Abstract
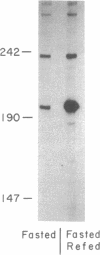
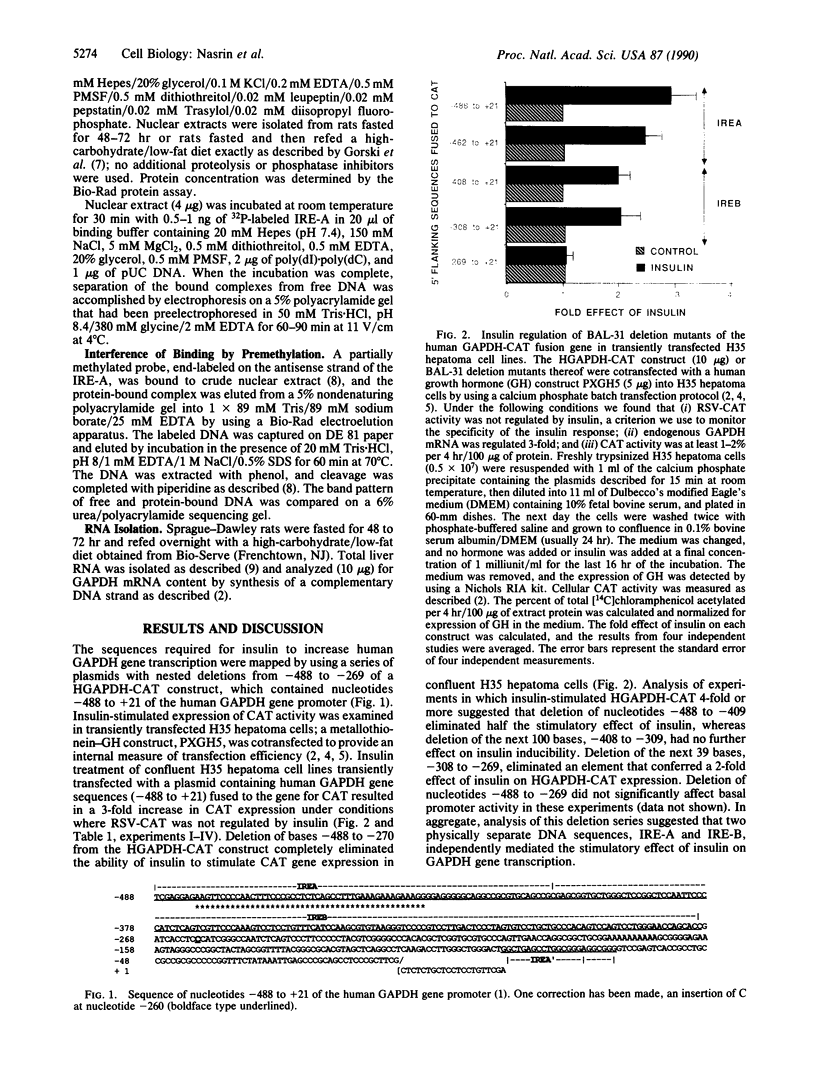
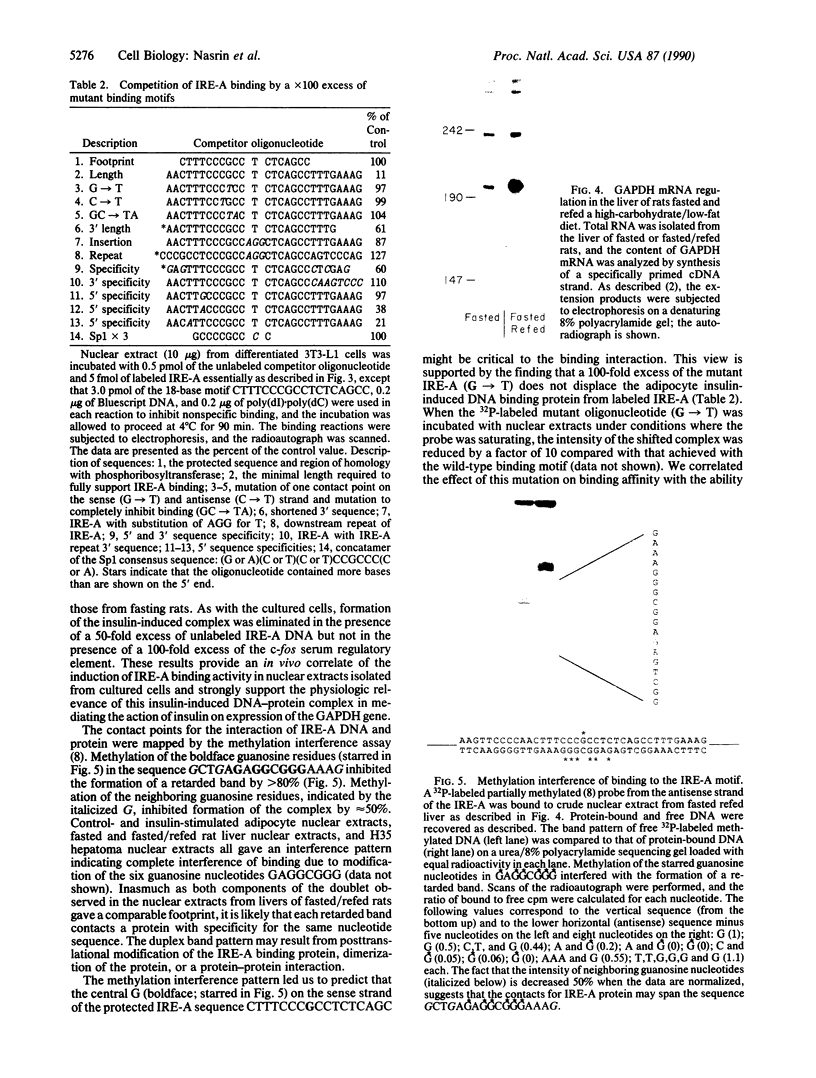
Two independent cis-acting insulin response elements (IREs) in the gene encoding glyceraldehyde-3-phosphate dehydrogenase [D-glyceraldehyde-3-phosphate: NAD+ oxidoreductase (phosphorylating), EC 1.2.1.12], designated IRE-A and IRE-B, are sufficient to direct insulin-inducible gene expression. Using the electrophoretic mobility shift assay, a 4-fold increase in the amount of IRE-A DNA bound to nuclear proteins was detected when extracts isolated from insulin-stimulated differentiated 3T3-L1 cells or from the liver of rats refed a high-carbohydrate/low-fat diet after a 72-hr fast were compared to control nuclear extracts. The points of contact between protein and IRE-A DNA may represent a sequence recognized by at least one class of insulin-sensitive transcription factor(s).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. C., Lomanto M., Nasrin N., Ramaika C. Insulin stimulates glyceraldehyde-3-phosphate dehydrogenase gene expression through cis-acting DNA sequences. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5092–5096. doi: 10.1073/pnas.85.14.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M., Curtis G., Avruch J., Goodman H. M. Insulin regulation of protein biosynthesis in differentiated 3T3 adipocytes. Regulation of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1985 Oct 5;260(22):11978–11985. [PubMed] [Google Scholar]
- Blackshear P. J. Insulin-stimulated protein biosynthesis as a paradigm of protein kinase C-independent growth factor action. Clin Res. 1989 Jan;37(1):15–25. