Abstract
Autoantibodies against ion channels are the cause of numerous neurologic autoimmune disorders. Frequently, such pathogenic autoantibodies have a restricted epitope-specificity. In such cases, competing antibody formats devoid of pathogenic effector functions (blocker antibodies) have the potential to treat disease by displacing autoantibodies from their target. Here, we have used a model of the neuromuscular autoimmune disease myasthenia gravis in rhesus monkeys (Macaca mulatta) to test the therapeutic potential of a new blocker antibody: MG was induced by passive transfer of pathogenic acetylcholine receptor-specific monoclonal antibody IgG1-637. The effect of the blocker antibody (IgG4Δhinge-637, the hinge-deleted IgG4 version of IgG1-637) was assessed using decrement measurements and single-fiber electromyography. Three daily doses of 1.7 mg/kg IgG1-637 (cumulative dose 5 mg/kg) induced impairment of neuromuscular transmission, as demonstrated by significantly increased jitter, synaptic transmission failures (blockings) and a decrease in the amplitude of the compound muscle action potentials during repeated stimulations (decrement), without showing overt symptoms of muscle weakness. Treatment with three daily doses of 10 mg/kg IgG4Δhinge-637 significantly reduced the IgG1-637-induced increase in jitter, blockings and decrement. Together, these results represent proof-of principle data for therapy of acetylcholine receptor-myasthenia gravis with a monovalent antibody format that blocks binding of pathogenic autoantibodies.
Introduction
Autoimmune disorders affect more than 5% of the general population, while for reasons that are poorly understood, the incidence of these disorders is currently increasing. In recent years, autoimmune mechanisms have been discovered in many diseases of the nervous system as being responsible for both neurological as well as psychiatric symptoms. Currently available broad-spectrum immunosuppressive drugs are routinely used to treat different autoimmune diseases, but often cause serious side-effects while generally taking four to fifteen months before achieving clinical remission. Antigen-specific therapies offer the possibility to avoid general immunosuppression and its associated risk for infections.
Myasthenia gravis (MG) is one of the best-understood autoimmune disorders and is characterized by muscle weakness as a result of impaired neuromuscular transmission. This is caused by autoantibodies against postsynaptic membrane proteins at the neuromuscular junction (NMJ). In most MG patients (~85%), the auto-antibodies are directed to the muscle nicotinic acetylcholine receptor (AChR)1 and induce loss of the AChR by means of complement-mediated lyses of the postsynaptic membrane2, 3 and cross-linking-induced degradation of the AChR (antigenic modulation)4. The muscle AChR is highly susceptible to antibody cross-linking for two reasons: First, the AChR is densely clustered5 and second, each AChR pentamer contains two identical alpha subunits, thus allowing cross-linking of various AChRs by bivalent antibodies against the alpha subunit. More than half of AChR-specific autoantibodies in MG patients is directed to the main immunogenic region (MIR) on the AChR alpha subunits6, as exemplified by the human monoclonal antibody IgG1-6377, 8. Although the MIR is important for expression and assembly of the AChR, MIR-specific autoantibodies do not interfere with AChR function, such as recognition of acetylcholine released from nerve terminals and opening of the receptor channel8. The exposed orientation of the MIRs of adjacent AChRs allows bivalent binding of MIR-antibodies, resulting in rapid AChR degradation. In contrast to the overall AChR antibody titer, the MIR antibody titer is strongly correlated to severity of muscle weakness in MG patients6. The pathogenicity and restricted epitope-specificity of AChR-specific autoantibodies makes the MIR an attractive target for blocker therapy with non-pathogenic antibodies9–11. Although autoantibodies can bind to various conformational epitopes within the MIR (e.g. 1–32 and 60–818) the binding area typically buried upon antibody binding is a circle with a diameter of 3.1 nm. Therefore, a blocking antibody has the potential to shield a large part of the AChR, which has a diameter of ~6 nm5. In passive transfer MG (PTMG) experiments using rats and mice, blocking antibody fragments directed against the MIR have been shown to prevent muscle weakness10, 12. In vitro, a single chain-Fv antibody fragment of IgG1–637 blocked binding of MG patient autoantibodies by 31.4% (range 2–77.4%)9. Similarly, varying degrees of competition of MG patients’ antibodies were observed with Fab-637 (ranging between <10% and 100%)7.
In rhesus monkeys, IgG1-637 causes destruction of the postsynaptic membrane, thereby inducing MG symptoms of muscle weakness11. In this model, an IgG4 version of the same antibody, IgG4-637, did not induce PTMG, and moreover, prevented IgG1-637-mediated muscle weakness11. This protective effect was explained by the inability of IgG4-637 to activate rhesus monkey complement, and the intrinsic ability of human IgG4 molecules to engage in Fab-arm exchange leading to functional monovalency; thus preventing antigenic modulation through cross-linking11, 13, 14.
