Abstract
Cardiac arrhythmias associated with intracellular calcium inhomeostasis are refractory to antiarrhythmic therapy. We hypothesized that late sodium current (I Na) contributed to the calcium-related arrhythmias. Monophasic action potential duration at 90% completion of repolarization (MAPD90) was significantly increased and ventricular arrhythmias were observed in hearts with increased intracellular calcium concentration ([Ca2+]i) by using Bay K 8644, and the increase became greater in hearts treated with a combination of ATX-II and Bay K 8644 compared to Bay K 8644 alone. The prolongations caused by Bay K 8644 and frequent episodes of ventricular tachycardias, both in absence and presence of ATX-II, were significantly attenuated or abolished by late I Na inhibitors TTX and eleclazine. In rabbit ventricular myocytes, Bay K 8644 increased I CaL density, calcium transient and myocyte contraction. TTX and eleclazine decreased the amplitude of late I Na, the reverse use dependence of MAPD90 at slower heart rate, and attenuated the increase of intracellular calcium transient and myocyte contraction. TTX diminished the phosphorylation of CaMKII-δ and Nav 1.5 in hearts treated with Bay K 8644 and ATX-II. In conclusion, late I Na contributes to ventricular arrhythmias and its inhibition is plausible to treat arrhythmias in hearts with increased [Ca2+]i
Introduction
An increase in intracellular calcium concentration ([Ca2+]i), i.e. calcium overload, is commonly seen in many pathological and pharmacological conditions, including tachycardia, acquired heart diseases (heart failure, ischemia/reperfusion, cardiomyopathy)1–3, inherited calcium channelopathies (long QT syndrome 8 and catecholaminergic polymorphic ventricular tachycardia, CPVT)4, 5, and post-administration of digitalis and catecholaminergic agents, etc6, 7. Increased [Ca2+]i-related diseases are characterized by distinctive cardiac electrical and mechanistic manifestations, including life-threatening ventricular arrhythmias which are difficult to evaluate and lack effective treatments8, 9. Recent clinical reports indicate that the late sodium current (late I Na) inhibitors ranolazine and mexiletine are effective, at least in part, in preventing or treating increased [Ca2+]i-associated cardiac arrhythmias, including LQTs 8 and myocardial hypertrophy10, 11, etc. The mechanisms underlying these treatments remain to be investigated.
Our previous study indicated that endogenous late I Na contributed to ventricular arrhythmias associated with bradycardia and administration of QT prolonging drugs that inhibits rapidly activated delayed rectifier K+ current (I Kr)12–14. Late I Na was increased in cardiomyocytes when [Ca2+]i was high via the subsequent activation of Ca2+/calmodulin dependent protein kinase (CaMK)II and protein kinase C (PKC) pathways, and the resulting increase in intracellular sodium concentration would further elevate [Ca2+]i via reversed sodium-calcium exchange15. This positive vicious cycle may enhance the proarrhythmic characteristics of increased [Ca2+]i in the heart. (S)-(-)-Bay K 8644 (Bay K 8644), a L-type Ca2+ channel agonist, accelerated Ca2+ current activation/inactivation kinetics and Ca2+ peak current amplitude by twofold16. In addition, sea anemone toxin (ATX)-II at concentrations of 1–3 nM increased late I Na, and while it did not cause any ventricular arrhythmias itself, it did potentiate the risk of proarrhythmic drugs in rabbit isolated hearts17.
In this study, we hypothesized that endogenous and enhanced late I Na might contribute to the intracellular Ca2+ overload-associated cardiac arrhythmias and myocardial dysfunction in a synergistic mode in association with upregulation of phosphorylation of CaMK-II and sodium channel. This effect is enhanced by the self-promoting effects of the previously described positive feedback cycle for late I Na. Increases in [Ca2+]i and late I Na were artificially achieved by infusions of Bay K 8644 and ATX-II, respectively18. The results of this study may prove helpful in explaining the mechanisms by which sodium channel inhibitors (mexiletine and flecainide) are effective in treating patients with LQTs 8 and CPVT. We speculate that selective late I Na inhibitors will be useful for preventing or treating cardiac arrhythmias associated with increased [Ca2+]i, as well as for cardiac dysfunction in clinical settings. Tetrodotoxin (TTX), at a concentration of 1 µM, has been reported to inhibit late I Na with minimal inhibition on peak I Na 19. Eleclazine (GS-6615), a novel selective late I Na inhibitor, has been shown to be potentially effective in treating arrhythmias in patients with LQTs 3 and other kinds of arrhythmias20. Therefore, TTX and eleclazine were used in this study to determine the contribution of late I Na.
Results
Prolongation of left ventricular monophasic action potential duration at 90% completion of repolarization (MAPD90) by Bay K 8644 was concentration-dependent, and was greater in hearts treated with ATX-II
In hearts paced at a cycle length (CL) of 1000 ms, left ventricular epicardial (epi-) and endocardial (endo-) MAPD90 were 173.8 ± 4.1 and 199.7 ± 5.1 ms, respectively. Bay K 8644 (10–300 nM, n = 11) increased both the epi- and endo-cardial MAPD90 in concentration-dependent manners by 42 ± 4 and 50 ± 5 ms, respectively.
In hearts with increased late I Na, i.e., after ATX-II administration, 3 nM ATX-II prolonged epi- and endo-MAPD90 by 68.5 ± 9.2 and 91.7 ± 11.8 ms, respectively (n = 7, Fig. 1(c) and (d)). In the continuous presence of 3 nM ATX-II, Bay K 8644 (10–200 nM) prolonged MAPD90 in concentration-dependent manners, and the increase of both epi- and endo- MAPD90 by Bay K 8644 (200 nM) were significantly greater in hearts treated with a combination of 3 nM ATX-II (above the value of ATX-II alone, equal to 70.4 ± 8.4 and 90.0 ± 9.4 ms, resp.), than that in hearts treated with Bay K 8644 alone (above the value of control, equal to 29.2 ± 3.3 and 37.3 ± 3.9 ms, resp. P <0.05) (Fig. 1(c) and (d)). Consequently, the slope of the concentration response curve for Bay K 8644 to increase MAPD90 was greater in the presence than in the absence of ATX-II (Fig. 1(c) and (d)). Representative AP traces in groups of control, ATX-II (3 nM), Bay K 8644 (200 nM), and ATX-II (3 nM) plus Bay K 8644 (200 nM) were shown in Fig. 1(a) and (b).
Figure 1.
