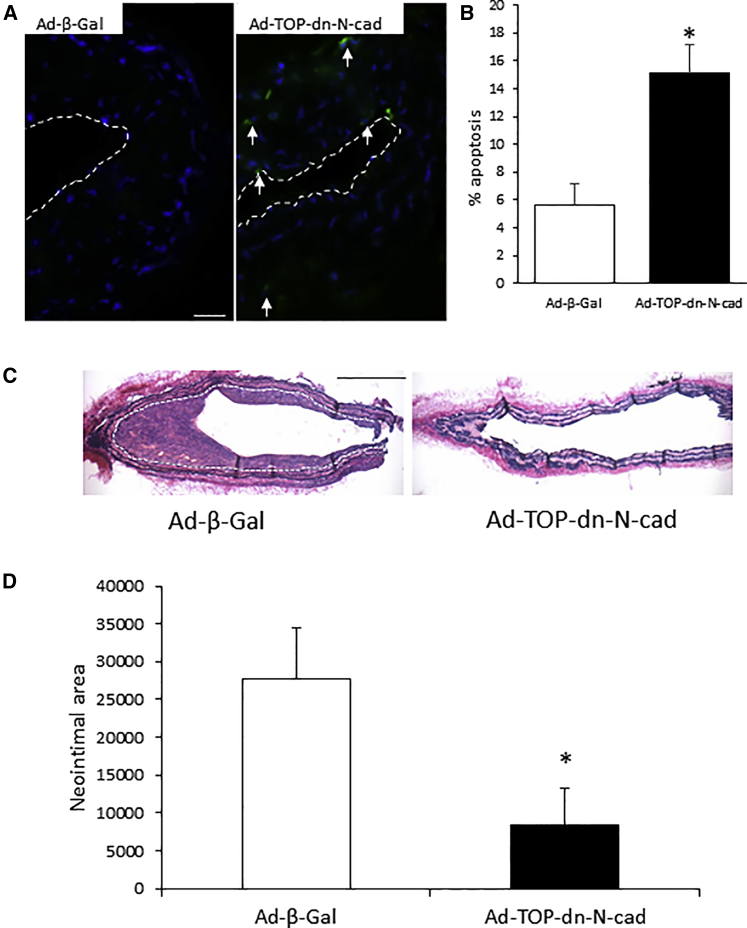
Ad-TOP-dn-N-cadherin transgene delivery to a ligated mouse carotid artery resulted in significantly increased numbers of apoptotic cells at 3 days after delivery (Figures 5A and 5B) and a significant reduction in intimal thickening at day 21 post-surgery in comparison to mice infected with Ad-β-gal (Figures 5C and 5D).
Figure 5.
Ad-TOP-dn-N-cadherin Transgene Delivery in a Mouse Carotid Artery Ligation Model Significantly Increased Apoptosis and Reduced Neointimal Thickening
Adenoviral delivery of Ad-β-Gal transgene in Pluronic F-127 gel to a ligated carotid artery and adenoviral delivery of Ad-TOP-dn-N-cad transgene. (A) Representative cleaved caspase-3 immunohistochemistry at 3 days. Arrows indicate positive cells (green); nuclei are stained blue with DAPI. Scale bar, 25 μm; applies to both panels. Dotted line indicates the lumenal surface. (B) Quantification of cleaved caspase-3 positive cells. * indicates a significant difference from Ad-β-Gal control, p < 0.05, Student’s t test, n = 4 per group. (C) Representative images show neointimal thickening in Miller’s EVG-stained sections at 21 days. Dotted line indicates the intimal:medial boundary. Scale bar, 200 μm; applies to both panels. (D) Quantification of neointimal area at 21 days. * indicates a significant difference from Ad-β-Gal control, p < 0.05, n = 12 (Ad-β-Gal) and n = 10 (Ad-TOP-dn-N-cad), Student’s t test. Error bars represent SEM.