Although these results are promising from a therapeutic point of view, Fab-arm exchange is a slow process in vivo 11, 15, which implies that therapeutic IgG4 would only gradually become functionally monovalent and thus would initially be able to cause harmful cross-linking-induced antigenic modulation, directly when administered to patients or animals. Moreover, indirect cross-linking through residual interactions of IgG4 with Fcγ receptors16–18 may represent an alternative route for antigenic modulation independent of functional monovalency. In addition, during Fab-arm exchange, bispecific antibodies will be generated with unknown partner-specificities, which may result in unpredictable pharmacodynamics. Taken together, these considerations suggest that for immunotherapy of MG an alternative antibody format with superior non-activating (no complement activation or Fcγ-receptor interaction) and non-cross-linking properties, compared to IgG4, is desired for clinical implementation.
In humans, the clinical presentation of AChR-MG may comprise fluctuating fatigability, drooping of eyelids (ptosis), difficulty in swallowing (dysphagia), shortness of breath (dyspnea) and proximal muscle weakness on repetitive use of the muscles19. Apart from the analysis of serum autoantibodies against the AChR, several functional tests are used for diagnosis, including injection of a cholinesterase inhibitor (Tensilon test), electromyography (EMG) and single-fiber electromyography (SFEMG). Of these tests, SFEMG is the most sensitive for detecting defects in neuromuscular transmission. In experimental autoimmune MG, rhesus monkeys display the same range of clinical symptoms as patients with MG (e.g. fatigability, hypoactivity, ptosis, dysphagia, anorexia), which can be scored by observing the behavior20, 21. Unlike in chronic models, in PTMG these symptoms are reversible and dose-dependent11.
In this study we evaluated hinge-deleted IgG4 (IgG4Δhinge) as therapeutic antibody format in PTMG in rhesus monkeys. By employing diagnostic EMG and SFEMG, we could study the therapeutic effects in this animal model and show that IgG4Δhinge protected the NMJ against a myasthenogenic autoantibody.
Results
Generation and functional characterization of IgG4Δhinge-637
In order to increase the non-activating and non-cross-linking properties of human IgG4, constructs lacking the genetic hinge exon were generated, resulting in hinge-deleted IgG4 (IgG4Δhinge) molecules (Fig. 1a). Especially the absence of inter heavy-chain disulfide linkages in IgG4Δhinge, as a result of C226 and C229 deletion, is thought to eliminate residual Fcγ receptor interactions, like observed for IgG1 and IgG322, 23. The hinge deletion in combination with the relatively weak CH3-CH3 interface interaction in human IgG4, minimizes heavy chain dimerization and thus antigen cross-linking24.
Figure 1.
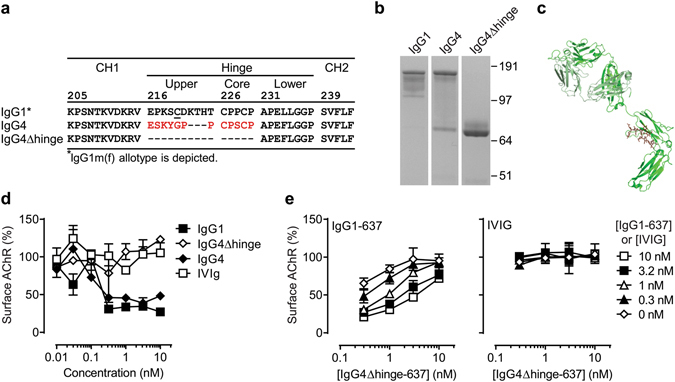
Design and in vitro characterization of IgG4Δhinge. (a) Sequence alignment of residues 205–234 (EU-numbering convention) of human IgG1, IgG4 and IgG4Δhinge. (b) Purified human IgG1, IgG4 and IgG4Δhinge variants of mAb 637 analyzed by non-reducing SDS-PAGE (image is cropped to show relevant bands, the complete gel is shown in supplementary Fig. 5). (c) In silico model of IgG4Δhinge based on crystal structures 1ADQ61 (CH2-CH3 domains), 1MCO62 (Δhinge region) and 1HZH63 (Fab region). HCΔhinge depicted in green, LC depicted in pale green. (d) AChR surface down-modulation on TE671 cells by purified variants of mAb 637 as indicated. Pooled intravenous immunoglobulin (IVIg) is included as negative control (e) Inhibition of (auto-) antibody-mediated AChR surface down-modulation on TE671 cells by IgG4Δhinge-637. Different concentrations of the challenge autoantibody (IgG1-637) or a negative control (IVIG) were tested in combination with IgG4Δhinge-637; each curve corresponds to a single concentration of challenge antibody (0–10 nM) as shown in the key on the right.