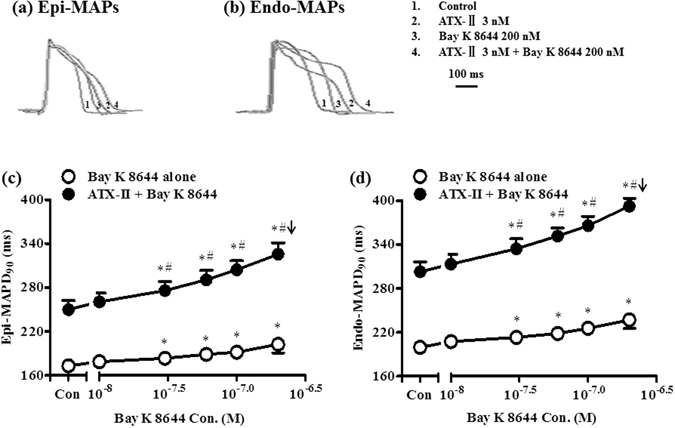
Prolongation of left ventricular monophasic action potential duration (MAPD90) by Bay K 8644 in the absence and presence of ATX-II. (a) and (b) Representative recordings of MAPs recorded from the epi- (a) and endo- (b) myocardium of the left ventricular wall in serially exposed to no drug (control), ATX-II (3 nM), Bay K 8644 (200 nM), and ATX-II (3 nM) plus Bay K 8644 (200 nM). (c) and (d) Concentration-dependent increases by Bay K 8644 of left ventricular epi- (c) and endo- (d) MAPD90 in absence (n = 11) and presence (n = 7) of ATX-II. * P < 0.05 compared with 0 nM Bay K 8644 either in absence or presence of ATX-II; # P < 0.05, the increase of MAPD90 by Bay K 8644 alone vs ATX-II plus Bay K 8644 at the same concentration. Arrows indicate ventricular tachycardia (VT) occurred at or above the concentration indicated.
Inhibition of late INa by TTX and eleclazine reduced the prolongation of MAPD90 by Bay K 8644 both in absence and presence of ATX-II
In hearts treated with Bay K 8644 alone (300 nM), TTX (at concentrations of 0.3, 0.6 and 1 µM) substantially reduced the increases of epi- and endo- MAPD90 (Fig. 2(b)). The reduction of epi- and endo- MAPD90 by 1 µM TTX were 24.1 ± 7.6 and 26.3 ± 1.9 ms, respectively (Fig. 2(b)). In hearts treated with both ATX-II (3 nM) and Bay K 8644 (200 nM), TTX (1 µM) caused significantly greater reduction in the prolongation of epi- and endo- MAPD90, with decreases of 61.7 ± 6.8 and 83.2 ± 10.1 ms, respectively (Fig. 2(c)). Similar to TTX, eleclazine also significantly decreased Bay K 8644-induced prolongation of epi- and endo- MAPD90 both in absence and presence of ATX-II (Fig. 2(c)). Compared with Bay K 8644 alone, the reduction of epi- and endo- MAPD90 by 10 µM eleclazine were 15.9 ± 2.6 and 23.7 ± 2.4 ms, resp. (Fig. 2(c), P < 0.05 vs. Bay K 8644 alone, n = 6). In the presence of ATX-II, the reduction of epi- and endo- MAPD90 by eleclazine were 64.3 ± 7.6 and 104.1 ± 3.6 ms, respectively (Fig. 2(c), P < 0.05 vs. ATX-II + Bay K 8644, n = 8). In addition, ATX-II plus Bay K 8644 significantly enhanced the transmural dispersion of MAPD90 (Δ Endo-Epi MAPD90), which was also reversed by TTX and eleclazine (Fig. 2(c)). Representative AP traces were shown in Fig. 2(a).
Figure 2.
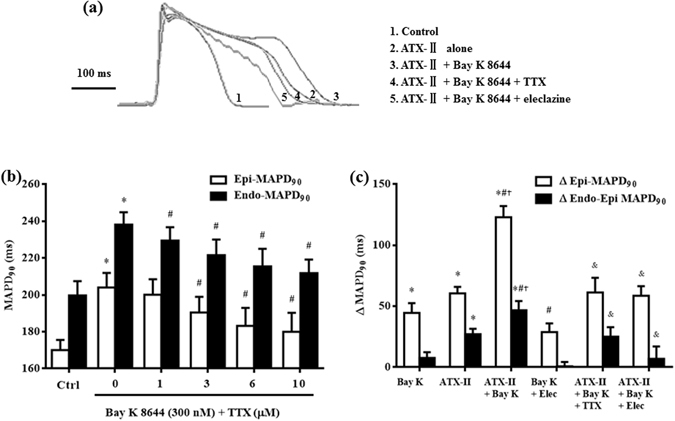
Reduction of MAPD90 prolongation by Tetrodotoxin (TTX) and eleclazine (Elec) in hearts treated with Bay K 8644 alone and ATX-II plus Bay K 8644. (a) Representative recordings of MAPs recorded from the epicardium of the left ventricular wall in serially exposed to no drug (control, curve 1), ATX-II (3 nM, curve 2), ATX-II plus Bay K 8644 (200 nM, curve 3), ATX-II plus Bay K 8644 plus TTX (1 µM, curve 4), and ATX-II plus Bay K 8644 plus eleclazine (10 µM, curve 5). (b) Concentration-dependent decreases by TTX of epi-MAPD90 and endo-MAPD90 in the presence of 300 nM Bay K 8644 (n = 7). (c) Effect of TTX (1 µM) and eleclazine (10 µM) on the increase of Δ epi-MAPD90 and Δ endo-epi MAPD90 induced by Bay K 8644 in absence and presence of ATX-II. * P < 0.05 vs. control (ctrl); # P < 0.05 vs. Bay K 8644; † P < 0.05 vs. ATX-II; & P < 0.05 vs. ATX-II plus Bay K 8644.
Inhibition of late INa by TTX abolished Bay K 8644-induced ventricular arrhythmias both in absence and in presence of ATX-II
Early after depolarization (EAD), extra-ventricular beats (EVBs), and ventricular tachycardia (VT) were not observed in any heart at control condition. Bay K 8644 alone caused EVBs in 10 out of 22 hearts (45.5%, Fig. 3(a) and (c)) and episodes of VT in 3 out of 22 hearts (13.6%, Fig. 3(c)) at a concentration of 200 nM, and frequent EVBs in 20 out of 22 hearts (90.9%) and episodes of VT in 6 out of 22 hearts (27.3%) at a concentration of 300 nM (Fig. 3(a) and (c)). In contrast, in the presence of 3 nM ATX-II, Bay K 8644 (200 nM) caused frequent EVBs in 14 out of 17 hearts (82.4%, Fig. 3(b) and (c)), and polymorphic VT in 13 out of 14 hearts (76.5%, Fig. 3(b) and (c)). These ventricular arrhythmias, including EVBs, and VT, but not ventricular fibrillation in 4 hearts, evoked by either Bay K 8644 alone or ATX-II + Bay K 8644, were attenuated and/or abolished by either 1 µM TTX (n = 14 for Bay K 8644 + TTX, and n = 9 for ATX-II + Bay K 8644 + TTX) or 10 µM eleclazine (n = 8 for Bay K 8644 + eleclazine, and n = 8 for ATX-II + Bay K 8644 + eleclazine) (Fig. 3(c)).
Figure 3.