Discussion
This study presents the development of an “activated” VSMC-targeted suicide gene approach to inhibit intimal thickening within a vein graft, similar to that described by Williams et al.4 In a previous study, we identified a significant increase in Wnt/β-catenin/TCF-activated VSMCs in the media at 3 days and in the media and intima at 28 days after carotid artery ligation. We have previously shown that Wnt/β-catenin/TCF-activated VSMCs display higher migratory and proliferative rates and thereby contribute to intimal thickening.6, 7 Williams et al.4 demonstrated that Ad-mediated delivery of the thymidine kinase (TK) gene driven by a Wnt/β-catenin/TCF-responsive promoter suppressed neointima formation in ligated carotid arteries of mice infused with ganciclovir. Selective death of Wnt/β-catenin/TCF-activated VSMCs was achieved by TK-dependent conversion of the prodrug ganciclovir into a toxic ganciclovir triphosphate. In this current study, we avoid the use of ganciclovir because it is not suitable for long-term use in humans and it has potential off-target effects. Instead, we employ dn-N-cadherin as a suicide gene to improve the potential for translation to a clinical setting. dn-N-cadherin was selected as the suicide gene because our previous works have determined that its overexpression in isolated VSMCs induced apoptotic cell death and thereby suppressed intimal thickening.3 We developed an Ad delivery of a dn-N-cadherin transgene under the control of a Wnt/β-catenin/TCF-specific promoter. This approach also confirms in both ex vivo and in vivo models that Wnt/β-catenin/TCF signaling is important for intimal thickening and apoptosis of Wnt/β-catenin/TCF-activated VSMCs effectively reduces intimal thickening.4
Mechanistically, we show in isolated VSMCs that overexpression of dn-N-cadherin, under the control of a Wnt/β-catenin/TCF-responsive promoter, increased apoptosis and reduced migration, but had no effect on proliferation. Expression of dn-N-cadherin in Ad-TOP-dn-N-cadherin-infected VSMCs was induced with LiCl, which inhibits the GSK-3β inhibitor and activates β-catenin; conversely, transactivation of Ad-TOP-dn-N-cadherin was abrogated with co-infection of Ad-dn-TCF-4 and Ad-ICAT, which both disrupt Wnt/β-catenin/TCF signaling. Thus, we demonstrated that expression of the pro-apoptotic dn-N-cadherin transgene required enhanced β-catenin signaling and so allowed selective killing of Wnt/β-catenin/TCF-activated VSMCs.
We examined the effects of the Ad-TOP-dn-N-cadherin on intimal thickening using a well-established HSV organ culture model.8, 9 After 7 days of culture, Ad-TOP-dn-N-cadherin significantly suppressed neointima formation and induced apoptotic cell death, but no effect on VSMC migration and proliferation was noted in organ cultures. We propose that dn-N-cadherin retarded neointima formation by inducing apoptosis in VSMCs that exhibit β-catenin/TCF-signaling activity. By inducing apoptosis of medial VSMCs, there are fewer VSMCs to migrate to the intima, where they proliferate and deposit matrix and cause neointimal thickening. This confirms our previously proposed pro-apoptotic approach for suppression of neointimal formation using TIMP-3 overexpression and dn-N-cadherin.3, 10 It further highlights that selection of activated VSMCs with β-catenin/TCF signaling retains the effectiveness of dn-N-cadherin to reduce neointima formation.
Damaged endothelium is detrimental in both early and late vein graft failure because it contributes to thrombosis formation in early vein graft failure, neointimal formation, and super-imposed atherosclerosis, which is the cause of late graft failure.11 Consequently, preservation of endothelial coverage of the vein graft is of great importance. Interestingly, we observed a significant increase in EC coverage in the Ad-TOP-dn-N-cadherin-infected vein, so performed in vitro studies to determine changes in EC behavior. This is in contrast to our previous observations with Ad-TIMP-3, in which EC coverage was unaffected by overexpression of this transgene.10 Despite a comparable reduction in the neointima area with Ad-TIMP-310 and Ad-TOP-dn-N-cadherin, we consider that the use of TOP-dn-N-cadherin is more advantageous because only Ad-TOP-dn-N-cadherin caused increased endothelial cell coverage, which may contribute to reduced thrombus formation and neointima development.
In cultured HUVECs, overexpression of dn-N-cadherin had no effect on apoptosis but significantly enhanced migration; this induction was lost with co-infection with Ad-dn-TCF4, a β-catenin/TCF signaling inhibitor. Interestingly, these observations in EC contrasted with the inhibitory effect on VSMC migration. We have previously demonstrated that dn-N-cadherin significantly retarded VSMC migration via induction of VSMC apoptosis3 because there are fewer VSMCs to migrate. Although ECs express N-cadherin on the basolateral surface, with which they interact with VSMCs, they predominantly express VE-cadherin to form homophilic contacts. We therefore propose the differing effects of dn-N-cadherin on these two cell types are due to the difference in cadherin expression.