To study this, an IgG4Δhinge version of mAb 637 was constructed and compared to IgG1-637 and IgG4-637 by non-reducing SDS-PAGE (Fig. 1b). Whereas IgG1-637 remained intact under non-reducing conditions, IgG4-637 displayed a mixture of intact (H2L2) and half-molecules (HL), as is characteristic for monoclonal wild-type human IgG4 molecules25–27. As expected, deletion of the genetic hinge region resulted in an exclusive population of non-covalently linked half-molecules (modeled in Fig. 1c).
The inability of IgG4Δhinge to activate complement was confirmed by C1q-binding ELISA (Supplementary Fig. 1a) and complement dependent cytotoxicity (CDC) assay (Supplementary Fig. 1b) using an IgG4Δhinge version of HuMab 7D828, directed against the CD20 antigen and shown to potently induce CDC as IgG1. Furthermore, elimination of residual FcγRI receptor interaction of IgG4Δhinge was confirmed by flow-cytometry (Supplementary Fig. 1c). Together, these data show that IgG4Δhinge has superior non-activating properties compared to IgG4.
Lack of inter heavy-chain disulfide linkage has been shown to influence antibody serum half-life29. Furthermore, as half-molecules contain only one instead of two binding sites for the neonatal Fc receptor (FcRn)30, their rescue from the IgG degradation pathway is likely to be impaired31. To determine the pharmacokinetics of IgG4Δhinge, single doses of IgG4-637 or IgG4Δhinge-637 were injected into Balb/c mice, cynomolgus monkeys (Macaca fascicularis) and human FcRn transgenic (Tg) mice32 and serum levels were followed over time (Supplementary Fig. 2a–c). As anticipated, IgG4Δhinge-637 was cleared 2.5, 2 and 3.2 times faster compared to IgG4-637 in the respective animal models (Supplementary Fig. 2d). IgG4Δhinge-637, however, was still protected from catabolic degradation as shown by the much faster clearance of the F(ab’)2 fragments (Supplementary Fig. 2a,d).
Protection against auto-antibody-induced AChR down-modulation in vitro
Bivalent targeting of the AChR, by either IgG1-637 or IgG4-637, induced AChR surface down-modulation (antigenic modulation) in vitro (Fig. 1d), as described4, 33–35. In contrast, IgG4Δhinge-637 did not reduce AChR surface expression, showing that IgG4Δhinge-637 is non-cross-linking (Fig. 1d). To investigate whether IgG4Δhinge-637 could protect against AChR surface down-modulation, serial dilutions of IgG4Δhinge-637 were co-incubated with a fixed optimal concentration of IgG1-637. Indeed, IgG4Δhinge-637 could effectively inhibit IgG1-637-mediated loss of AChR expression (Fig. 1e). Pooled intravenous immunoglobulin (IVIg), included as a negative control, did not affect AChR expression by itself, and no significant changes in AChR expression were observed when co-incubated with IgG4Δhinge-637 (Fig. 1e). These data showed that IgG4Δhinge-637 is able to neutralize IgG1-637-mediated down modulation of the AChR in vitro and limited the loss of AChR expression.
Passive transfer MG and clinical evaluation in rhesus monkey model
Since establishment of the PTMG model11, improved animal housing conditions had resulted in increased weight, muscle mass and fitness of experimental rhesus monkeys. Therefore, a pilot study with six female rhesus monkeys was initiated in order to check the validity of the experimental conditions for monkeys raised under the new housing conditions. The animals were each challenged intravenously with 1.7 mg/kg/day IgG1-637 on three consecutive days, adding up to a cumulative dose of 5 mg/kg IgG1-637, which had been shown to result in clinical symptoms of MG in a previous study using lighter animals11. In the present study, neuromuscular transmission was investigated using single fiber electromyography (SFEMG), to increase sensitivity of the analysis. Furthermore, clinical observation, blood sampling, decrement measurements of the compound muscle action potential (CMAP) and intercostal biopsies were all included in the clinical evaluation.
Intravenous (i.v.) injections with IgG1-637 were well tolerated and no acute adverse effects were observed. Based on clinical observations, none of the animals showed muscle weakness. Baseline (before antibody treatment) mean consecutive difference (MCD) or “jitter” values were recorded in these animals ranging from 9–19 µs (Supplementary Fig. 3), similar to those found in healthy humans (typically between 10 and 20 µs36, 37). Seven days after the first injection of the three daily doses of IgG1-637, the jitter values in the six rhesus monkeys increased significantly, with MCD values ranging from 39 to 130 μs (Supplementary Fig. 3).