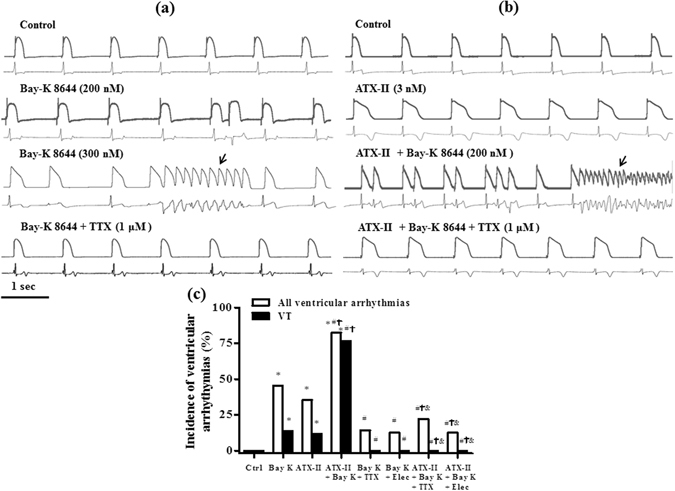
TTX and eleclazine (Elec) abolished ventricular arrhythmias induced by Bay K 8644 both in absence (a) and presence (b) of ATX-II. Representative recordings of MAPs (upper records in each panel) and ECG (lower records in each panel) were recorded simultaneously in control, Bay K 8644 (200 and 300 nM, a) or ATX-II-treated hearts (b) before and after treatment with TTX. Arrows indicate an episode of VT. The incidence of all ventricular arrhythmias and VT are presented in panel c. * P < 0.05 vs. ctrl; # P < 0.05 vs. Bay K 8644; † P < 0.05 vs. ATX-II; & P < 0.05 vs. ATX-II plus Bay K 8644.
TTX and eleclazine decreased the reverse use dependence (RUD) of Bay K 8644 on left ventricular MAPD90, and abolished Bay K 8644-induced ventricular arrhythmias at longer CL
In the absence of drug (control), MAPD90 of the left ventricular epicardium (epi-) MAPD90 at CL of 400 ms was 150.8 ± 2.3 ms. As the pacing CL was prolonged to 500, 667, 1000, 1333 and 2000 ms, epi-MAPD90 was significantly increased in an RUD manner by 10.1 ± 1.0, 27.4 ± 1.3, 39.7 ± 2.3, 49.5 ± 2.3 and 58.4 ± 4.1 ms to 160.9 ± 3.2, 178.2 ± 3.3, 190.5 ± 4.1, 200.3 ± 3.7 and 209.2 ± 5.2 ms, respectively (n = 11, P <0.05, Figs 4(a) and Supplementary Fig. S1).
Figure 4.
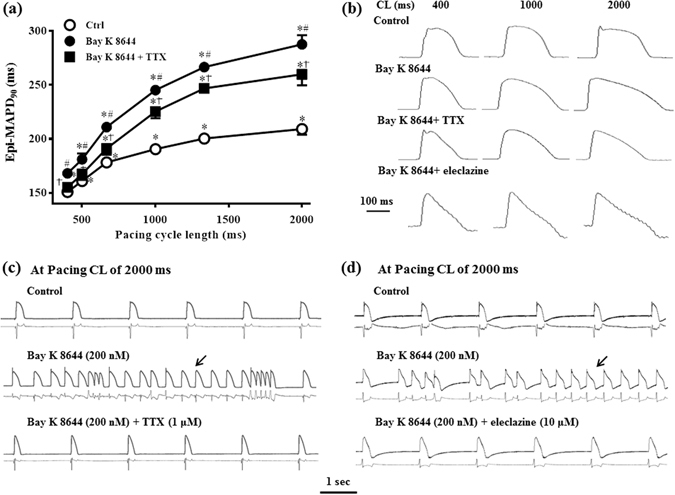
Effects of TTX and eleclazine on the reverse use dependence (RUD) of MAPD90 caused by Bay K 8644. (a) Values of MAPD90 were measured in the absence (Ctrl) and presence of Bay K 8644 (200 nM), Bay K 8644 (200 nM) plus TTX (n = 11). * P < 0.05 vs. cycle length (CL) of 400 ms; # P < 0.05 vs. Ctrl at the same CL; † P < 0.05 vs. Bay K 8644 alone at the same CL. (b) Representative AP traces obtained when hearts were paced at CLs of 400 ms, 1000 ms and 2000 ms in control, Bay K 8644 alone, Bay K 8644 plus TTX, and Bay K 8644 plus eleclazine. (c) and (d) Representative recordings of MAPs (upper records in each panel) and ECG (lower records in each panel) were recorded at pacing CL of 2000 ms in control, Bay K 8644 (200 nM), Bay K 8644 + TTX (1 µM), and Bay K 8644 + eleclazine (10 µM). Arrows indicate an episode of VT.
In hearts treated with Bay K 8644 (200 nM), the prolongation of epi-MAPD90 were increased at all stimulation rates (n = 11, P < 0.05, Fig. 4(a)), and the increase was greater at longer CLs (e.g., 78.5 ± 5.5 ms at a CL of 2000 ms) than at shorter CLs (17.3 ± 2.3 ms at a CL of 400 ms, P < 0.05).
In the continued presence of Bay K 8644 in same group of hearts, late I Na inhibitors TTX and eleclazine significantly attenuated the RUD of epi-MAPD90, especially at longer CLs (n = 11 and 5, P < 0.05, Fig. 4(a) and Supplementary Fig. S1, representative AP traces were shown in Fig. 4(b)).
EADs, EVBs, and VT were not observed in all hearts at shorter CLs of 400, 500, and 667 ms in absence and presence of Bay K 8644. At CLs of 1000, and 1333 ms, Bay K 8644 (200 nM) only caused EVBs and a few episodes of VT. In contrast, frequent EVBs and episodes of VT in 10 out of 13 hearts (76.9%) were induced by Bay K 8644 at a CL of 2000 ms. These ventricular arrhythmias, including EADs, EVBs, VTs evoked by Bay K 8644 at all pacing CLs were attenuated by TTX and eleclazine (Fig. 4(c) and (d)).
Attenuation by TTX of the enhanced myocardial contraction and the increase of intracellular calcium transient induced by Bay K 8644 in single ventricular myocytes
The average sarcomere lengths at diastolic and systolic phase in single ventricular myocytes in control condition were 1.85 ± 0.01 and 1.78 ± 0.01 µm, respectively. Bay K 8644 (300 nM) significantly shortened the diastolic and systolic sarcomere lengths to 1.83 ± 0.01 and 1.59 ± 0.02 µm, respectively (n = 25, P < 0.05). Additionally, myocyte contraction amplitude was enhanced when treated with 300 nM Bay K 8644, from 0.07 ± 0.01 to 0.24 ± 0.01 μm (Fig. 5(a) and (c)). TTX at 1 µM depressed the increases by Bay K 8644 on myocyte contraction amplitude by 27.8%, to 0.17 ± 0.02 µm (Fig. 5(c), representative sarcomere lengths were shown in Fig. 5(a)).
Figure 5.
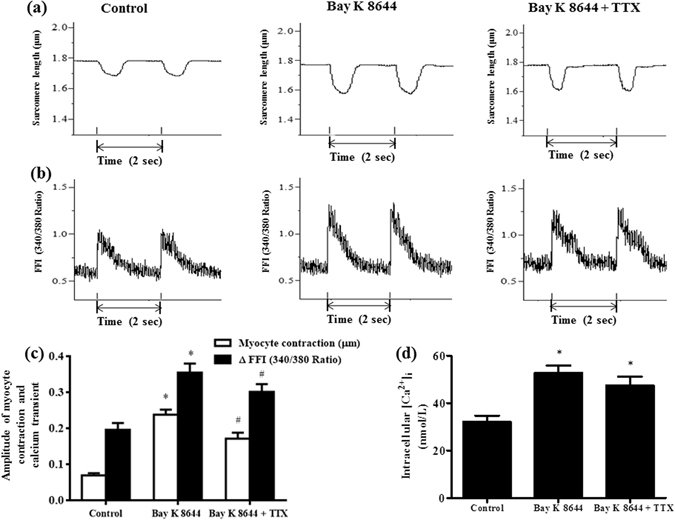
Effects of TTX (1 µM) on cardiomyocyte contraction function and intracellular calcium transient in the presence of Bay K 8644 (300 nM). (a) and (b) Representative recordings of myocardial sarcomere length (a) and intracellular calcium transients (b) under different conditions. (c) The myocardial contraction amplitude (n = 25), and calcium transient amplitude (n = 16) in control, Bay K 8644, and Bay K 8644 plus TTX. (d) The intracellular calcium concentration ([Ca2+]i) in myocardial cells in control, Bay K 8644, and Bay K 8644 plus TTX, n = 16. * P < 0.05 vs. control; # P < 0.05 vs. Bay K 8644.
