Abstract
People with spinal cord injury (SCI) experience bone and muscle loss in their paralyzed limbs that is most rapid and severe in the first 3 years after injury. Restoration of mechanical loading through therapeutic physical activity may potentially slow or reverse post-SCI bone loss, however, therapeutic targets cannot be developed without accurate biomechanical models. Obesity is prevalent among SCI population, and it alters body composition and further affects parameters of these models. Here, clinical whole body dual-energy X-ray absorptiometry data from people with acute (n=39) and chronic (n=61) SCI were analyzed to obtain anthropometric parameters including segment masses, center of mass location, and radius of gyration for both obese and non-obese individuals. Chronic SCI was associated with higher normalized trunk mass of 3.2 %BW and smaller normalized leg mass of 1.8 %BW in males, but no significant changes in segment centers of mass or radius of gyration. People with chronic SCI had 58.6% lean mass in the trunk, compared to 66.6% lean mass in those with acute SCI (p=0.01), with significant changes in all segments. Obesity was associated with an increase in trunk mass proportion of 3.1 %BW, proximal shifts in thigh and upper arm center of mass, and changes to thigh and shank radius of gyration. The data presented here can be used to accurately represent the anthropometrics of SCI population in biomechanical studies, considering obesity and injury duration.
Keywords: Anthropometry, dual energy x-ray absorptiometry, biomechanics, kinetics, inertia, Rehabilitation Medicine
Introduction
Approximately 276,000 people live with spinal cord injury (SCI) in the United States, and 80% of them are male (National SCI Statistical Center, 2015). People with SCI experience rapid bone loss in their paralyzed limbs, which leads to severe osteoporosis and fracture risk of 40.0% in the long term (Morse et al., 2009; Tan et al., 2013). Physical activities that restore mechanical loading have been proposed to slow, or even reverse bone loss (Tan et al., 2013), although clinical evidence is inconclusive (Giangregorio and McCartney, 2006; Hammond et al., 2014; Panisset et al., 2015). Such activities include functional electrical stimulation-assisted rowing or cycling, and weight supported walking (Giangregorio et al., 2005; Lauer et al., 2011; Tan et al., 2013). To maximize rehabilitation potential and ensure safety for the SCI population, biomechanical analyses are needed to quantify the loads applied to the musculoskeletal system during proposed therapeutic activities.
Biomechanical models used to perform inverse dynamics calculations regard humans as a system of linked rigid body segments. Each segment is characterized by its mass, center of mass location (COM), and moment of inertia. These body segment parameters are used for kinetic analyses, and they affect calculations of net joint loads and moments. Just as it has long been recognized that body segment parameters change with growth (Jensen, 1986), there has been a growing recognition that the most commonly used anthropometric data sets may not adequately represent specific clinical populations of interest to the biomechanics community. One recent study compared net joint moment calculations on healthy male subjects using six different sets of anthropometric data, and reported discrepancies in hip moment of up to 20.1% (Rao et al., 2006). In amputees, using cadaveric estimations of segment parameters resulted in significant overestimations of knee and hip moments during the swing phase of gait (Goldberg et al., 2008). Compared to population-specific measurements, traditional regressions resulted in as much as 11% difference in segment radius of gyration (ROG) in older adults (Durkin and Dowling, 2003). This highlights the need for accurate, population-specific anthropometric data.
Body segment parameters are influenced by body composition, which varies with clinical and demographic factors including age, sex, and race. For example, in older men and women, trunk and arm mass represent approximately 2.5% less of total body mass, while leg mass represents around 3% more in females compared to males (Chambers et al., 2010). The same study found that older obese men and women carried 1.5% to 5% more of their mass in the trunk compared to non-obese individuals. Another study found that in males, upper arm and thigh moments of inertia decreased with age, while the center of mass (COM) of the arm moved more distally (Muri et al., 2008). Because SCI is associated with changes in body composition, the available anthropometric data generated primarily in older cadavers or healthy college students (de Leva, 1996; Dempster, 1955) are unlikely to be generalizable to SCI-specific biomechanical analyses. SCI is associated with higher rates of obesity than the general population (Pelletier et al., 2016), which independently affects segment parameters. Moreover, the most profound changes in body composition occur within the first 3 years of SCI, thus anthropometric characteristics may change as a result of injury duration.
Therefore, the purpose of this study was to determine the association between injury duration and obesity on body segment parameters after SCI. We hypothesized that people with chronic SCI would have smaller lower extremity mass and greater trunk and upper extremity mass compared to those with acute SCI, and that individuals who were obese would have greater proportional trunk and arm mass compared to leaner individuals.
Methods
Subjects
One hundred and seventy-nine patients with SCI received a total body dual-energy X-ray absorptiometry (DXA) scan between June 2012 and October 2015 as part of clinical care. We excluded those with fixation rods or metal artifact that prevented accurate segment parameter calculation, and those whose body parts (trunk, or both arms, or both legs) were incomplete in the scans (n=10). We then studied a convenience sample of 78 male and 22 female patients, reflecting the typical male/female ratio of individuals with SCI (National SCI Statistical Center, 2015). This study was conducted according to our institutional review board (IRB) approved protocol.
Demographic and clinical information were obtained by medical record review. Injury severity was classified as motor complete SCI (AIS A/B) or motor incomplete SCI (AIS C/D). Injury level was considered as paraplegia or tetraplegia. Usual mobility mode (more than 50% of the time) was considered as wheelchair use (motorized wheelchair or hand-propelled wheelchair) or walking (with aid such as crutch, cane or walk without assistance). Body mass index (BMI) was used to define non-obese (< 25 kg/m2) or obese (≥25 kg/m2) categories using SCI-specific cut points (Laughton et al., 2009). Duration of injury was considered to be acute (< 3 years since injury) or chronic (≥3 years since injury).