As a considerable effect on jitter could be measured by SFEMG, without evident muscle weakness, the 5 mg/kg IgG1-637 dose was chosen as a model for subclinical MG. Since jitter values above 91 μs are strongly predictive for respiratory muscle weakness in MG patients38, we considered it possible that moderate clinical symptoms were not observed due to the natural behavior of rhesus monkeys to avoid showing weakness in order to preserve social hierarchy39. No higher IgG1-637 dose was attempted for our studies to avoid the risk of inducing a myasthenic crisis. All other recorded data were blinded, included in the subsequent study and (re-) analyzed.
Electrophysiological evaluation of IgG4Δhinge-637 treatment in passive transfer MG
Subclinical MG was induced in seven additional animals to assess the therapeutic effect of IgG4Δhinge-637. For this, the animals received three daily intravenous doses of 10 mg/kg IgG4Δhinge-637 (n = 6) or PBS (n = 1), six hours prior to challenge with IgG1-637. The dosing of IgG4Δhinge-637 was based on the protective dose of IgG4-637 (three daily doses of 5 mg/kg) in a previous study11, compensated for the two-fold faster clearance compared to IgG4 as determined in cynomolgus monkeys (Supplementary Fig. 2). Two additional control animals only received IgG4Δhinge-637 and no IgG1-637 challenge. Clinical signs of MG were monitored as described above (Fig. 2a).
Figure 2.
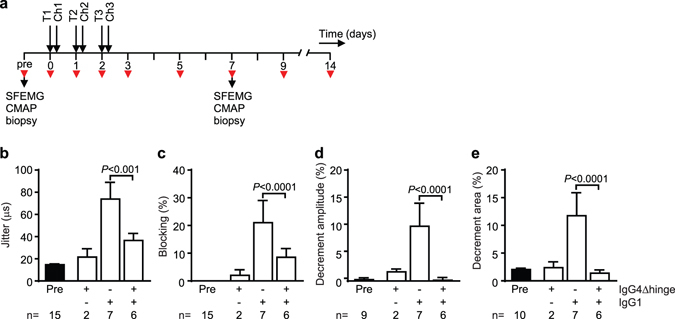
Electrophysiological evaluation of IgG4Δhinge-637 treated rhesus monkeys. (a) Treatment schedule. Monkeys were treated with PBS or IgG4Δhinge-637 (IgG4Δhinge) on three consecutive days (T1-T3), 6 hours prior to challenge (Ch1-Ch3) with PBS or IgG1-637 (IgG1). Intercostal muscle biopsies, single fiber electromyography (SFMG) and compound muscle action potential (CMAP) measurements during repetitive nerve stimulation were performed before (pre) and 7 days after start of treatment. Red arrowheads indicate blood sampling. Mean consecutive difference (MCD) of the delay between motor nerve stimulation and muscle fiber action potential (Jitter) (b) and neuromuscular transmission failures (Blockings) (c) in the orbicularis oculi muscle in different treatment groups. Decrement amplitude (d) and area (e) in the CMAP of the extensor digitorum brevis muscle in different treatment groups. Reference values were recorded before start of treatment (Pre). Data represent mean ± SEM.
Blinded (re-)analysis of the SFEMG data of the untreated rhesus monkeys (seven animals total) yielded control values for jitter in rhesus monkeys. The mean MCD obtained was 14.83 ± 3.39 μs (range 9–19 μs; Fig. 2B) with an upper limit (mean ± 3 SD) of 24.99 μs. The mean control MCD for all individual potentials (n = 352) obtained was 14.88 ± 6.45 μs (range 5–55 μs) with an upper limit of 34.23 μs. Administration of IgG4Δhinge-637 alone did not significantly increase the jitter (Fig. 2b). Challenge with IgG1–637 significantly (P < 0.001) increased the jitter compared to controls (Fig. 2b). Animals receiving IgG4Δhinge-637 treatment prior to pathogenic challenge with IgG1-637 showed significantly (P < 0.001) reduced jitter compared to the group challenged with IgG1-637 alone (Fig. 2b).
Subsequently, we analyzed the frequency of neuromuscular blockings, the failure to elicit an action potential in the muscle fiber upon stimulation of the motor nerve (Fig. 2c). No blocking events were observed in the animals before antibody administration. A few events (4%) of blockings were observed in one out of two animals injected with the IgG4Δhinge-637 alone, indicating a possible minor effect on synaptic transmission. In six out of seven animals challenged with IgG1-637, blocking events were observed with an average blocking rate per animal of 24.1% (Fig. 2c). Similarly, blocking was observed in five out of six rhesus monkeys treated with IgG4Δhinge-637 six hours prior to injection with IgG1-637. In these animals, the number of blockings induced by IgG1-637 was significantly (P < 0.0001) reduced to 8.6% by treatment with IgG4Δhinge-637 (Fig. 2c).