Diastolic and systolic calcium fura-2 AM fluorescence intensities (FFI, 340/380 Ratio), and the intracellular [Ca2+]i were increased after administration of 300 nM Bay K 8644 (Fig. 5(c) and (d), n = 16, P < 0.05). At this concentration, Bay K 8644 significantly increased the FFI difference between diastolic and systolic phases (ΔFFI) (Fig. 5(b) and (c)). Bay K 8644 increased the intracellular calcium transient amplitude and intracellular [Ca2+]i from 0.20 ± 0.02 (ΔFFI, 340/380 ratio) and 32.17 ± 2.61 nM to 0.36 ± 0.03 (ΔFFI, 340/380 ratio) and 52.76 ± 3.23 nM, respectively (n = 16, P < 0.05). In the continued presence of Bay K 8644, 1 µM TTX inhibited the increases in calcium transient amplitude by 15.0% (Fig. 5(c), representative intracellular calcium transient in each group was shown in Fig. 5(b)).
Bay K 8644 increased ICaL but not late INa, and endogenous and ATX-II-augmented late INa were attenuated by both TTX and eleclazine
In isolated ventricular myocytes, Bay K 8644 (100 and 300 nM) significantly enhanced I CaL in a concentration dependent manner (Fig. 6(a) and (b), n = 5). The current density of I CaL was increased from 11.41 ± 1.29 to 17.06 ± 1.11 and 18.04 ± 1.72 pA/pF, respectively, by 100 and 300 nM Bay K 8644 (P < 0.05, n = 5). Late I Na was significantly increased by ATX-II (3 nM) from 0.42 ± 0.02 to 1.34 ± 0.10 pA/pF (P < 0.05 vs. ctrl, n = 10), but not by 300 nM Bay K 8644 (0.49 ± 0.04 pA/pF, P > 0.05, n = 9). TTX and eleclazine significantly decreased late I Na to 0.11 ± 0.01 and 0.09 ± 0.01 pA/pF, respectively (n = 5 for both groups, P < 0.05, vs. ctrl), to 0.65 ± 0.07 and 0.54 ± 0.04 pA/pF, respectively (n = 4 and 6, P < 0.05, vs. ATX-II), and to 0.18 ± 0.03 and 0.10 ± 0.01 pA/pF, respectively (n = 4 and 5, P < 0.05, vs. Bay K 8644).
Figure 6.
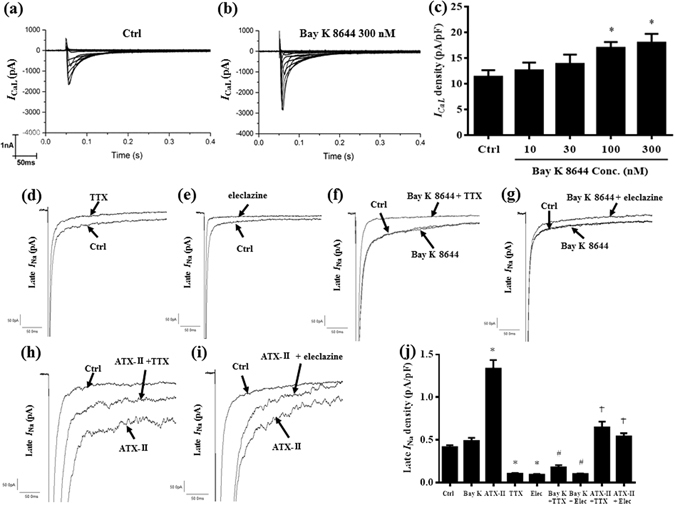
Bay K 8644 increased I CaL. TTX and eleclazine reduced both endogenous and enhanced late I Na. (a) and (b) Representative recordings of I CaL in control (Ctrl, a) and in a cell treated with Bay K 8644 (300 nM, b). (c) Summarized data of various concentrations of Bay K 8644 to increase the density of I CaL in rabbit ventricular cells. * P < 0.05 vs. control. (d) and (e) Representative recordings of late I Na in a cell in Ctrl, and after treatment with either TTX (1 µM, d) or eleclazine (Elec, 10 µM, e). (f) and (g) Representative recordings of late I Na in a cell in Ctrl, before (Bay K 8644 300 nM alone) and after treatment with either TTX (f) or Elec (g). (h) and (i) Representative recordings of late I Na in a cell in Ctrl, before (ATX-II 3 nM) and after treatment with either TTX (1 µM, h) or Elec (10 µM, i) in the continued presence of ATX-II. (j) Summarized data of TTX and Elec on the density of late I Na in Ctrl and in cells treated with either Bay K 8644 (300 nM) or ATX-II (3 nM). * P < 0.05 vs. Ctrl; # P < 0.05 vs. Bay K 8644; † P < 0.05 vs. ATX-II.
TTX reduced the phosphorylation of CaMK II-δ and the expression of Nav 1.5 in hearts treated with Bay K 8644 and/or ATX-II
The phosphorylation of CaMK II-δ and the expression of Nav 1.5 in left ventricular myocardium was significantly enhanced in heart treated with either Bay K 8644 or ATX-II alone (n = 5, P < 0.05 compared with control, Fig. 7). The upregulation of p-CaMK II-δ and the expression of p-Nav 1.5 were substantially greater in hearts treated with the combination of ATX-II and Bay K 8644 than in hearts treated with either Bay K 8644 or ATX-II alone. Inhibition of late I Na by TTX attenuated the Bay K 8644-induced phosphorylation in CaMK II-δ and the expression of Nav 1.5 (Fig. 7). Representative protein bands expression of p-CaMK II-δ and p-Nav 1.5 in each group were displayed in Fig. 7(a).
Figure 7.