In order to evaluate the translational potential of our gene therapy in terms of reducing intimal thickening, we tested Ad-TOP-dn-N-cadherin in an in vivo mouse model. Due to the lack of a suitable mouse model of vein grafting that adequately represents the human remodeling process and neointima formation, we consider that the carotid artery ligation model is the most appropriate for testing the efficacy of TOP-dn-N-cadherin. Unfortunately, the mouse vein graft model12 is not suited for this study because the majority of the medial VSMCs in the graft die within the first few days after graft implantation, and intimal thickening in this model is the result of repopulation of the graft material. This is in contrast to the remodeling of human vein grafts, in which complete loss of medial VSMCs does not occur. Consequently, our approach to induce death of graft medial cells would not work in this model and therefore we selected to utilize the carotid artery ligation model, which is well established for investigating VSMC migration and proliferation and vascular remodeling at the molecular level.13 Blood flow in the left common carotid artery is disrupted by ligating the vessel near the distal bifurcation. This causes vascular remodeling, promoting VSMC migration and proliferation and the development of an intimal lesion (intimal thickening). Compared to Ad-β-gal, Ad-TOP-dn-N-cadherin significantly reduced intimal thickening. The media of ligated vessels appeared structurally normal. Interestingly, neointimal area was more effectively attenuated by Ad-TOP-dn-N-cadherin (∼70%) than by Ad-TK (∼40%).4
In summary, we have demonstrated that Ad delivery of a dn-N-cadherin transgene, under the control of a Wnt/β-catenin/TCF-specific promoter (Ad-TOP-dn-N-cadherin), increased VSMC apoptosis and reduced intimal thickening both ex vivo and in vivo. Intra-operative gene transfer of dn-N-cadherin for selective death of Wnt/β-catenin/TCF-activated VSMCs may hold promise for reducing incidences of restenosis after CABG. This procedure is particularly amenable to gene therapy because the vein can be infected ex vivo prior to implantation, which avoids in situ or systemic delivery and restricts exposure to the Ad.
Materials and Methods
Generation of Wnt/β-catenin/TCF-Responsive dn-N-cadherin Construct Ad-TOP-dn-N-cadherin
The original Xenopus dn-N-cadherin cDNA was obtained from a cvpSP72ΔNcadC construct, a kind gift from Dr. L. Weitzmann, Washington University. The dn phenotype was generated by deletion of the extracellular domain, retaining the transmembrane and cytosolic domains.14 The dn-N-cadherin cDNA was removed from the cvpSP272 vector by XhoI and EcoRV digestion, yielding a 1,201-nt fragment. In order to clone dn-N-cadherin into the pDC512 destination vector (Microbix), dn-N-cadherin was sub-cloned into a pGEMT Easy Vector (Promega) by TA cloning. The dn-N-cadherin fragment was removed by EcoRI digestion and ligated into pDC512 (also EcoRI digested and de-phosphatased by calf intestinal phosphatase [CIP] [NEB]). The 250-nt minimal cytomegalovirus (minCMV) promoter was generated from murine CMV (MCMV) cDNA of the pDC516 vector (Microbix) and amplified by PCR (incorporating HindIII and NheI restriction sites) using KOD (high fidelity) polymerase and the following primers:
(forward) 5′-AAGCTTTAGGGCCAGTACATAAGGTCAATAG-3′ and
(reverse) 5′-GCTAGCCTGACTGCATTAGTTTCAATAGC-3′.
Amplicons were cloned into the pGEMT Easy Vector by TA cloning and ligated using the Roche Ligation Kit (Roche Life Science). The minCMV/pGEMT construct was digested with HindIII, blunted with mung bean nuclease, and digested with NheI (high fidelity) to remove the minCMV and produce compatible ends for ligation. The ΔNcadCpDC512 was digested with BglII, blunted with mung bean nuclease, and digested with NheI before treatment with CIP and ligation with the minCMV. The M50 Super 8x TOPFlash promoter (Addgene plasmid 12456) containing seven TCF/lymphoid enhancing factor-1 (LEF-1)-binding sites was amplified by PCR to generate a 147-nt amplicon using the following primers:
(forward) 5′- GCGTCTAGAGGTACCGAGCTCTTACGCG-3′ and
(reverse) 5′-GCGGTCGACCCCTCTAGAGTCTAGATCTCG-3′.