During repetitive nerve stimulation, the amount of neurotransmitter that is released at each subsequent nerve impulse decreases, especially at higher stimulation frequencies. In combination with low levels of AChRs, this leads to increasing numbers of neuromuscular blocking events during repetitive nerve stimulation. As a result, the CMAP decreases during repetitive stimulations of the muscles, which is referred to as a decrement of the CMAP response. In clinical practice a decrement of 10% or more at a stimulation frequency of 3 Hz (including both the amplitude and the area under the curve) is considered confirmation of the diagnosis of MG in individual patients40. More recently, a 7–8% cutoff was found to increase sensitivity of this test for MG diagnosis by 6–11%, while preserving high specificity of >95%41. CMAPs were recorded from the extensor digitorum brevis (EDB) muscle of the foot during repeated stimulation of the peroneal nerve below the fibular head. Four out of seven animals had 7% decrement or more in the untreated IgG1-637-challenged group (see Supplemental Table 2). None of the animals in the other experimental groups reached these levels. There were significant differences of the average decrement values between the groups (Fig. 2d and e) that paralleled the aforementioned changes in jitter values (Fig. 2b). Amplitude and area decrement values measured in animals in the untreated and in the IgG4Δhinge-637 control groups ranged between −3% and +5%. Administration of IgG1-637 significantly increased average decrement values to 11% for amplitude and 13% for area decrement. Treatment with IgG4Δhinge-637 significantly reduced these IgG-637-induced decrement values to −0.3% and 1.4%, respectively (both P < 0.0001) (Fig. 2d and e).
Electron microscopic analysis of intercostal neuromuscular junctions
Electron micrographs were used for qualitative and quantitative evaluation of the degree of postsynaptic damage at synaptic boutons in intercostal muscle biopsies taken before, and one week after the first antibody administration (Fig. 3a–f). In line with the mild clinical symptoms, no significant differences of the folding index (a measure for folding complexity of the postsynaptic membrane) were observed between the experimental groups (Fig. 3g). Nonetheless, a widening of the primary and secondary synaptic cleft (indicating ultrastructural damage of the NMJ) was frequently observed (see Fig. 3e). Because no quantitative morphometric analysis is currently available for this change, all images were subjected to blind qualitative scoring (Fig. 3h). This indicated a significant widening of the primary synaptic cleft in the IgG1-637-challenged muscles as compared to the untreated control muscles. In contrast, significant cleft widening was not observed in combination with the IgG4Δhinge-637 treatment (Fig. 3h).
Figure 3.
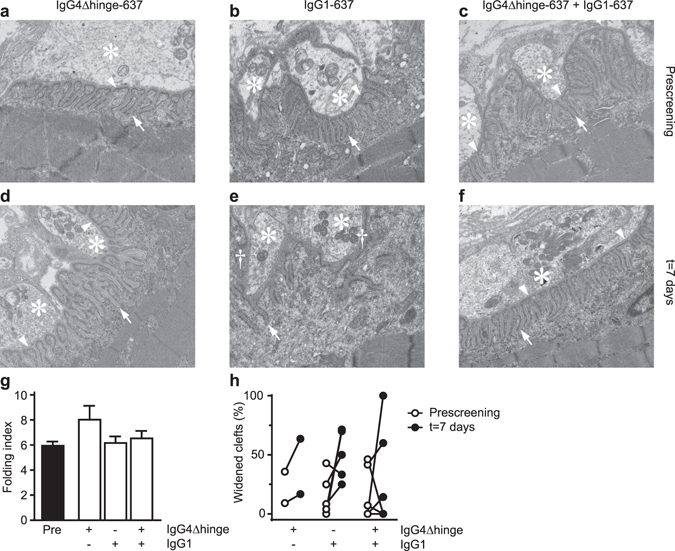
Electron microscopic analyses of intercostal neuromuscular junctions in rhesus monkeys. Transmission electron micrographs showing representative nerve boutons of 3 animals, either before (‘Prescreening’, a,b,c) or seven days after challenge with IgG4Δhinge-637 (d), IgG1-637 (e) or the combination of IgG4Δhinge-637 and IgG1-637 (f). Each micrograph has the dimension of 5 × 6 µm. Asterisks indicate nerve terminals/boutons and arrowheads point at the (intact) primary synaptic clefts. Arrows point to normal secondary postsynaptic clefts/folds; the daggers in panel e indicate widening of the primary synaptic cleft, where the presynaptic and the postsynaptic membrane were separated from each other. (g) The folding index (length of postsynaptic membrane/length of the corresponding presynaptic membrane), a measure of the degree of postsynaptic folding. (h) Blinded scoring of the normal versus widened synaptic clefts.