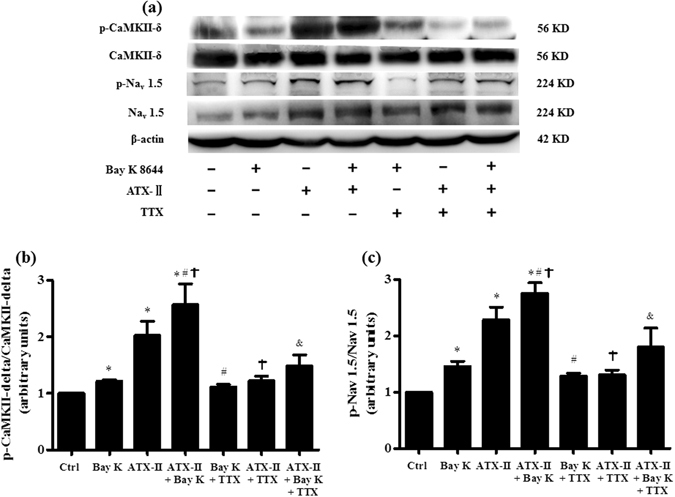
TTX (at concentration of 1 µM) reduced the phosphorylation of CaMK II-δ and Nav 1.5 in hearts pretreated with Bay K 8644 (200 nM) in both absence and presence of ATX-II (3 nM). (a) Representative protein expression of p-CaMK II-δ, CaMK II-δ, p-Nav 1.5 and Nav 1.5 in rabbit left ventricular myocardium under conditions indicated. (b) and (c) The quantitative analysis of p-CaMK II-δ (b) and p-Nav 1.5 (c) protein expression. The corresponding intensity was normalized to expression in Ctrl. * P < 0.05 vs. Ctrl; # P < 0.05 vs. Bay K 8644; † P < 0.05 vs. ATX-II; & P < 0.05 vs. ATX-II plus Bay K 8644.
Discussion
The findings described in the present study suggest that endogenous and enhanced late I Na play important roles in the electrical and mechanistic dysfunctions associated with increased [Ca2+]i by activating CaMK-II and sodium channel activities. Bay K 8644 treatment, by increasing I CaL, induced prolongation of MAPD90, enhanced RUD, increased calcium transient and upregulation of phosphorylated CaMK II-δ and Nav 1.5, finally led to cardiac arrhythmias and contractile dysfunction. When late I Na was amplified by ATX-II, Bay K 8644 caused greater increases in MAPD90 in a synergistic manner, higher incidence of ventricular arrhythmias, and greater phosphorylation of CaMK II-δ and Nav 1.5. TTX and eleclazine, at concentration that inhibits late I Na, attenuated the pro-arrhythmic effects of Bay K 8644.
Cardiac Ca2+ homeostasis plays crucial roles in the maintenance of cardiac excitation and contraction function, and inhomeostasis of intracellular [Ca2+]i is common in a number of pathological conditions. Ca2+ release from SR leads to arrhythmias in the setting of LQTs, CPVT and digoxin toxicity21. The increase in transmembrane inward I CaL caused by mutations of L-type calcium channel or RYR2, is associated with LQTs 811 and CPVT22 in clinic. Therefore, L-type calcium channel activator Bay K 8644 was used to mimic pathological conditions associated with increased [Ca2+]i by amplifying I CaL and stimulating Ca2+ influx, which could also reduce the repolarization reserve23, 24. Similar to our results, study from optical mapping data has indicated that abnormal Ca2+ handling caused arrhythmias in models of both repolarization impairment and bradycardia. The regional Ca2+ heterogeneities in Ca2+ handling leading to temporal instability of AP duration were considered one of the mechanisms for arrhythmias in the study25.
ase proarrhthmic in hearts when Endogenous late I Na is small in amplitude (about 20–80 pA) in a normal heart and will be increased in many physiological (e.g., slow heart rate), pharmacological and pathological conditions26, and then the increase in endogenous late I Na contributes to the arrhythmogenic activities in hearts with reduced repolarization reserve. Enhanced late I Na caused by the mutation of SCN5A was initially reported to be contributed to arrhythmias in patients with LQTs 3. The “gain-of-function” of sodium channel prolonged ventricular repolarization and caused malignant arrhythmias. Heterogeneous distribution of late I Na was also responsible for interregional differences in rate adaptation of repolarization, and is arrhythmogenic when rate dependence of repolarization was augmented27. Our previous study has revealed that the endogenous late I Na is arrhythmogenic in hearts after use of I Kr inhibitors, when repolarization reserve was reduced by decrease of outward current19. The role of endogenous late I Na should be of concern in patients with calcium-related arrhythmias, especially in patients with bradycardia. RUD model of APD prolongation is an implicit property of several drugs that either increase inward current of repolarization or decrease outward current of repolarization. Our previous study has proved that endogenous late I Na contributes to the increased RUD of APD and beat-to-beat variability of repolarization (BVR) induced by I Kr inhibitors, and to bradycardia-related ventricular arrhythmias14. In the present study, the contribution of late I Na was also found on enhanced RUD induced by Bay K 8644. The results suggesting that RUD augment of late I Na is the basis of the increased RUD of MAPD90 in hearts with increased [Ca2+]i. The rate-dependent augment of late I Na is the basis of the increased RUD of APD under repolarization reserve reduced condition, may explain the clinical phenomenon that LQTs 8 patients are often characterized by bradycardia, and late I Na inhibitor is effective to treat LQTs 811.
At the concentration of 3 µM or lower, TTX has been shown to be selective in inhibiting late I Na with minimal inhibition on peak I Na in rabbit cardiomyocytes28, which was confirmed in the present study that 1 µM TTX apparently decreased late I Na in ventricular myocytes in both absence and presence of Bay K 8644 or ATX-II. In accordance with other’s report20, eleclazine exhibited a similar effect to TTX, inhibited late I Na, shortened the prolongation of MAPD, and attenuated ventricular arrhythmias. The shortening of MAPD and related antiarrhythmic activities induced by TTX and eleclazine in both absence and presence of either Bay K 8644 or ATX-II indicated that both endogenous and enhanced late I Na were pro-arrhythmic in hearts when repolarization reserve was reduced by increase of Ca2+ current by Bay K 8644. Inhibition of endogenous late I Na was sufficient in eliciting antiarrhythmic effects in hearts with increased [Ca2+]i. The underlying mechanism may be that endogenous late I Na is, at least in part, responsible for the prolongation of MAP, which enhances the arrhythmogenic effects from an increase in [Ca2+]i. However, because the IC50 of TTX on Nav 1.5 has been reported as 1 µM, peak I Na may also be potentially affected and contribute to the decrease in Nav 1.5 and the changes of a cardiac EP, if a concentration above 1 µM was used in the study.
Enhanced late I Na synergistically increased the risk of arrhythmias in hearts with Bay K 8644 by activating CaMK II-δ and Nav1.5. ATX-II, at concentrations of 3 nM or lower, increases late I Na in rabbit isolated myocytes, prolongs APD in the intact heart model17, 29. Therefore, 3 nM ATX-II was used to enhance late I Na in this study. The results clearly showed that, in hearts treated with 3 nM ATX-II, Bay K 8644 (200 nM) caused greater prolongation of MAPD in association with an increased incidence of VT. This suggests a synergistic effect between increased [Ca2+]i and enhanced late I Na in potentiating the arrhythmogenic activities. The results in this study demonstrated that either Bay K 8644 or ATX-II increased the phosphorylation of CaMK II-δ and Nav1.5. TTX, at low concentration with inhibition of late I Na but relatively minimal inhibition of peak I Na, reversed the activation of CaMK II-δ and Nav1.5. The increase in the phosphorylation of Nav 1.5 and CaMKII induced by Bay K 8644 may cause enhancement of late I Na. However, the increase in the density of late I Na after Bay K 8644 administration in this single cell study was small and insignificant. One of the possible reasons underlying the disparity between Nav 1.5 and late I Na may be attributed to the difference in exposure time of Bay K 8644. The exposure time of Bay K 8644 for late I Na recording from single cells was 3 minutes which is shorter than the perfusion time of 30 minutes for Nav 1.5 and CaMKII phosphorylation assays from whole heart experiments. Further study is needed to determine the time course for Bay K 8644 to cause a steady-state maximal effect on late I Na.