Amplicons were digested with SalI. The dn-N-cadherinpDC512minCMV was digested with SalI and de-phosphatased with CIP. The TOPFlash and dn-N-cadherinpDC512minCMV fragments were ligated to generate a TOPminCMVdn-N-cadherin construct. All recombinants were confirmed by direct sequencing. The plasmid map is shown in Figure S1.
Ad Vectors
Replication-defective Ads based on the Ad-5 vector were used for this study: Ad-TOP-dn-N-cadherin, control Ad-β-gal, Ad-ICAT, and Ad-dn-TCF4. Ad-TOP-dn-N-cadherin consists of a Wnt/β-catenin/TCF-responsive promoter (TOP) controlling the dn (truncated) N-cadherin. Ad-β-gal encodes the bacterial lacZ gene under the control of the CMV immediate early promoter and was used as a control adenovirus. Ad-ICAT and Ad-dn-TCF4 encode ICAT and dnTCF-4, respectively, and are described previously.15 Plaque-pure viruses were propagated on HEK293 cells, purified by caesium chloride density gradient centrifugation, and titered using the end-point dilution assay. Recombinant Ads were tested for replication deficiency by titration on non-permissive HeLa cells. In addition, all preparations of Ads were tested for functional transgene production and lack of toxicity in isolated VSMCs in vitro. Stocks were stored at −70°C in aliquots and thawed immediately before use.
Cell Culture
Saphenous vein segments surplus to surgical requirements were obtained from patients undergoing coronary artery bypass surgery (REC number 12/SW/0306). HSV VSMCs were grown as described previously.16 VSMCs were maintained in growth media (DMEM supplemented with 100 μg/mL of penicillin, 100 IU/mL streptomycin, 2 mM L-glutamine, and 10% [v/v] fetal calf serum [FCS]). When required, (canonical) Wnt signaling was induced with 10 μM LiCl. Four batches of pooled populations of HUVECs were purchased from Promocell and cultured as recommended by the supplier.
Total Protein Extraction
VSMC proteins were extracted from cells with 1% (w/v) SDS lysis buffer. Each well was scraped with a pipette tip and lysates were homogenized by a syringe needle and centrifuged at 13,000 × g for 5 min to remove cell debris. The protein concentration of the supernatant was determined using the bicinchoninic acid (BCA) protein assay kit (Thermo Scientific). Equal protein concentrations were then subjected to western blotting.
Infection with Ads
Cells were seeded in 12-well tissue culture plates at a density of 8 × 104 cells/well. For overexpression of Ad-dn-N-cadherin, cells were infected with 100, 300, and 1,000 plaque-forming units (pfu) per cell (0.1 × 108, 0.33 × 108, and 1 × 108 per mL) of Ad-TOP-dn-N-cadherin or Ad-β-gal (control virus) in growth media. For nuclear inhibition of Wnt/β-catenin/TCF signaling, cells were also infected with 300 pfu/cell of Ad-ICAT or Ad-dn-TCF-4. After 18 hr, fresh growth media was added to induce Wnt/β-catenin/TCF signaling for a further 24 hr before cell lysis for western blot analysis.
Western Blotting
Cell lysates were loaded on 3%–8% polyacrylamide Mini-PROTEAN Precast Gels (BIO-RAD) and transferred using the Trans-Blot Turbo System. Nitrocellulose membranes were blocked in 2% (w/v) BSA in Tris-buffered saline, 0.1% Tween 20 (TBS-T). Blots were incubated overnight at 4°C with 0.1 μg/mL of mouse anti-N-cadherin antibody (BD Transduction Laboratories, 610921), which recognizes the C-terminal (intracellular) domain. Bound antibodies were detected using goat anti-mouse horseradish peroxidase (HRP) conjugated antibody (DAKO) diluted 1:2,000 in 2% BSA in TBS-T and enhanced chemiluminescence (Luminata Forte Western HRP Substrate, Merck Millipore). To assess equal protein loading, blots were subsequently incubated with 0.11 μg/mL of mouse anti-β-actin antibody (Sigma, A5316). Bound antibodies were detected with rabbit anti-mouse HRP (DAKO) and enhanced chemiluminescence as described above. Densitometry using a GS-690 densitometer (BIO-RAD) was used to quantify detected bands (optical density [OD] × mm2).