Plasma and NMJ levels of active complement
In addition to antigenic modulation, the pathogenicity of IgG1-637 may be attributed to antibody-mediated complement activation. To investigate the role of complement in the PTMG model in rhesus monkey, levels of C4b/c, as a measure for overall complement activation, were quantified at different time-points in the plasma of all monkeys. No significant changes in C4b/c levels were observed in time or between groups (Supplementary Fig. 4). Moreover, we analyzed formation of membrane attack complex (MAC, C5b-9) in intercostal muscle biopsies taken before and after antibody treatment (Fig. 4). While little or no MAC staining was found in endplates of unchallenged and IgG4Δhinge-637-treated monkeys, a consistently very strong MAC staining colocalized with human IgG and alpha-bungarotoxin staining in IgG1-637-treated monkeys (Fig. 4a). Treatment with IgG4Δhinge-637 prevented MAC deposition in endplates of IgG1-637 challenged animals by 70% (P < 0.01) (Fig. 4b). Since total human IgG levels (IgG1 + IgG4Δhinge) were comparable between these groups (Fig. 4c), these data suggest that IgG4Δhinge partially blocked binding of IgG1 at the intercostal NMJs.
Figure 4.
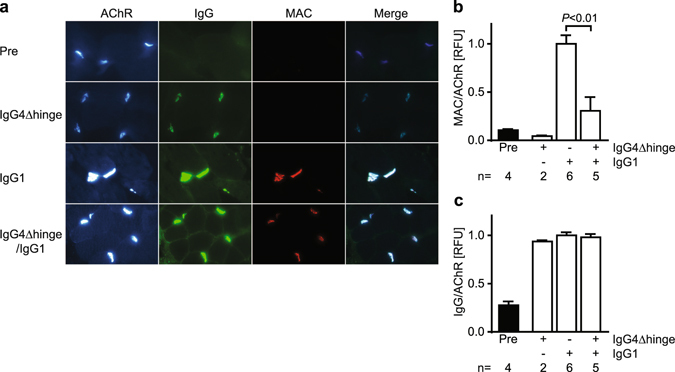
Quantitative Immunofluorescent analysis of NMJ endplates in rhesus monkey intercostal biopsies. Biopsies were obtained from each animal before (“Pre”) or 7 days after antibody challenge with IgG4Δhinge-637, IgG1-637 or the combination of both. (a) Representative photomicrographs from the four groups showing staining of the AChR (detected by alpha-bungarotoxin fluorescence), IgG (IgG + IgG4Δhinge), the membrane attack complex (MAC) and a merged image to show colocalization. Relative fluorescence intensities (RFU) of (b) MAC staining and (c) human IgG staining was normalized with AChR expression in individual endplates. Intensities were quantitated in a total of 589 endplates (5-158 endplates per biopsy) and averaged per biopsy. The number n indicates the number of animals/biopsies analyzed for each condition. Data represent means ± SEM.
Discussion
The presence of circulating autoantibodies is a hallmark of autoimmune disease (AID). In some organ-specific AIDs, like MG, the autoantibodies are solely responsible for the elicitation of pathogenic effects and frequently display restricted epitope-specificity7, 42, 43. In such cases, displacing pathogenic autoantibodies from their target with blocker antibodies (competing antibody formats devoid of pathogenic effector functions) represents a potential treatment strategy. The validity of this concept has been demonstrated previously with MIR-specific antibody formats in AChR MG7, 11 and an aquaporin-4 (AQP4)-specific antibody format in neuromyelitis optica44.
In MG, the autoantibody-mediated pathogenic effector functions include antigenic modulation through auto-antibody-mediated receptor cross-linking4 and complement-mediated lysis of the post-synaptic membrane2, 3. The therapeutic potential of Fab fragments and post-exchange IgG4 has been shown before7, 11, as both can displace pathogenic serum auto-antibodies without being able to activate complement or induce receptor cross-linking, due to their (functional) monovalency. However, the rapid clearance of Fab fragments from the circulation and the potential of IgG4 for indirect cross-linking via residual Fc-receptor interactions are clear disadvantages of these approaches16–18. In addition, IgG4 only gradually becomes functionally monovalent through Fab-arm exchange upon administration in patients and thus could be pathogenic by inducing cross-linking through bivalent interaction at the initiation of therapy11, 15. The present study describes the use of IgG4Δhinge as format for blocker therapy of MG in a non-human primate model. The patient-derived IgG4Δhinge blocked cell surface AChR binding of a pathogenic IgG1 and prevented antigenic modulation in vitro. Treatment of pathogenic IgG1-challenged rhesus monkeys with IgG4Δhinge significantly improved the neuromuscular transmission defects in vivo as measured by electromyography and microscopic analysis of muscle biopsies. The relatively long half-life in combination with monovalent interaction makes this non-pathogenic antibody format superior to Fab fragments7, post-exchange IgG411 or silenced IgG1 formats44 and suitable for clinical applications in MG.