DXA Image Processing
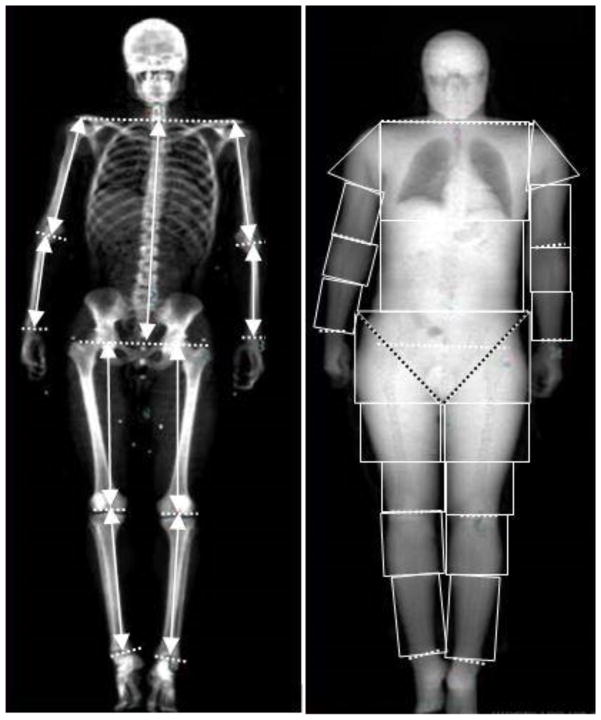
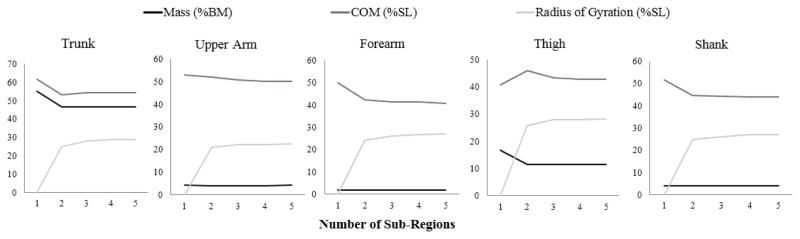
A DXA scan (5th generation Lunar iDXA, GE Healthcare, UK) with software (enCORE 12.3, GE Healthcare, UK) was used to assess body composition clinically. Whole-body frontal plane DXA images containing the skeleton and soft tissue were obtained. Trunk, upper arm, forearm, thigh, and shank were identified as segments of interest. Segment lengths were defined similar to de Leva (de Leva, 1996) and Chambers et al. (Chambers et al., 2010) using anatomical landmarks (Figure 1, Table 1). Segment boundaries were manually defined by a single investigator (YF) using polygon sub-regions (usually rectangles). The left and right borders of each sub-region were determined such that they enclosed the soft tissue along the length of each segment. The bottom border of trunk segment and top border of thigh segment were defined using trunk/thigh planes, which extended inferior and medial from the anterior superior iliac spine to the ischial tuberosity of the pelvis (Chambers et al., 2010) (Figure 1b). For each subregion, bone mass, lean mass, fat mass, dimension, and centroid were provided by the software. The number of sub-regions necessary to accurately measure each segment was determined by two sensitivity analyses. First, thigh normalized mass, COM and ROG were calculated for 5 subjects ranging from 1.57 to 1.77 m in height and 58–96 kg in mass, using up to 9 sub-regions. These parameters did not differ significantly depending on the number of sub-regions used (Table 2). Next, normalized mass, COM and ROG were examined in all segments of a typical subject as a function of number of sub-regions (Figure 2). The calculated parameters for each segment did not change more than 1.5% when more than 3 sub-regions were used. Based on these analyses, each segment was broken into three sub-regions for analysis of the complete data set.
Figure 1.
Whole body DXA scan showing a) skeleton with segment lengths (solid line with arrow) and the plane (dashed line) where anatomical landmarks locate, and b) soft tissue with regions of interest. Each segment was divided into polygon sub-regions. The black dashed line indicates trunk/thigh plane.
Table 1.
Definition of the superior and inferior ends of segment length.
| Superior | Inferior | |
|---|---|---|
| Trunk | Midpoint of the right and left acromion | Midpoint of the hip joint center |
| Upper Arm | Acromion | Elbow joint center |
| Forearm | Elbow joint center | Wrist joint center |
| Thigh | Hip joint center | Knee joint center |
| Shank | Knee joint center | Ankle joint center |
Table 2.
Mass, center of mass (COM), and radius of gyration (ROG) of thigh when using 3, 4, and 9 subregions. P-values was reported after a repeated measures analysis of variance test between using 3 different sub-regions among 5 subjects.
| Subject | Number of Sub-regions | Mass (% Body Mass) | COM (% Segment Length) | ROG (% Segment Length) |
|---|---|---|---|---|
| 1 | 3 | 10.8 | 42.3 | 28.7 |
| 4 | 11.1 | 42.4 | 28.5 | |
| 9 | 10.3 | 41.9 | 29.2 | |
| 2 | 3 | 6.5 | 43.3 | 25.9 |
| 4 | 6.5 | 43.0 | 26.9 | |
| 9 | 6.3 | 44.5 | 26.9 | |
| 3 | 3 | 7.3 | 44.7 | 26.2 |
| 4 | 7.5 | 43.8 | 26.5 | |
| 9 | 7.6 | 44.4 | 27.1 | |
| 4 | 3 | 9.1 | 42.9 | 26.9 |
| 4 | 9.0 | 41.2 | 28.7 | |
| 9 | 8.7 | 42.3 | 27.9 | |
| 5 | 3 | 7.2 | 45.8 | 27.9 |
| 4 | 7.0 | 45.0 | 28.4 | |
| 9 | 7.0 | 45.3 | 28.5 | |
| p value | 0.973 | 0.708 | 0.457 | |
Figure 2.