Apoptosis
VSMCs were fixed as previously described above. Apoptotic VSMCs were identified by ISEL and cleaved caspase-3 immunocytochemistry as previously described.17 The number of positive (apoptotic) cells was expressed as a percentage of the total number of cells in 15 0.25 mm2 fields using the Media Cybernetics Image Pro Plus version 3 image analysis system (Data Cell).
Cell Proliferation
Cells were seeded onto coverslips in 24-well tissue culture plates at a density of 4 × 104 cells/well and cultured in growth media for 30 hr. VSMCs were then infected with 1,000 pfu/cell of Ad-TOP-dn-N-cadherin or Ad-β-gal control virus in growth media. After 18 hr, the media was replaced with fresh growth media supplemented with 10 μM BrdU. The cells were fixed after 24 hr with ice-cold methanol for 5 min. Hydrochloric (HCl) acid (4N) was added to each well and incubated for 30 min at 37°C. VSMCs were then incubated with 1 μM mouse anti-BrdU, (Sigma, B2531) in 1% (v/v) goat serum and 1% (w/v) BSA/PBS at 4°C overnight. Cells were then incubated with biotinylated goat anti-mouse antibody diluted 1:200 in 1% BSA/PBS for 30 min at room temperature and then Extravidin-HRP diluted 1:200 in 1% BSA/PBS for 30 min at room temperature. The color was developed with 3,3′-diaminobenzidine (DAB) solution as described above by incubating for 10 min. VSMCs were counterstained with hematoxylin and washed well with tap water. Coverslips were mounted onto glass slides with polyvinylpyrrolidone (PVP). Proliferation was measured by counting the number of BrdU-positive (brown) cells and dividing by the total number of cells in 20 0.25 mm2 fields using image analysis.
Migration
VSMCs and HUVECs were seeded onto coverslips in 24-well tissue culture plates at a density of 4 × 104 cells/well and cultured in growth media for 30 hr. Cells were then infected with 1,000 pfu/cell of Ad-TOP-dn-N-cadherin or Ad-β-gal control virus in growth media. After 18 hr, cells were subjected to wounding by rubbing a 1 mL pipette tip across the layer twice. The media was replaced with fresh growth media supplemented with 2 mM hydroxyurea to suppress proliferation, as previously described.3 A total of six images of wounded and non-wounded areas were captured at 0 hr and 24 hr using Image Pro Plus version 3. The distance migrated was measured at 20 points along the wound edge using image analysis.
Organ Culture
HSV surplus segments were collected as detailed previously.8 The vein was placed in wash medium (20 mM HEPES-buffered RPMI 1640 supplemented with 2 mM L-glutamine, 100 IU/mL penicillin, and 100 μg/mL of streptomycin). The adventitial layer was removed, and the vein was cut into three equal segments. One segment was infected with Ad-TOP-dn-N-cadherin, and another segment was infected with Ad-β-gal control virus. The vein was infused with 100 μL of 1.2 × 1010 pfu/mL of Ad-dn-N-cadherin or Ad-β-gal for a period of 1 hr, as described previously.10, 18, 19 All three segments were then opened longitudinally and cut transversely into three 5- to 10 mm segments. Vein segments were cultured separately, with the luminal surface facing upward for up to 14 days in organ culture growth media (RPMI 1640 supplemented with 30% [v/v] FCS, 2 mM L-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10 μM BrdU). The tissue culture growth media was changed every 2 days. After 4, 7, or 14 days of culture, the vein segments were washed in PBS and then fixed in 10% (v/v) formalin in PBS prior to embedding in paraffin wax, and the analysis was performed on at least three different sections. Infection efficiency was monitored by X-gal staining for the β-galactosidase transgene, as described previously.18
Apoptosis: ISEL in Saphenous Vein Organ Cultures
Briefly, 3-μm deparaffinized, rehydrated sections were incubated with 5 μg/mL of proteinase K for 15 min at room temperature. Sections were then incubated in reaction buffer containing 0.01 mM dATP, dCTP, dGTP, and biotin-16-dUTP, 8 U/mL DNA polymerase I (Klenow) large fragment, 50 mM Tris Cl pH 7.2, 10 mM MgSO4, and 0.1 mM DTT for 15 min at 37°C. After treating with 3% hydrogen peroxide for 5 min, slides were incubated in extravidin HRP diluted 1:200 in 10% FCS in PBS for 30 min at room temperature and then color developed with DAB solution for 10 min prior to counterstaining with hematoxylin for 2 min. Slides were washed well with tap water and then dehydrated and cleared prior to mounting in DPX. The number of positive (brown nuclei) cells (on the intimal surface and in the media) was expressed as a percentage of the total number of cells across the entire length of the vein segment using image analysis.