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercolani L., Florence B., Denaro M., Alexander M. Isolation and complete sequence of a functional human glyceraldehyde-3-phosphate dehydrogenase gene. J Biol Chem. 1988 Oct 25;263(30):15335–15341. [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Siegfried Z., Ziff E. B. Mutation of the c-fos gene dyad symmetry element inhibits serum inducibility of transcription in vivo and the nuclear regulatory factor binding in vitro. Mol Cell Biol. 1987 Mar;7(3):1217–1225. doi: 10.1128/mcb.7.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. L., Isham K. R., Johnson A., Kenney F. T. Insulin enhances transcription of the tyrosine aminotransferase gene in rat liver. Arch Biochem Biophys. 1986 Aug 1;248(2):597–603. doi: 10.1016/0003-9861(86)90513-8. [DOI] [PubMed] [Google Scholar]
- Magnuson M. A., Quinn P. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase-chloramphenicol acetyltransferase fusion genes. Insulin's effects oppose those of cAMP and dexamethasone. J Biol Chem. 1987 Nov 5;262(31):14917–14920. [PubMed] [Google Scholar]
- Osborn L., Rosenberg M. P., Keller S. A., Ting C. N., Meisler M. H. Insulin response of a hybrid amylase/CAT gene in transgenic mice. J Biol Chem. 1988 Nov 15;263(32):16519–16522. [PubMed] [Google Scholar]
- Selden R. F., Howie K. B., Rowe M. E., Goodman H. M., Moore D. D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986 Sep;6(9):3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Stewart T. N., Gilman M. Z., Blackshear P. J. Identification of c-fos sequences involved in induction by insulin and phorbol esters. J Biol Chem. 1988 Feb 5;263(4):1611–1614. [PubMed] [Google Scholar]
- Taub R., Roy A., Dieter R., Koontz J. Insulin as a growth factor in rat hepatoma cells. Stimulation of proto-oncogene expression. J Biol Chem. 1987 Aug 5;262(22):10893–10897. [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Weinstock R. S., Messina J. L. Transcriptional regulation of a rat hepatoma gene by insulin and protein kinase-C. Endocrinology. 1988 Jul;123(1):366–372. doi: 10.1210/endo-123-1-366. [DOI] [PubMed] [Google Scholar]