Without covalent disulfide linkages in the hinge region, human IgG4 half-molecules are non-covalently associated through relatively weak interactions at the CH3-CH3 interface24, 45, 46. As a consequence, monovalent IgG4Δhinge molecules are still able to dimerize in a concentration dependent manner and high target expression levels can promote local dimerization and induce crosslinking. Although this effect is not evident from the results, the 4% blocking observed in 1 animal that only received IgG4Δhinge, compared to untreated animals, could thus be explained. Likewise, local IgG concentrations in the endosomes could promote IgG4Δhinge dimerization and increase the avidity for FcRn. This could explain the discrepancy between the clearance rate of IgG4 half-molecules observed in Balb/c mice in our study and that reported by Wilkinson et al.46, i.e. a 2.5-fold faster clearance of IgG4Δhinge relative to wild-type IgG4 in this study, compared to a 10–20-fold faster clearance for IgG4 half-molecules engineered to be more monovalent. Although the pharmacokinetic (PK) profile of IgG4Δhinge is superior to that of Fab fragments, functionally monovalent formats with regular IgG PK would still be preferred. A non-glycosylated one-armed format like the one described for c-Met47, a non-activating functionally monovalent bispecific IgG format (e.g. containing one dummy arm)48, or even a non-activating bispecific IgG containing two non-overlapping AChR-specific arms that supplement each other in blocking auto-antibodies but incapable of inducing AChR-crosslinking, would be worth investigating.
In our study we used a monoclonal antibody to induce disease in our model, while in patients the AChR autoantibodies are polyclonal49. The competition between the therapeutic IgG4Δhinge-637 antibody and poly-clonal patient IgG is variable, based on experiments with the scFv-6379 and Fab-6377. Such an in vitro competition assay could be used to identify patients that might benefit from IgG4Δhinge blocker antibodies prior to therapy. Moreover, the blocker therapy could be extended with a number of other human AChR monoclonal antibodies that are now available7, 49–52.
With the present study efficacy of the therapy could not be tested in a model of severe clinical disease symptoms since we used non-human primates. Therefore our results do not allow definitive conclusions about the safety and efficacy in severe disease, e.g. a myasthenic crisis. Since the NMJ has a remarkable capacity for regeneration and the AChR has a high turnover of ~1 week, we believe that even during pre-existing disease it is meaningful to prevent antibody-binding to newly-formed AChRs.
Specific conditions in which MG blocker therapy might potentially be beneficial include drug-resistant MG, neonatal myasthenia or arthrogryposis, where passive transfer occurs from mother to child via placental transfer. Furthermore, the IgG4Δhinge format might be useful for treatment of other antibody-driven autoimmune channelopathies53, such as neuromyelitis optica44, or organ-specific AIDs in general, where complement activation or antigenic modulation are major pathogenic mechanisms.
Taken together, these results demonstrate that IgG4Δhinge shows good therapeutic efficacy in a well-characterized model for PTMG in rhesus monkeys, which is highly supportive of further investigations into the development of antigen-specific therapies.
Materials and Methods
Cells
FreeStyle™ 293 F (HEK-293F) and FreeStyle™ CHO-S (CHO-S) cells were cultured in FreeStyle™ 293 expression medium and FreeStyle™ CHO expression medium, respectively (Invitrogen, Carlsbad, CA). TE671 (human rhabdomyosarcoma54) cells were cultured in IMDM (Gibco) supplemented with 10% (v/v) heat-inactivated FCS (Bodinco, Alkmaar, the Netherlands), 1% (v/v) penicillin/streptomycin (Gibco), 1 mM pyruvate (Gibco) and 2.5 μM dexamethasone (Sigma).
Cloning and expression of antibodies
Construction of expression vectors for IgG1-637 (pIgG-637) and IgG4-637 (pTomG4MG and pConLamMG) have been described previously11. For the construction of the IgG4Δhinge-637 heavy chain expression vector, the IgG4-637 heavy chain coding sequence was codon optimized with deletion of amino acid residues 216-ESKYGPPCPSCP-230 (EU-numbering conventions are used throughout the manuscript), constituting the genetic IgG4 hinge exon. The whole construct was synthesized de novo by Geneart AG (Regensburg, Germany) and cloned in expression vector pEE6.4 (Lonza Biologics, Slough, UK), resulting in pHG-MG.