Sensitivity test showing the change in results using different number of sub-regions for all segments in one subject.
Variable Definitions and Statistical Analysis
Segment mass, COM, and frontal plane ROG were calculated for each segment, and lean mass proportion was calculated for trunk, leg, arm, and whole body using following equations (Ganley and Powers, 2004; Winter, 2009),
| (1) |
| (2) |
| (3) |
| (4) |
where m1 and li are the mass and centroid of each sub-region. Segment mass was expressed as a percent of body weight (%BW); COM was the longitudinal distance from the superior (trunk) or proximal (other segments) end to the COM location of the segment, expressed as a percent of segment length (%SL); ROG was also expressed as a percent of segment length (%SL). Dependent variables were grouped in the following seven categories: segment mass (kg), normalized segment mass expressed as a percentage of body weight (%BW), COM (%SL), ROG (%SL), total mass (kg), lean mass (kg), and lean mass proportion (%).
Statistical analyses were performed using SPSS software (Chicago, IL, USA). For males, a 2 × 2 multivariate analysis of variance (MANOVA) was performed with time since injury (acute versus chronic) and obesity (yes/no) as factors within each category. Post hoc t-tests with Bonferroni corrections were performed when appropriate. Due to the small number of females, obesity was not considered as a separate variable. Instead, all variables were compared using student's t-test between acute injury group and chronic injury group. Due to the heterogeneity of the SCI population, secondary comparisons were made between other subgroups within the sample population. Subgroups that were compared included: gender (male versus female), paralysis level (tetraplegia versus paraplegia), and wheelchair use (yes versus no). For each secondary analysis, ANOVA was used to compare the variables of segment mass, COM, and ROG between the two subgroups. An alpha criterion of 0.05 was used to assess significance.
Results
Subject Characteristics
Seventy-eight males and twenty-two females with SCI were included for final analysis. Subjects in the acute injury group were 1.0 ± 0.7 years post injury, and those in the chronic injury group were 14.8 ± 12.9 years post injury. Average BMI of non-obese and obese groups were 21.4 and 30.9 kg/m2, respectively (Table 3).
Table 3.
Subject characteristics: mean ± standard deviation.
| Acute (N=39) | Chronic (N=61) | |||
|---|---|---|---|---|
|
|
|
|||
| BMI < 25 (N=24) | BMI≥25 (N=15) | BMI < 25 (N=28 ) | BMI≥25 (N=33) | |
| Sex (female/male) | 4/20 | 3/12 | 7/21 | 8/25 |
| Age (years) | 38.0 ± 16.7 | 42.8 ± 13.9 | 39.5 ± 13.3 | 45.8 ± 13.9 |
| Years Since Injury (years) | 1.1 ± 0.8 | 0.9 ± 0.5 | 13.9 ± 13.7 | 15.6 ± 12.5 |
| Height (cm) | 175.9 ± 9.4 | 171.2 ± 12.4 | 175.2 ± 12.8 | 174.0 ± 11.2 |
| Mass (kg) | 67.5 ± 11.5 | 90.2 ± 17.2 | 66.9 ± 15.7 | 93.0 ± 17.9 |
| BMI (kg/m2) | 21.7 ± 2.4 | 30.9 ± 5.7 | 21.2 ± 2.7 | 30.9 ± 4.7 |
| Motor Complete | 12 | 4 | 20 | 22 |
| Wheelchair User | 17 | 12 | 27 | 21 |
| Tetraplegia | 18 | 12 | 15 | 19 |
The acute and chronic groups did not differ in terms of age (p = 0.309) or height (p = 0.832).
Effect of Obesity on Anthropometric Parameters in Males with SCI
MANOVA showed a main effect for obesity in all categories. Obese males had significantly greater normalized mass in the trunk (p = 0.001; Table 4) and lower lean mass proportion in all segments (p≤0.005) compared to non-obese males (Table 5). Males with BMI < 25 possessed more proximally located thigh COM, more distally located upper arm COM, greater thigh ROG, and smaller shank ROG than those with BMI≥25 (p≤0.019; Table 4).
Table 4.
Segment mass, center of mass (COM), radius of gyration (ROG) of segments of interest for male subjects with acute and chronic SCI. COM is expressed as the longitudinal distance from proximal (superior end of trunk) end to the COM location of each segment as a percentage of segment length. Mean ± SD are reported.