Intimal Thickening
3-μm transverse sections were stained with Miller’s EVG. The intimal area and length of each vein segment was measured by image analysis. The average intimal thickness was determined by dividing the intimal area by the length of each vein segment.
Cell Proliferation and Migration in Saphenous Vein Organ Cultures
Proliferation was assessed in paraffin wax-embedded sections by immunohistochemistry for incorporated BrdU. Sections (3 μm) were incubated with 1% (v/v) goat serum and mouse 8.6 μg/mL of anti-BrdU antibody (Sigma, B2531) in 1% BSA/PBS at 4°C overnight. Slides were incubated with biotinylated goat anti-mouse secondary antibody, diluted 1:200 in 1% BSA/PBS for 30 min at room temperature and then extravidin-HRP diluted 1:200 in 1% BSA/PBS for 30 min at room temperature. Color was developed by incubation with DAB for 10 min. VSMCs were counterstained with hematoxylin and washed with tap water. Coverslips were mounted with PVP. Quantification of the BrdU incorporation was achieved by calculating the percentage of BrdU-positive VSMCs in the media and intima. Migration was estimated by counting the number of BrdU-negative cells in the intima and dividing by the length of the vein segment using image analysis, as in our previous studies.3, 10, 18, 19, 20, 21, 22 A representative image is shown in Figure S2.
EC Coverage
EC coverage was assessed by QBend-10 immunocytochemistry. Deparaffinized, rehydrated sections (3 μm) were incubated with 3% (v/v) hydrogen peroxide for 5 min at 4°C before blocking in 20% (v/v) goat serum in PBS for 30 min at room temperature. Sections were incubated with 0.1 μg/mL anti-QBend-10 antibody (Abcam, ab8536) in 1% (w/v) BSA in PBS overnight at 4°C. Slides were then incubated with biotinylated goat anti-mouse diluted 1:200 in 1% (w/v) BSA/PBS for 30 min at room temperature and then extravidin-HRP diluted 1:200 in 1% (w/v) BSA/PBS for 30 min at room temperature. Slides were incubated with DAB for 10 min as above. VSMCs were counterstained with hematoxylin and washed with tap water. Coverslips were mounted with PVP. EC coverage was quantified by counting positive cells along the luminal surface and dividing by the length of the segment.
In Vivo Analysis of Ad-dn-N-cadherin Transgene Delivery and Effects on Intimal Thickening
The housing and care of all the animals and the procedures used in these studies were performed in accordance with the guidelines and regulations of the University of Bristol and the United Kingdom Home Office. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US NIH (Publication No. 85-23, revised 1996). Male, 8-week-old C57BL/6 mice (n = 30) were subjected to a left common carotid artery ligation surgical procedure involving dissection and ligation near the carotid bifurcation to induce intimal thickening, as described previously.6 To assess the effects of Ad-TOP-dn-N-cadherin transgene delivery and intimal thickening in vivo, mice were randomly divided into two groups (n = 15 per group). One group received 2.2 × 1012 pfu/mouse of Ad-TOP-dn-N-cadherin in 30% (v/v) Pluronic F-127 gel (Sigma Aldrich). The other group received the same concentration of Ad-β-gal control virus. The Ads were pipetted onto the ligated artery immediately after carotid artery ligation using an ice-cold pipette tip. After surgery, the mice were housed within their experimental group as trios for 21 days and fed a normal chow diet. At day 3 and 21, mice were euthanized and left and right (non-ligated) carotid arteries were harvested, fixed in 10% formalin for 24 hr, and embedded in paraffin wax in a longitudinal orientation for 21-day samples and transversely for 3-day samples, as previously described.6 3-μm longitudinal sections were stained with EVG. Intimal thickening was quantified in the 21-day samples using image analysis, and the lumen area was expressed as a percentage of the area within the internal elastic lamina. Apoptotic cells and β-galactosidase protein were identified in 3 μm transverse sections at 3 days using immunohistochemistry for cleaved caspase-3 and β-galactosidase, as previously described.6, 23
Statistical Analysis
Data are presented as n = independent experiments, as noted in the figure legends. For analysis of two groups, paired or unpaired Student’s t tests were employed. ANOVA and the Student Newman Keuls post hoc test were performed when three or more groups of data were analyzed. Data were considered statistically significant when p < 0.05.