The proteins interaction between sodium channel activities and Ca2+ handling may contribute to the synergistic effect of ATX-II and Bay K 8644 on the prolongation of MAPD and ventricular arrhythmias. Changes of key Ca2+-handling proteins associated with arrhythmias involve activation of CaMK II, and phosphorylation of L-type Ca2+ channels, RyR2 (promoting Ca2+ release) and Ca2+-ATPase SERCA2a. CaMK II has been identified as an important activator of late I Na. In normal ventricular myocyte, Ca2+ overload at concentrations higher (0.6–1 µM) than that in this study could increase the late I Na via activating CaMK II and PKC pathway15. Others showed Nav1.5-dependent persistent Na+ influx could activate CaMK II, which in turn phosphorylated Nav1.5, RyR2, and L-type Ca2+ channels, further promoting Na+ influx and Ca2+ release30. Thus, the interaction of late I Na and calcium handling forms a positive feedback loop with progressive increases in intracellular calcium and sodium concentration, leading to spatial heterogeneity of calcium transients and trigger activities. Various studies have revealed that Ca2+-handling proteins are regulated by the balance between serine/threonine protein kinases and phosphatase activity, and several potential CaMK II phosphorylation sites have been identified. Using Scn 5a knock-in mouse models, sodium channel phosphorylated at Ser 571 was revealed to promote abnormal repolarization and intracellular Ca2+ handling, and increase susceptibility to arrhythmia31. Importantly, the phosphorylated antibody used in the present study could recognize Nav1.5 at serine 573 and threonine 17 loci, which is different from the results in mouse study30.
Besides late I Na, there are also other factors regulated calcium-related arrhythmias. Exchange protein directly activated by cAMP (Epac)2 has been recently recognized as key mediators that regulated arrhythmogenic SR Ca leak via CaMKII delta-dependent signaling pathway. A recent study demonstrated that the underlying mechanism of impaired Epac2 signaling-induced arrhythmias is due to ROS dependent activation of late I Na 32. Therefore, selective inhibition of late I Na provides a strategy to prevent arrhythmias under the condition of intracellular calcium overload.
The findings of the present study may explain why I Na inhibitor is able to attenuate arrhythmias of LQTs 811. Further research will be necessary in the mechanistic study of cardiac arrhythmias in models of myocardial ischemia/reperfusion and oxidative stress2, atrial fibrillation with intracellular Ca2+ handling abnormalities, because late I Na was reported to be increased under these conditions33. Subsequent inhibition of endogenous and enhanced late I Na shows feasible antiarrhythmic results. Novel late I Na inhibitors, e.g., GS-967 or 661534, may thus be useful in treating ventricular arrhythmias in patients with intracellular Ca2+ overload. However, as the conditions of Ca2+ overload and late I Na amplification may come together, application of late I Na inhibitors only might attenuate the part of repolarization abnormality from the contribution of late I Na and therefore only reverse part of the complex conditions but may be sufficient to be antiarrhythmic in experimental models, as well as in clinic. Increased intracellular [Ca2+]i in LQT 3 cardiomyocytes in which late I Na was already enhanced has been reported with decreased amplitude of late I Na 35, suggesting that the relationships between the [Ca2+]i and late I Na need to be further determined under pathological conditions. Indeed, data from an ischemia-reperfusion model (in supplement of this study) indicated a decreased infarct area in hearts treated with a late I Na inhibitor ranolazine. Therefore, further study to evaluate the role of late I Na in various disease conditions and the possible application of drugs that inhibit both late I Na and Ca2+ handling is needed.
Methods
Female Rabbit Isolated Whole Heart Model
New Zealand White female rabbits, weighing 2.5–3.5 kg, were sedated using 6 mg/kg xylazine i.m. and 40 mg/kg ketamine i.m. and then anesthetized by a “cocktail” (ketamine 15 mg/kg + xylazine 4 mg/kg in 1.5 mL saline) i.v. via the marginal ear vein. The heart was excised and perfused in Langendorff mode at a rate of 20 mL/min with modified Krebs-Henseleit (K-H) solution warmed to 37 °C. The solution contains (in mM): 118 NaCl, 2.8 KCl, 1.2 KH2PO4, 2.5 CaCL2, 0.5 MgSO4, 2.0 sodium pyruvate, 5.5 glucose, and 25 NaHCO3. The atrioventricular (AV) nodal area was thermally ablated, and hearts were paced on the ventricular septum (close to His bundle)14. The use of animals in this study conformed to the “Guide for the Care and Use of Laboratory Animals” published by the United States National Institutes of Health (NIH publication No. 85–23, revised 2011), and the study protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Peking University First Hospital (J201325).
MAP and ECGs recording
Epi- and endo- MAP from left ventricle were recorded. MAPs signals were displayed in real time and calculated by Cambridge Electronics Device Spike II software to determine the MAPD90. A pseudo 12-lead ECG was recorded using a circular Einthoven-Goldberger ECG electrode system (Harvard Apparatus, Inc., Holliston, MA, USA) connected to a Biopac Wilson ECG amplifier (Biopac, Goleta, CA).
Determination of arrhythmic activities
MAP and ECGs were monitored continuously, as reported previously19. An EAD was defined as the depolarization during phase 2 and/or 3 of an MAP signal. An EVB was defined as a spontaneous beat occurring earlier than the next paced beat. VT was defined as a sequence of three or more consecutive spontaneous ventricular beats at a rate exceeding the pacing rate. A torsade de pointes ventricular tachycardia (TdP) was defined as a VT wherein the morphology of the QRS complexes in a 12-channel ECG record was continuously changing.
Determination of concentration-response relationships of Bay K 8644 on electrophysiological parameters in the absence and presence of ATX-II
Hearts were paced at the pacing rate of 1.0 Hz. (S)-(-)-Bay K 8644 (Bay K 8644, 1546, Tocris Bioscience, USA) of 10, 30, 60, 100, 200 and 300 nM was administrated to the heart for 8 minutes or longer at each concentration until a steady state maximal effect was observed either in absence or presence of 0.1, 0.3, 0.6 and 1 µM TTX (1078, Tocris Bioscience, USA), and 10 µM eleclazine (synthesized at Gilead Sciences, USA). To test the effects of Bay K 8644 in the presence of ATX-II (Alomone labs, Cat# STA-700, USA), hearts were perfused with ATX-II for 20 min and then exposed to Bay K 8644 and TTX.
Determination of RUD of APD induced by Bay K 8644
To measure the RUD of APD, hearts were paced at increasing CLs of 400, 500, 667, 1000, 1333 and 2000 ms for 2 to 4 minutes at each CL. After exposed to K-H buffer (control), hearts were treated with 200 nM Bay K 8644 in the absence and presence of 1 µM TTX and 10 µM eleclazine, allowing 10 minutes between increases in drug concentration to record a maximal steady-state effect. The stimulation protocol at CLs from 400 to 2000 ms and measurement of MAPD90 was repeated before and after each drug treatment.