| Acute (< 3 years) | Chronic (≥3 years) | p-value (N=78) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Segment | BMI <25 (N=20) | BMI≥25 (N=12) | Total (N=32) | BMI <25 (N=21 ) | BMI≥25 (N=25) | Total (N=46) | Acute vs. Chronic | Obese vs. non-obese |
| Mass (kg) | ||||||||
|
|
||||||||
| Trunk | 33.0 ± 5.7 | 47.7 ± 9.9 | 38.5 ± 10.4 | 33.7 ± 5.1 | 52.0 ± 10.9 | 43.7 ± 12.6 | 0.258 | <0.001 |
| Thigh | 7.8 ± 1.4 | 10.7 ± 3.0 | 8.9 ± 2.5 | 7.7 ± 2.0 | 11.0 ± 2.7 | 9.5 ± 2.9 | 0.788 | <0.001 |
| Shank | 2.6 ± 0.4 | 3.2 ± 0.8 | 2.8 ± 0.7 | 2.5 ± 0.6 | 3.2 ± 0.7 | 2.9 ± 0.7 | 0.883 | <0.001 |
| Upper Arma | 2.5 ± 0.7 | 2.8 ± 0.6 | 2.6 ± 0.6 | 2.4 ± 0.6 | 3.6 ± 0.7 | 3.0 ± 0.9 | 0.004 | <0.001 |
| Forearma | 1.0 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.5 ± 0.3 | 1.3 ± 0.3 | 0.004 | <0.001 |
|
| ||||||||
| Mass (%Body Mass) | ||||||||
|
|
||||||||
| Trunk | 47.7 ± 2.7 | 50.6 ± 4.0 | 48.8 ± 3.5 | 50.7 ± 2.3 | 53.1 ± 3.6 | 52.0 ± 3.3 | <0.001 | 0.001 |
| Thigh | 11.6 ± 1.2 | 12.4 ± 1.1 | 11.9 ± 1.2 | 10.5 ± 1.4 | 10.8 ± 1.6 | 10.7 ± 1.5 | <0.001 | 0.111 |
| Shank | 3.8 ± 0.4 | 3.7 ± 0.4 | 3.8 ± 0.4 | 3.4 ± 0.3 | 3.2 ± 0.6 | 3.3 ± 0.5 | <0.001 | 0.059 |
| Upper Arm | 3.4 ± 0.7 | 3.0 ± 0.3 | 3.3 ± 0.6 | 3.7 ± 0.8 | 3.7 ± 0.9 | 3.7 ± 0.8 | 0.009 | 0.281 |
| Forearm | 1.5 ± 0.2 | 1.4 ± 0.1 | 1.4 ± 0.2 | 1.6 ± 0.4 | 1.4 ± 0.3 | 1.5 ± 0.3 | 0.094 | 0.073 |
|
| ||||||||
| Center of Mass (%Segment Length) | ||||||||
|
|
||||||||
| Trunk | 54.6 ± 1.7 | 55.2 ± 1.4 | 54.8 ± 1.6 | 54.9 ± 1.3 | 54.4 ± 1.6 | 54.6 ± 1.5 | 0.488 | 0.099 |
| Thigh | 42.5 ± 1.8 | 40.2 ± 2.7 | 41.6 ± 2.5 | 42.3 ± 2.5 | 40.8 ± 2.3 | 41.5 ± 2.5 | 0.677 | 0.001 |
| Shank | 43.4 ± 1.2 | 42.8 ± 0.9 | 43.2 ± 1.1 | 43.4 ± 1.3 | 43.8 ± 4.6 | 43.6 ± 3.5 | 0.432 | 0.862 |
| Upper Arm | 53.7 ± 1.8 | 56.3 ± 2.8 | 54.7 ± 2.5 | 53.6 ± 2.3 | 55.9 ± 3.3 | 54.8 ± 3.1 | 0.633 | <0.001 |
| Forearm | 43.2 ± 1.0 | 43.1 ± 1.0 | 43.2 ± 1.0 | 42.8 ± 1.8 | 42.9 ± 2.0 | 42.9 ± 1.9 | 0.462 | 0.978 |
|
| ||||||||
| Radius of Gyration (%Segment Length) | ||||||||
|
|
||||||||
| Trunk | 28.4 ± 0.7 | 28.0 ± 1.0 | 28.2 ± 0.8 | 28.0 ± 1.0 | 27.8 ± 1.1 | 27.9 ± 1.1 | 0.193 | 0.231 |
| Thigh | 27.8 ± 1.0 | 29.1 ± 2.1 | 28.3 ± 1.6 | 28.2 ± 1.0 | 29.0 ± 1.3 | 28.6 ± 1.2 | 0.676 | 0.001 |
| Shank | 24.2 ± 0.7 | 23.7 ± 0.8 | 24.0 ± 0.7 | 24.1 ± 0.6 | 23.7 ± 1.1 | 23.9 ± 0.9 | 0.654 | 0.019 |
| Upper Arm | 20.2 ± 1.0 | 19.8 ± 1.7 | 20.1 ± 1.3 | 20.5 ± 0.6 | 20.3 ± 1.8 | 20.4 ± 1.4 | 0.217 | 0.351 |
| Forearm | 24.2 ± 0.5 | 24.4 ± 0.9 | 24.2 ± 0.7 | 24.8 ± 0.9 | 24.5 ± 1.0 | 24.7 ± 1.0 | 0.062 | 0.952 |
Obesity*time interaction
Table 5.
Total mass, lean mass, and lean mass proportion as a percentage of total mass of arm, leg, trunk, and whole body for male subjects. Mean ± SD are reported.