Author Contributions
Conceptualization, S.J.G.; Methodology, S.J.G., H.W., and G.B.S.-N.; Formal Analysis, S.J.G., S.H.-C., and K.S.W.; Investigation, S.H.-C., H.W., and K.S.W.; Writing – Original Draft, S.H.-C. and S.J.G.; Writing – Review and Editing, S.J.G., S.H.-C., H.W., and K.S.W.; Visualization, S.J.G., S.H.-C., and K.S.W.; Supervision, S.J.G., H.W., and G.B.S.-N.; Project Administration, S.J.G.; Funding Acquisition, S.J.G.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was funded by Heart Research UK (project grant: RG2622) and the NIHR Bristol Cardiovascular Biomedical Research Unit and NIHR Bristol Biomedical Research Centre. We thank Sue Finerty and Jill Tarlton for assistance with molecular cloning and adenovirus production.
Footnotes
Supplemental Information includes four figures and can be found with this article online at http://dx.doi.org/10.1016/j.omtm.2017.04.009.
Supplemental Information
References
- 1.Halabi A.R., Alexander J.H., Shaw L.K., Lorenz T.J., Liao L., Kong D.F., Milano C.A., Harrington R.A., Smith P.K. Relation of early saphenous vein graft failure to outcomes following coronary artery bypass surgery. Am. J. Cardiol. 2005;96:1254–1259. doi: 10.1016/j.amjcard.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 2.Rodés-Cabau J., Facta A., Larose E., DeLarochellière R., Déry J.-P., Nguyen C.M., Roy L., Proulx G., Gleeton O., Barbeau G. Predictors of aorto-saphenous vein bypass narrowing late after coronary artery bypass grafting. Am. J. Cardiol. 2007;100:640–645. doi: 10.1016/j.amjcard.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 3.Lyon C.A., Koutsouki E., Aguilera C.M., Blaschuk O.W., George S.J. Inhibition of N-cadherin retards smooth muscle cell migration and intimal thickening via induction of apoptosis. J. Vasc. Surg. 2010;52:1301–1309. doi: 10.1016/j.jvs.2010.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams H., Slater S., George S.J. Suppression of neointima formation by targeting β-catenin/TCF pathway. Biosci. Rep. 2016;36:e00427. doi: 10.1042/BSR20160229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwong K.Y., Zou Y., Day C.-P., Hung M.-C. The suppression of colon cancer cell growth in nude mice by targeting β-catenin/TCF pathway. Oncogene. 2002;21:8340–8346. doi: 10.1038/sj.onc.1206050. [DOI] [PubMed] [Google Scholar]
- 6.Tsaousi A., Williams H., Lyon C.A., Taylor V., Swain A., Johnson J.L., George S.J. Wnt4/β-catenin signaling induces VSMC proliferation and is associated with intimal thickening. Circ. Res. 2011;108:427–436. doi: 10.1161/CIRCRESAHA.110.233999. [DOI] [PubMed] [Google Scholar]
- 7.Williams H., Tsaousi A., George S. WNT/beta-catenin signalling regulates intimal thickening and smooth muscle cell migration. Atherosclerosis. 2010;213:e15. [Google Scholar]
- 8.George S.J., Williams A., Newby A.C. An essential role for platelet-derived growth factor in neointima formation in human saphenous vein in vitro. Atherosclerosis. 1996;120:227–240. doi: 10.1016/0021-9150(95)05717-x. [DOI] [PubMed] [Google Scholar]
- 9.Soyombo A.A., Angelini G.D., Bryan A.J., Jasani B., Newby A.C. Intimal proliferation in an organ culture of human saphenous vein. Am. J. Pathol. 1990;137:1401–1410. [PMC free article] [PubMed] [Google Scholar]
- 10.George S.J., Lloyd C.T., Angelini G.D., Newby A.C., Baker A.H. Inhibition of late vein graft neointima formation in human and porcine models by adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-3. Circulation. 2000;101:296–304. doi: 10.1161/01.cir.101.3.296. [DOI] [PubMed] [Google Scholar]