Measurement of myocytes contraction function and calcium transient
Rabbit left ventricular cardiomyocytes were isolated enzymatically, as described previously36. Then cardiomyocytes stored in regular Tyrode’s solution containing 1.8 mM CaCl2 were loaded with 0.5 μM fura-2-AM (Sigma) for 10 min at 25 °C. Myocyte contraction function and calcium transient were measured using IonWizard’s Transient system (IonOptix LLC, Milton, USA). The myocardial contraction function was evaluated by the magnitude of myocyte shortening/re-lengthening. The intracellular calcium transient was determined with the ratio of fluorescence intensity at 340 and 380 nm (FFI = 340/380 ratio). Intracellular Ca2+ fluorescence measurements were assessed using the electrically stimulated rise in intracellular Ca2+ (ΔFFI)36. The maximal fluorescence (Fmax) with 20 μmol/L ionomycin and the minimal fluorescence (Fmin) with 20 mmol/L EGTA were determined, respectively. The formula to calculate the intracellular Ca2+ concentration ([Ca2+]i) is as follows: [Ca2+]i = Kd (R − Rmin)Fmin/(Rmax − R)Fmax 37.
Recordings of ICaL and late INa using whole cell patch-clamp techniques
Left ventricular cardiomyocytes were isolated from rabbit hearts. Whole cell I CaL and late I Na were obtained with a patch-clamp amplifier (EPC-10, Heka Electronic, Lambrecht, Pfalz, Germany), filtered at 1 kHz, and digitized at 10 kHz. All patch-clamp experiments were performed at a room temperature of 23 ± 1 °C. For I CaL recording, the pipette solution contained (in mM): 80 CsCl, 60 CsOH, 0.65 CaCl2, 5 disodium creatine phosphate, 5 MgATP, 40 aspartic acid, 10 EGTA, and 5 HEPES (pH 7.3). The bath solution was the Tyrode’s solution. I CaL was elicited by a 150-ms prepulse to −40 mV from a holding potential of −80 mV followed by a 300-ms depolarizing pulse from −40 mV to 0 mV (0.2 Hz). I CaL was determined as the difference between peak inward current and the current remaining at the end of the 300-ms pulse15. For late I Na recording, the pipette solution contained (in mM): 120 CsCl2, 1 CaCl2, 5 MgCl2, 5 Na2ATP, 10 TEA-Cl, 11 EGTA, and 10 HEPES (pH 7.3, adjusted with CsOH). The bath solution contained (in mM): 135 NaCl, 5.4 CsCl2, 1.8 CaCl2, 1 MgCl2, 0.3 BaCl2, 0.33 NaH2PO4, 10 glucose, 10 HEPES, and 0.001 nicardipine (pH 7.4). Late I Na was recorded by a 300-ms depolarizing pulse to −20 mV from a holding potential of −90 mV14. The amplitude of late I Na was measured at 200 ms after initiation of the depolarization step before (control, no drug), and 3 minutes after exposure to 3 nM ATX-II or 300 nM Bay K 8644 in absence and presence of TTX and eleclazine, respectively.
Immunoprecipitation and Western Blotting Assay
To determine the levels of phosphorylation of CaMK II-δ and the expression of Nav 1.5, 7 groups of heart tissue were properly used and analyzed after perfusion with either K-H solution in absence (control) or presence of Bay K 8644 (200 nM), ATX-II(3 nM), ATX-II (3 nM) + Bay K 8644 (200 nM), Bay K 8644 (200 nM) + TTX (1 μM), ATX-II (3 nM) + TTX (1 μM), ATX-II (3 nM) + Bay K 8644 (200 nM) + TTX (1 μM). Total protein of left ventricular myocardium was extracted, immunoprecipitation and Western blotting analysis were performed using a standard method. In brief, tissue lysates were first incubated with magnetic beads covalently conjugated with anti-phospho-Akt substrate (RXXS*/T*) rabbit mAb (Cell Signaling Technology, #8050) and anti-phospho-PKA substrate antibody (RRXS*/T*) Ab (Cell Signaling Technology, #9621) at 4 °C overnight, respectively. Sample buffer mixed with the washed beads was heated at 95 °C for 5 min and subjected to SDS-PAGE. Then the proteins were blotted with rabbit anti- CaMK II-δ Ab (Abcam, ab90445), and anti-Nav 1.5 Ab (Abcam, ab56240), respectively. Blotted antibodies were visualized by HRP-conjugated mouse anti-rabbit IgG mAb (Cell Signaling Technology, #5127) and ECL detection system (Millipore). Densitometric analyses of blots were performed using the Quantity One image analyzer software (Bio-Rad, Richmond CA, USA).
Statistical Analysis
Data of MAPD90 were plotted and analyzed with Prism version 5 (GraphPad Software, San Diego, CA) and all parameters were expressed as mean ± SEM. The significance of differences in measures before and after interventions in the same hearts was determined by repeated measures one-way or two-way ANOVA followed by the Student-Newman-Keuls test. P < 0.05 was defined as significant difference.
Electronic supplementary material
Acknowledgements
This work was supported by the National Science Foundation of China (NSFC-81470454, 81170156 and 81430098). The authors thank Drs. Ming Xu, Ming Zheng, Chenglin Zhang and Dingfang Bu at Peking University Health Science Center for their support on single cell studies and immunoprecipitation assay.
Author Contributions
X.-H.W., Y.H., and L.W. constructed the concept and designed the experimental protocol; X.-H.W., S.-D.Y., L.R., S.-H.H., Q.-M.Y., P.W., Y.-P.C., and W.Y. performed experiments; X.-H.W., L.R., and S.-H.H. analyzed data; X.-H.W., Y.-S.D., and L.W. interpreted results of experiments; X.-H.W. prepared figures; X.-H.W. drafted this manuscript; X.-H.W. and L.W. edited and revised this manuscript; X.-H.W., S.-D.Y., L.R., S.-H.H., Q.-M.Y., P.W., Y.-P.C., W.Y., Y.-S.D., Y.H., and L.W. approved the final version of this manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01056-0
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yong Huo, Email: huoyong@263.net.cn.
Lin Wu, Email: wuepgroup@126.com.