| Acute (< 3 years) | Chronic (≥3 years) | p-value (N=78) | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Segment | BMI <25 (N=20) | BMI≥25 (N=12) | Total (N=32) | BMI <25 (N=21 ) | BMI≥25 (N=25) | Total (N=46) | Acute vs. Chronic | Obese vs. non-obese |
| Total Mass (kg) | ||||||||
|
|
||||||||
| Arm | 4.1 ± 1.1 | 4.8 ± 0.9 | 4.4 ± 1.1 | 4.4 ± 1.4 | 5.7 ± 1.8 | 5.1 ± 1.7 | 0.066 | 0.004 |
| Leg | 11.3 ± 2.2 | 14.2 ± 1.9 | 12.4 ± 2.5 | 10.3 ± 2.9 | 13.3 ± 3.9 | 11.9 ± 3.8 | 0.188 | <0.001 |
| Trunk | 33.0 ± 6.6 | 46.2 ± 10.9 | 38.0 ± 10.6 | 37.7 ± 8.8 | 51.3 ± 10.6 | 45.1 ± 11.9 | 0.027 | <0.001 |
| Whole Body | 69.7 ± 10.8 | 90.6 ± 15.5 | 77.5 ± 16.2 | 71.9 ± 13.8 | 97.3 ± 17.0 | 85.7 ± 20.0 | 0.194 | <0.001 |
|
| ||||||||
| Lean Mass (kg) | ||||||||
|
|
||||||||
| Arm | 2.9 ± 0.9 | 3.0 ± 0.5 | 2.9 ± 0.8 | 2.9 ± 1.1 | 3.6 ± 1.3 | 3.3 ± 1.3 | 0.219 | 0.196 |
| Leg | 7.6 ± 1.4 | 8.1 ± 1.8 | 7.8 ± 1.6 | 6.4 ± 1.7 | 7.8 ± 2.2 | 7.1 ± 2.0 | 0.086 | 0.032 |
| Trunk | 23.1 ± 3.8 | 26.3 ± 2.9 | 24.3 ± 3.8 | 23.7 ± 4.2 | 27.3 ± 4.8 | 25.7 ± 4.9 | 0.399 | 0.001 |
| Whole Body | 48.5 ± 6.3 | 54.2 ± 4.5 | 50.6 ± 6.3 | 47.6 ± 8.9 | 55.3 ± 8.6 | 51.8 ± 9.5 | 0.956 | <0.001 |
|
| ||||||||
| Lean Mass Proportion (%) | ||||||||
|
|
||||||||
| Arm | 70.2 ± 8.9 | 62.5 ± 6.6 | 67.3 ± 8.8 | 66.1 ± 12.5 | 61.3 ± 6.7 | 63.5 ± 10.0 | 0.224 | 0.005 |
| Leg | 67.9 ± 8.3 | 57.5 ± 13.8 | 64.0 ± 11.7 | 62.6 ± 6.5 | 58.9 ± 6.4 | 60.6 ± 6.6 | 0.335 | 0.001 |
| Trunk | 71.3 ± 11.9 | 58.6 ± 9.6 | 66.6 ± 12.5 | 64.4 ± 10.1 | 53.8 ± 6.8 | 58.6 ± 9.9 | 0.011 | <0.001 |
| Whole Body | 70.3 ± 8.7 | 60.9 ± 8.0 | 66.8 ± 9.5 | 66.9 ± 10.0 | 57.2 ± 5.3 | 61.6 ± 9.1 | 0.064 | <0.001 |
Effect of Injury Duration on Anthropometric Parameters after SCI
There was a main effect for time since injury on normalized segment mass (p < 0.001). Chronic SCI was associated with smaller trunk lean mass ratio among males (p = 0.011; Table 5). Females in the chronic group had greater normalized upper arm mass than those in the acute group by 0.40 %BW (p = 0.039; Table 6).
Table 6.
Anthropometric parameters of females with SCI. P-values refer to comparison between acute and chronic groups using student's t-test.
| Acute (N=7) | Chronic (N=15) | Total (N=22) | p-value (Acute vs. Chronic) | |
|---|---|---|---|---|
| Mass (kg) | ||||
|
|
||||
| Trunk | 35.1 ± 13.3 | 35.6 ± 11.3 | 35.4 ± 11.6 | 0.937 |
| Thigh | 8.1 ± 4.0 | 8.5 ± 2.5 | 8.4 ± 3.0 | 0.770 |
| Shank | 2.5 ± 1.2 | 2.3 ± 0.7 | 2.4 ± 0.9 | 0.628 |
| Upper Arm | 2.2 ± 0.9 | 2.3 ± 0.7 | 2.3 ± 0.7 | 0.748 |
| Forearm | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.9 ± 0.2 | 0.442 |
|
| ||||
| Mass (%Body Mass) | ||||
|
|
||||
| Trunk | 47.2 ± 4.0 | 50.0 ± 4.2 | 49.1 ± 4.2 | 0.157 |
| Thigh | 11.6 ± 2.7 | 12.4 ± 1.3 | 12.1 ± 1.8 | 0.457 |
| Shank | 3.7 ± 1.1 | 3.4 ± 0.5 | 3.5 ± 0.8 | 0.340 |
| Upper Arm | 2.9 ± 0.4 | 3.3 ± 0.5 | 3.2 ± 0.5 | 0.039 |
| Forearm | 1.2 ± 0.2 | 1.3 ± 0.2 | 1.3 ± 0.2 | 0.128 |
|
| ||||
| Center of Mass (%Segment Length) | ||||
|
|
||||
| Trunk | 55.2 ± 2.3 | 55.2 ± 1.5 | 55.2 ± 1.8 | 0.985 |
| Thigh | 40.3 ± 2.6 | 38.7 ± 3.5 | 39.2 ± 3.3 | 0.284 |
| Shank | 42.0 ± 1.8 | 42.8 ± 1.7 | 42.6 ± 1.7 | 0.324 |
| Upper Arm | 53.2 ± 7.9 | 55.4 ± 2.9 | 54.7 ± 4.9 | 0.342 |
| Forearm | 43.4 ± 0.9 | 43.0 ± 2.7 | 43.1 ± 2.3 | 0.698 |
|
| ||||
| Radius of Gyration (%Segment Length) | ||||
|
|
||||
| Trunk | 28.1 ± 1.3 | 27.9 ± 1.5 | 28.0 ± 1.4 | 0.732 |
| Thigh | 29.1 ± 0.8 | 30.0 ± 1.9 | 29.7 ± 1.7 | 0.244 |
| Shank | 23.2 ± 1.0 | 23.7 ± 0.8 | 23.5 ± 0.9 | 0.290 |
| Upper Arm | 20.9 ± 2.8 | 20.0 ± 1.1 | 20.3 ± 1.8 | 0.284 |
| Forearm | 24.5 ± 0.8 | 24.6 ± 1.0 | 24.6 ± 0.9 | 0.936 |
|
| ||||
| Total Mass (kg) | ||||
|
|
||||
| Arm | 2.9 ± 0.9 | 3.5 ± 1.0 | 3.3 ± 1.0 | 0.188 |
| Leg | 12.1 ± 4.8 | 10.7 ± 3.0 | 11.1 ± 3.6 | 0.389 |
| Trunk | 32.9 ± 13.0 | 33.5 ± 12.0 | 33.3 ± 12.0 | 0.925 |
| Whole Bodyb | 70.4 ± 24.3 | 66.6 ± 18.8 | 67.8 ± 20.2 | 0.693 |
|
| ||||
| Muscle Mass (kg) | ||||
|
|
||||
| Arm | 1.7 ± 0.4 | 1.9 ± 0.5 | 1.8 ± 0.5 | 0.306 |
| Leg | 6.6 ± 1.6 | 5.2 ± 1.5 | 5.7 ± 1.6 | 0.054 |
| Trunk | 20.2 ± 4.3 | 17.4 ± 4.3 | 18.3 ± 4.4 | 0.176 |
| Whole Body | 42.0 ± 6.6 | 35.3 ± 7.8 | 37.4 ± 8.0 | 0.062 |
|
| ||||
| Muscle Mass Proportion (%) | ||||
|
|
||||
| Arm | 59.0 ± 12.0 | 54.8 ± 6.3 | 56.1 ± 8.5 | 0.284 |
| Leg | 57.2 ± 7.9 | 49.8 ± 8.6 | 52.2 ± 9.0 | 0.069 |
| Trunk | 65.6 ± 16.1 | 55.2 ± 11.8 | 58.5 ± 13.8 | 0.104 |
| Whole Body | 62.7 ± 11.1 | 54.5 ± 8.3 | 57.1 ± 9.8 | 0.066 |
Effect of Gender, Paralysis Level, and Wheelchair use on Anthropometric Parameters after SCI
A significant gender effect was found in most parameters. Males had normalized mass that was 5.0 % greater in the trunk, 11.6% greater in the upper arm, and 19.3% greater in the forearm compared to females (p≤0.032). Similarly, muscle mass proportion in males was 13.5% greater in the arm and 16.6% greater in the leg (p = 0.008 and p = 0.002, respectively). Males possessed 7.4% more distally located thigh COM, with 4.8% smaller thigh ROG versus females (p < 0.001, and p = 0.001, respectively), and their shank ROG was 2.2% greater than that of females (p = 0.017).