- 11.de Vries M.R., Simons K.H., Jukema J.W., Braun J., Quax P.H.A. Vein graft failure: from pathophysiology to clinical outcomes. Nat. Rev. Cardiol. 2016;13:451–470. doi: 10.1038/nrcardio.2016.76. [DOI] [PubMed] [Google Scholar]
- 12.Zou Y., Dietrich H., Hu Y., Metzler B., Wick G., Xu Q. Mouse model of venous bypass graft arteriosclerosis. Am. J. Pathol. 1998;153:1301–1310. doi: 10.1016/S0002-9440(10)65675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A., Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler. Thromb. Vasc. Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 14.Uglow E.B., Slater S., Sala-Newby G.B., Aguilera-Garcia C.M., Angelini G.D., Newby A.C., George S.J. Dismantling of cadherin-mediated cell-cell contacts modulates smooth muscle cell proliferation. Circ. Res. 2003;92:1314–1321. doi: 10.1161/01.RES.0000079027.44309.53. [DOI] [PubMed] [Google Scholar]
- 15.Quasnichka H., Slater S.C., Beeching C.A., Boehm M., Sala-Newby G.B., George S.J. Regulation of smooth muscle cell proliferation by BETA-catenin/T-cell factor signaling involves modulation of cyclin D1 and p21 expression. Circ. Res. 2006;99:1329–1337. doi: 10.1161/01.RES.0000253533.65446.33. [DOI] [PubMed] [Google Scholar]
- 16.Dwivedi A., Sala-Newby G.B., George S.J. Regulation of cell-matrix contacts and β-catenin signaling in VSMC by integrin-linked kinase: implications for intimal thickening. Basic Res. Cardiol. 2008;103:244–256. doi: 10.1007/s00395-007-0693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koutsouki E., Beeching C.A., Slater S.C., Blaschuk O.W., Sala-Newby G.B., George S.J. N-cadherin-dependent cell-cell contacts promote human saphenous vein smooth muscle cell survival. Arterioscler. Thromb. Vasc. Biol. 2005;25:982–988. doi: 10.1161/01.ATV.0000163183.27658.4b. [DOI] [PubMed] [Google Scholar]
- 18.George S.J., Johnson J.L., Angelini G.D., Newby A.C., Baker A.H. Adenovirus-mediated gene transfer of the human TIMP-1 gene inhibits smooth muscle cell migration and neointimal formation in human saphenous vein. Hum. Gene Ther. 1998;9:867–877. doi: 10.1089/hum.1998.9.6-867. [DOI] [PubMed] [Google Scholar]
- 19.George S.J., Baker A.H., Angelini G.D., Newby A.C. Gene transfer of tissue inhibitor of metalloproteinase-2 inhibits metalloproteinase activity and neointima formation in human saphenous veins. Gene Ther. 1998;5:1552–1560. doi: 10.1038/sj.gt.3300764. [DOI] [PubMed] [Google Scholar]
- 20.Lyon C.A., Wadey K.S., George S.J. Soluble N-cadherin: A novel inhibitor of VSMC proliferation and intimal thickening. Vascul. Pharmacol. 2016;78:53–62. doi: 10.1016/j.vph.2015.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George S.J., Johnson J.L., Angelini G.D., Jeremy J.Y. Short-term exposure to thapsigargin inhibits neointima formation in human saphenous vein. Arterioscler. Thromb. Vasc. Biol. 1997;17:2500–2506. doi: 10.1161/01.atv.17.11.2500. [DOI] [PubMed] [Google Scholar]
- 22.George S.J., Angelini G.D., Capogrossi M.C., Baker A.H. Wild-type p53 gene transfer inhibits neointima formation in human saphenous vein by modulation of smooth muscle cell migration and induction of apoptosis. Gene Ther. 2001;8:668–676. doi: 10.1038/sj.gt.3301431. [DOI] [PubMed] [Google Scholar]
- 23.Lyon C.A., Johnson J.L., Williams H., Sala-Newby G.B., George S.J. Soluble N-cadherin overexpression reduces features of atherosclerotic plaque instability. Arterioscler. Thromb. Vasc. Biol. 2009;29:195–201. doi: 10.1161/ATVBAHA.108.178087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.