References
- 1.Perennec J, Willemin M, Pocholle P, Hatt PY, Crozatier B. Cardiac ultrastructural abnormalities in Syrian hamsters with spontaneous cardiomyopathy or subjected to cardiac overloads. Basic research in cardiology. 1992;87:54–64. doi: 10.1007/BF00795390. [DOI] [PubMed] [Google Scholar]
- 2.Prunier F, et al. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation. 2008;118:614–624. doi: 10.1161/CIRCULATIONAHA.108.770883. [DOI] [PubMed] [Google Scholar]
- 3.Zhang C, et al. Microtubule-mediated defects in junctophilin-2 trafficking contribute to myocyte transverse-tubule remodeling and Ca2+ handling dysfunction in heart failure. Circulation. 2014;129:1742–1750. doi: 10.1161/CIRCULATIONAHA.113.008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin G, et al. Arrhythmogenic calmodulin mutations disrupt intracellular cardiomyocyte Ca2+ regulation by distinct mechanisms. Journal of the American Heart Association. 2014;3:e000996–e000996. doi: 10.1161/JAHA.114.000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Matthews GD, Lei M, Huang CL. Abnormal Ca(2+) homeostasis, atrial arrhythmogenesis, and sinus node dysfunction in murine hearts modeling RyR2 modification. Front Physiol. 2013;4:150. doi: 10.3389/fphys.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priori SG, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.CIR.0000020013.73106.D8. [DOI] [PubMed] [Google Scholar]
- 7.Splawski I, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Venetucci L, Denegri M, Napolitano C, Priori SG. Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nature reviews. Cardiology. 2012;9:561–575. doi: 10.1038/nrcardio.2012.93. [DOI] [PubMed] [Google Scholar]
- 9.Sikkel MB, Hayward C, MacLeod KT, Harding SE, Lyon AR. SERCA2a gene therapy in heart failure: an anti-arrhythmic positive inotrope. British journal of pharmacology. 2014;171:38–54. doi: 10.1111/bph.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coppini R, et al. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation. 2013;127:575–584. doi: 10.1161/CIRCULATIONAHA.112.134932. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, et al. Inhibition of late sodium current by mexiletine: a novel pharmotherapeutical approach in timothy syndrome. Circulation. Arrhythmia and electrophysiology. 2013;6:614–622. doi: 10.1161/CIRCEP.113.000092. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, et al. Role of late sodium current in modulating the proarrhythmic and antiarrhythmic effects of quinidine. Heart rhythm: the official journal of the Heart Rhythm Society. 2008;5:1726–1734. doi: 10.1016/j.hrthm.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu L, et al. Augmentation of late sodium current unmasks the proarrhythmic effects of amiodarone. Cardiovasc Res. 2008;77:481–488. doi: 10.1093/cvr/cvm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu L, et al. Late sodium current contributes to the reverse rate-dependent effect of IKr inhibition on ventricular repolarization. Circulation. 2011;123:1713–1720. doi: 10.1161/CIRCULATIONAHA.110.000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, et al. Calmodulin kinase II and protein kinase C mediate the effect of increased intracellular calcium to augment late sodium current in rabbit ventricular myocytes. American journal of physiology. Cell physiology. 2012;302:C1141–1151. doi: 10.1152/ajpcell.00374.2011. [DOI] [PubMed] [Google Scholar]
- 16.Sanguinetti MC, Krafte DS, Kass RS. Voltage-dependent modulation of Ca channel current in heart cells by Bay K8644. The Journal of general physiology. 1986;88:369–392. doi: 10.1085/jgp.88.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Shryock JC, Song Y, Belardinelli L. An increase in late sodium current potentiates the proarrhythmic activities of low-risk QT-prolonging drugs in female rabbit hearts. The Journal of pharmacology and experimental therapeutics. 2006;316:718–726. doi: 10.1124/jpet.105.094862. [DOI] [PubMed] [Google Scholar]
- 18.Lai D, et al. Stretch current-induced abnormal impulses in CaMKIIdelta knockout mouse ventricular myocytes. J Cardiovasc Electrophysiol. 2013;24:457–463. doi: 10.1111/jce.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, et al. Reduction of repolarization reserve unmasks the proarrhythmic role of endogenous late Na(+) current in the heart. Am J Physiol Heart Circ Physiol. 2009;297:H1048–1057. doi: 10.1152/ajpheart.00467.2009. [DOI] [PubMed] [Google Scholar]
- 20.Zablocki JA, et al. Discovery of Dihydrobenzoxazepinone (GS-6615) Late Sodium Current Inhibitor (Late INai), a Phase II Agent with Demonstrated Preclinical Anti-Ischemic and Antiarrhythmic Properties. Journal of medicinal chemistry. 2016;59:9005–9017. doi: 10.1021/acs.jmedchem.6b00939. [DOI] [PubMed] [Google Scholar]
- 21.Lei M, Wang X, Ke Y, Solaro RJ. Regulation of Ca(2+) transient by PP2A in normal and failing heart. Front Physiol. 2015;6:13. doi: 10.3389/fphys.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsiao PY, et al. Gene mutations in cardiac arrhythmias: a review of recent evidence in ion channelopathies. The application of clinical genetics. 2013;6:1–13. doi: 10.2147/TACG.S29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolkowicz P, et al. Inhibitors of arachidonate-regulated calcium channel signaling suppress triggered activity induced by the late sodium current. Eur J Pharmacol. 2014;724:92–101. doi: 10.1016/j.ejphar.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Satoh, H., Hayashi, H., Blatter, L. A. & Bers, D. M. BayK 8644 increases resting calcium spark frequency in ferret ventricular myocytes. Heart and vessels Suppl 12, 58–61 (1997). [PubMed]
- 25.Nemec J, Kim JJ, Salama G. The link between abnormal calcium handling and electrical instability in acquired long QT syndrome - Does calcium precipitate arrhythmic storms? Prog Biophys Mol Biol. 2016;120:210–221. doi: 10.1016/j.pbiomolbio.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaza A, Belardinelli L, Shryock JC. Pathophysiology and pharmacology of the cardiac “late sodium current”. Pharmacol Ther. 2008;119:326–339. doi: 10.1016/j.pharmthera.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Qi D, et al. Heterogeneous distribution of INa-L determines interregional differences in rate adaptation of repolarization. Heart rhythm: the official journal of the Heart Rhythm Society. 2015;12:1295–1303. doi: 10.1016/j.hrthm.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, et al. Sophocarpine attenuates the Na(+)-dependent Ca2(+) overload induced by Anemonia sulcata toxin-increased late sodium current in rabbit ventricular myocytes. Journal of cardiovascular pharmacology. 2012;60:357–366. doi: 10.1097/FJC.0b013e318262c932. [DOI] [PubMed] [Google Scholar]
- 29.Wu L, et al. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. The Journal of pharmacology and experimental therapeutics. 2004;310:599–605. doi: 10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- 30.Yao L, et al. Nav1.5-dependent persistent Na+ influx activates CaMKII in rat ventricular myocytes and N1325S mice. American journal of physiology. Cell physiology. 2011;301:C577–586. doi: 10.1152/ajpcell.00125.2011. [DOI] [PubMed] [Google Scholar]
- 31.Glynn P, et al. Voltage-Gated Sodium Channel Phosphorylation at Ser571 Regulates Late Current, Arrhythmia, and Cardiac Function In Vivo. Circulation. 2015;132:567–577. doi: 10.1161/CIRCULATIONAHA.114.015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z, et al. Epac2-Rap1 signaling regulates reactive oxygen species production and susceptibility to cardiac arrhythmias. Antioxidants & redox signaling. 2016 doi: 10.1089/ars.2015.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. Journal of the American College of Cardiology. 2014;63:2335–2345. doi: 10.1016/j.jacc.2014.02.555. [DOI] [PubMed] [Google Scholar]
- 34.Antzelevitch C, et al. The role of late I Na in development of cardiac arrhythmias. Handbook of experimental pharmacology. 2014;221:137–168. doi: 10.1007/978-3-642-41588-3_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potet F, Beckermann TM, Kunic JD, George AL., Jr. Intracellular calcium attenuates late current conducted by mutant human cardiac sodium channels. Circulation. Arrhythmia and electrophysiology. 2015;8:933–941. doi: 10.1161/CIRCEP.115.002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian C, et al. Resveratrol attenuates the Na(+)-dependent intracellular Ca(2+) overload by inhibiting H(2)O(2)-induced increase in late sodium current in ventricular myocytes. PloS one. 2012;7:e51358. doi: 10.1371/journal.pone.0051358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. The Journal of biological chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