For people with different paralysis levels, most parameters were not significantly different except for upper body mass and shank ROG. Those with tetraplegia had 17.8 % smaller normalized upper arm and 12.5 % smaller normalized forearm mass compared to those with paraplegia (p < 0.001, and p < 0.001, respectively). Shank ROG was also smaller by 0.3% BW (p = 0.012) in individuals with tetraplegia.
People who used wheelchairs had significantly greater normalized shank mass by 4.7 %BW (p = 0.002) and greater trunk lean mass ratio by 7.2 % (p = 0.036) than those who did not use a wheelchair.
Discussion
The aims of this study were to quantify anthropometric parameters in people with SCI and to investigate the impact of injury duration and obesity on all parameters. Our results support the hypothesis that mass distribution and tissue composition vary over time after the injury and are significantly affected by obesity. Compared to acute SCI, chronic injury is associated with a shift in mass proportion from lower limbs to the trunk and upper arms, and decreased lean mass proportion, especially in the trunk. Obesity strongly affects the anthropometric characteristics of all males with SCI and is associated with a higher proportion of the body mass located in the trunk and away from the distal limbs. This is associated with a proximal shift in COM and increased ROG at the thigh, a distal shift in upper arm COM, and a decreased ROG at the shank. These changes show similar trends to the anthropometric characteristics of an able-bodied obese population (Chambers et al., 2010; Matrangola et al., 2008).
Overall, chronic injury is associated with significant decreases in lean mass proportion throughout the body. Males in the chronic phase had greater normalized mass in the upper body than those in the acute phase. Additionally, lean mass proportion was smaller by 8.0 %BW in the trunk and 3.8 %BW in the arm among people with chronic SCI compared to those with acute SCI. This indicates either fat accumulation or muscle loss in the long term post injury. In the present cohort of females, the chronic injury group had similar trunk total mass to the acute group, but less lean mass, suggesting that at this site females may become fatter, but not heavier, with increased injury duration. However, the number of subjects included in this comparison was small, and further research is needed. Consistent with our data, others have reported higher fat proportion in the trunk and waist region in people with SCI compared to able-bodied controls (Beck et al., 2014; Emmons et al., 2011), and the difference was greater for those with motor complete vs. motor incomplete injury, and in those with tetraplegia vs. paraplegia (Spungen et al., 2003). Among individuals with SCI, trunk mass proportion was higher for those who are obese. Mass accumulation in the trunk causes central obesity, increasing the risk of coronary heart disease. However, it does not necessarily lead to a high BMI value. Studies have shown that individuals with SCI who had central obesity were not recognized as obese based on BMI standard for the general population (Sabour et al., 2011). Therefore, waist circumference and an SCI-specific BMI value of 25 were suggested to be used to identify obese for individuals with SCI (Buchholz and Bugaresti, 2005; Laughton et al., 2009).
Compared to the acute injury group, we observed higher absolute and normalized mass in the upper extremities in the chronic injury group. Interestingly, although lean mass proportion decreased over time after the injury, absolute lean mass was similar in the chronic group, and was increased in the obese subset. Similar to at the trunk, this suggests increased fat accumulation in the arms with longer injury duration. It is likely that exercise from daily activities and rehabilitation contributes to maintenance of lean tissue mass in the arms. Others have shown that people with paraplegia have greater arm lean tissue and forearm extensor cross-sectional area than those with tetraplegia and able-bodied individuals. They suggested that manual wheelchair propulsion and transfer using arms can offset muscle loss (Gorgey et al., 2014; Yarar-Fisher et al., 2013). This is further confirmed by our data; when subjects were analyzed by injury level, those with paraplegia had greater normalized mass and lean proportion in the arms than those with tetraplegia. Additionally, people who used a wheelchair had significantly greater normalized mass and lean mass proportion in the trunk. Unfortunately, our database did not specify the type of wheelchair used, although it is likely that most individuals with paraplegia used manual wheelchairs and would consequently have greater lean mass in their upper limbs and trunk.
Spinal cord injury is followed by well documented muscle atrophy and bone loss in the lower limbs after paralysis (Giangregorio and McCartney, 2006; Wilmet et al., 1995). Our data are consistent with this understanding, demonstrating a smaller normalized mass and lean mass proportion in the legs in the chronic group. Absolute mass and lean mass are higher, but lean mass proportion is lower among subjects who are obese, indicating greater muscle and fat in the legs among obese subjects. Because a greater portion of the tissue in the legs is located proximally, we expected that obesity-related gain of muscle and fat would increase the proximal mass within the thigh and shank, shifting the COM proximally. This was supported by the data in the thigh as people in the obese group had a more proximally located thigh COM. A similar finding was observed in the able-bodied population (Chambers et al., 2010; Matrangola et al., 2008).
Our study had several limitations. We did not calculate parameters for the head, hands, and feet, therefore we were not able to verify that the sum of the normalized segment masses equals 100% of body mass. However, using published able-bodied mass proportions for the hands (0.6%), feet (1.3%), and head (7.7%) (Durkin and Dowling, 2003), plus the summed mass for our studied segments (88.9 %BW), our average summed mass would be 100.4 %BW, suggesting that our measured segment masses are realistic. As an additional verification, we compared the subset of our present cohort (n=15) who received a DXA scan within six months of becoming spinal cord injured with published able-bodied data also measured with DXA (Chambers et al., 2010). Most of the variables were within 1 %BW or 1 %SL between the two groups, although the difference in upper arm parameters was nearly 5%. This can be attributed to slightly different segment definitions, combined with a high rate of obesity in the SCI group, which is associated with fat accumulation around and under the shoulders and upper arms. DXA scans are performed with subjects lying supine, which may cause soft tissue deformation and can slightly affect COM and ROG calculations, especially at the upper arm. Additionally, our validated methods utilized fewer sub-regions to define each segment than other studies, although our sensitivity analyses indicated that this did not significantly influence the results.
Although ROG was calculated in the frontal plane only, it is commonly assumed that the lower and upper limbs are circular in cross-section, making sagittal and frontal plane ROG equivalent. For the trunk, ROG was calculated considering the trunk as an elliptical cylinder with the depth equals to 70% of its width (Marras et al., 2001), and the difference between frontal plane and sagittal plane values was within 0.1% of the trunk length. Therefore trunk ROG in the frontal plane can be used for the sagittal plane. The small number of SCI females in the current study limited our ability to examine the effect of obesity in females, however, it is not substantially different from the 4:1 male/female ratio reported in the general SCI population (National SCI Statistical Center, 2015). Finally, the patients included in this study were all from a single rehabilitation hospital location in a large urban area, and may not represent individuals with SCI in other settings.
In summary, we have calculated anthropometric parameters for individuals with acute and chronic spinal cord injury and have examined the effect of obesity on these parameters. In general, people with chronic SCI carry a lower proportion of their mass in the lower limbs, and a greater proportion in the trunk and upper limbs. This is accompanied by increases in trunk fat, particularly in obese subjects. SCI-related muscle loss and increases in fat were observed in most body parts after the injury. Small magnitude differences in inverse dynamics calculations have been shown to be clinically important in other contexts such as knee OA (Kaufman, 2001), emphasizing the need for accurate anthropometric models. The data presented here can be used to accurately represent the anthropometrics of SCI population considering obesity and injury phase.
Acknowledgments
This study received support from the Department of Defense (W81XWH-10-1-1043), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (1R01AR059270-01), and the Department of Education, National Institute on Disability and Rehabilitation Research (H133N110010).
Footnotes
Conflict of Interest Disclosure None of the authors have any real or apparent conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck LA, Lamb JL, Atkinson EJ, Wuermser LA, Amin S. Body composition of women and men with complete motor paraplegia. The journal of spinal cord medicine. 2014;37:359–365. doi: 10.1179/2045772313Y.0000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal cord. 2005;43:513–518. doi: 10.1038/sj.sc.3101744. [DOI] [PubMed] [Google Scholar]
- Chambers AJ, Sukits AL, McCrory JL, Cham R. The effect of obesity and gender on body segment parameters in older adults. Clin Biomech (Bristol, Avon) 2010;25:131–136. doi: 10.1016/j.clinbiomech.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leva P. Adjustments to Zatsiorsky-Seluyanov's segment inertia parameters. Journal of biomechanics. 1996;29:1223–1230. doi: 10.1016/0021-9290(95)00178-6. [DOI] [PubMed] [Google Scholar]
- Dempster WT. Space requirements of the seated operator: geometrical, kinematic, and mechanical aspects of the body, with special reference to the limbs. WADC Technical Report. 1955:55–159. [Google Scholar]
- Durkin JL, Dowling JJ. Analysis of body segment parameter differences between four human populations and the estimation errors of four popular mathematical models. Journal of biomechanical engineering. 2003;125:515–522. doi: 10.1115/1.1590359. [DOI] [PubMed] [Google Scholar]
- Emmons RR, Garber CE, Cirnigliaro CM, Kirshblum SC, Spungen AM, Bauman WA. Assessment of measures for abdominal adiposity in persons with spinal cord injury. Ultrasound in medicine & biology. 2011;37:734–741. doi: 10.1016/j.ultrasmedbio.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Ganley KJ, Powers CM. Anthropometric parameters in children: a comparison of values obtained from dual energy x-ray absorptiometry and cadaver-based estimates. Gait & posture. 2004;19:133–140. doi: 10.1016/S0966-6362(03)00038-9. [DOI] [PubMed] [Google Scholar]
- Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. The journal of spinal cord medicine. 2006;29:489–500. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangregorio LM, Hicks AL, Webber CE, Phillips SM, Craven BC, Bugaresti JM, McCartney N. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal cord. 2005;43:649–657. doi: 10.1038/sj.sc.3101774. [DOI] [PubMed] [Google Scholar]
- Goldberg EJ, Requejo PS, Fowler EG. The effect of direct measurement versus cadaver estimates of anthropometry in the calculation of joint moments during above-knee prosthetic gait in pediatrics. Journal of biomechanics. 2008;41:695–700. doi: 10.1016/j.jbiomech.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Gorgey AS, Timmons MK, Michener LA, Ericksen JJ, Gater DR. Intra-rater reliability of ultrasound imaging of wrist extensor muscles in patients with tetraplegia. PM & R : the journal of injury, function, and rehabilitation. 2014;6:127–133. doi: 10.1016/j.pmrj.2013.08.607. [DOI] [PubMed] [Google Scholar]
- Hammond ER, Metcalf HM, McDonald JW, Sadowsky CL. Bone mass in individuals with chronic spinal cord injury: associations with activity-based therapy, neurologic and functional status, a retrospective study. Archives of physical medicine and rehabilitation. 2014;95:2342–2349. doi: 10.1016/j.apmr.2014.07.395. [DOI] [PubMed] [Google Scholar]
- Hartkopp A, Murphy RJ, Mohr T, Kjaer M, Biering–Sorensen F. Bone fracture during electrical stimulation of the quadriceps in a spinal cord injured subject. Archives of physical medicine and rehabilitation. 1998;79:1133–1136. doi: 10.1016/s0003-9993(98)90184-8. [DOI] [PubMed] [Google Scholar]
- Jensen RK. Body segment mass, radius and radius of gyration proportions of children. Journal of biomechanics. 1986;19:359–368. doi: 10.1016/0021-9290(86)90012-6. [DOI] [PubMed] [Google Scholar]
- Kaufman Kenton R, et al. Gait characteristics of patients with knee osteoarthritis. Journal of biomechanics. 2001;34(7):907–915. doi: 10.1016/s0021-9290(01)00036-7. [DOI] [PubMed] [Google Scholar]
- Lauer RT, Smith BT, Mulcahey MJ, Betz RR, Johnston TE. Effects of cycling and/or electrical stimulation on bone mineral density in children with spinal cord injury. Spinal cord. 2011;49:917–923. doi: 10.1038/sc.2011.19. [DOI] [PubMed] [Google Scholar]
- Laughton GE, Buchholz AC, Martin Ginis KA, Goy RE. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal cord. 2009;47:757–762. doi: 10.1038/sc.2009.33. [DOI] [PubMed] [Google Scholar]
- Marras WS, Jorgensen MJ, Granata KP, Wiand B. Female and male trunk geometry: size and prediction of the spine loading trunk muscles derived from MRI. Clin Biomech (Bristol, Avon) 2001;16:38–46. doi: 10.1016/s0268-0033(00)00046-2. [DOI] [PubMed] [Google Scholar]
- Matrangola SL, Madigan ML, Nussbaum MA, Ross R, Davy KP. Changes in body segment inertial parameters of obese individuals with weight loss. Journal of biomechanics. 2008;41:3278–3281. doi: 10.1016/j.jbiomech.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse LR, Battaglino RA, Stolzmann KL, Hallett LD, Waddimba A, Gagnon D, Lazzari AA, Garshick E. Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20:385–392. doi: 10.1007/s00198-008-0671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muri J, Winter SL, Challis JH. Changes in segmental inertial properties with age. Journal of biomechanics. 2008;41:1809–1812. doi: 10.1016/j.jbiomech.2008.03.002. [DOI] [PubMed] [Google Scholar]
- National SCI Statistical Center. Spinal cord injury facts and figures at a glance. The journal of spinal cord medicine. 2015;37:659–660. doi: 10.1179/1079026814Z.000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panisset MG, Galea MP, El-Ansary D. Does early exercise attenuate muscle atrophy or bone loss after spinal cord injury? Spinal cord. 2015 doi: 10.1038/sc.2015.150. [DOI] [PubMed] [Google Scholar]
- Pelletier CA, Miyatani M, Giangregorio L, Craven BC. Sarcopenic Obesity in Adults with Chronic Spinal Cord Injury: A Cross-Sectional Study. Archives of physical medicine and rehabilitation. 2016 doi: 10.1016/j.apmr.2016.04.026. [DOI] [PubMed] [Google Scholar]
- Rao G, Amarantini D, Berton E, Favier D. Influence of body segments' parameters estimation models on inverse dynamics solutions during gait. Journal of biomechanics. 2006;39:1531–1536. doi: 10.1016/j.jbiomech.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Sabour H, Javidan AN, Vafa MR, Shidfar F, Nazari M, Saberi H, Rahimi A, Razavi HE. Obesity predictors in people with chronic spinal cord injury: an analysis by injury related variables. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences. 2011;16:335–339. [PMC free article] [PubMed] [Google Scholar]
- Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Jr, Waters RL, Bauman WA. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol (1985) 2003;95:2398–2407. doi: 10.1152/japplphysiol.00729.2002. [DOI] [PubMed] [Google Scholar]
- Tan CO, Battaglino RA, Morse LR. Spinal Cord Injury and Osteoporosis: Causes, Mechanisms, and Rehabilitation Strategies. International journal of physical medicine & rehabilitation. 2013:1. [PMC free article] [PubMed] [Google Scholar]
- Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33:674–677. doi: 10.1038/sc.1995.141. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanics and motor control of human movement. John Wiley & Sons; 2009. [Google Scholar]
- Yarar-Fisher C, Chen Y, Jackson AB, Hunter GR. Body mass index underestimates adiposity in women with spinal cord injury. Obesity (Silver Spring) 2013;21:1223–1225. doi: 10.1002/oby.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]