All antibodies were produced under serum-free conditions (FreeStyle™ medium) by cotransfecting relevant heavy and light chain expression vectors in HEK-293F cells, using 293fectin (Invitrogen), or CHO-S cells using FreeStyle™ MAX Reagent (Invitrogen), both according to the manufacturer’s instructions. Stable CHO-K1SV clones expressing IgG1-637, IgG4-637 or IgG4Δhinge-637 were obtained after selection with 50 μM MSX.
Antibodies were purified by Protein A affinity chromatography (rProtein A FF, GE Healthcare, Uppsala, Sweden), dialyzed overnight to PBS and filtered-sterilized over 0.2 µM dead-end filters. Concentration of purified IgGs was determined by nephelometry and absorbance at 280 nm. Purified proteins were analyzed by SDS-PAGE, mass spectrometry and glycoanalysis. Batches of IgG were tested by size-exclusion chromatography and shown to be at least 94% monomeric. Endotoxin levels of batches used in vivo were below 0.1 EU/mg IgG.
Antigenic modulation of AChR
Antibody-induced degradation of surface AChR (antigenic modulation) was measured as described11. In short, confluent TE671 cells were incubated for 3 h at 37 °C with serial dilutions of IgG1-637, IgG4Δhinge-637, human intravenous immunoglobulin (IVIg; Immunoglubulin I.V., Sanquin, the Netherlands). The antibodies were diluted in DMEM containing 40 μM cycloheximide (blocking de novo AChR synthesis). After washing of the cells, remaining AChR expression was determined by incubating for 1 h at 37 °C with an excess of 125I-labeled α-bungarotoxin in the same medium (without antibody), washing three times with PBS and assessing the amount of radioactivity bound. Nonspecific binding was measured by incubating cells with unlabeled α-bungarotoxin prior to incubation with 125I-labeled α-bungarotoxin.
Passive transfer myasthenia gravis in rhesus monkeys
Experiments were approved by the institutional ethical Committee on Animal Welfare (protocol DEC-590 BPRC) of the Biomedical Primate Research Center (Rijswijk, the Netherlands). All animal experimental procedures complied with applicable guidelines, regulations and laws of the Netherlands. Female rhesus monkeys (Macaca mulatta) of 3.5 to 8.0 kg were pre-screened for the presence of preexisting anti-human IgG responses and were found negative (data not shown). Antibodies were given as three doses (injected on consecutive days) to guarantee animal safety and enable acute therapy in case of a myasthenic crisis12. Intravenous injections with IgG1-637 and IgG4Δhinge-637 were well tolerated and no acute adverse effects were observed. MG was induced by administration of IgG1-637 at doses of 1.7 mg/kg/day resulting in a total cumulative dose of 5 mg/kg. The effect of IgG4Δhinge-637 was tested alone or in combination with IgG1-637 and was administered 6 hours prior to each IgG1-637 injection at 10 mg/kg/day (total cumulative dose of 30 mg/kg). In the case where IgG4Δhinge-637 was tested alone, a dose of saline was administered instead of the IgG1-637. Whole blood samples were taken on different days and collected in clot activator tubes with gel separator (Greiner) and tubes containing a mix of protease inhibitors and anti-coagulation factors55. The clot activator tubes were centrifuged at room temperature for 10 min at 2000 × g. The resulting serum was collected and used for clinical chemistry or stored at −80 °C. The tubes containing protease inhibitors and anti-coagulation factors were centrifuged at room temperature for 10 min at 1000 × g, no brake. The resulting plasma was aliquoted into 3 tubes and stored at −80 °C. Each animal was used for an experiment only once to avoid the effect of a primate anti-human antibody response. In total fifteen monkeys (Supplementary Table 1) were used which all survived the experiments and were not sacrificed for analysis.
Clinical observation
Body weight was recorded each day the animals were sedated. Clinical muscle weakness was defined by at least two of the following symptoms: difficulty to walk, difficulty to climb, difficulty to eat or swallow, drooping of the eyelids (ptosis), more than 5% weight loss. If any one of the aforementioned tasks (walking, climbing or eating/swallowing) could not be performed at all, weakness was also demonstrated. In this study, none of these symptoms were observed.
Analysis of neuromuscular transmission by single fiber electromyography (SFEMG)
Rhesus monkeys were anesthetized with 10 mg/kg ketamine without the use of muscle relaxants. Stimulated SFEMG was performed in the orbicularis oculi (OO) muscles. As a stimulation electrode, a monopolar needle electrode was placed lateral to the lateral canthus of the eye to stimulate facial nerve branches. Stimulus duration was 0.02 ms and stimulus intensity was increased until a visible movement of the upper eyelid was obtained during 3 Hz stimulation. The recording SF-needle electrode was placed in the orbital part of the OO. As soon as muscle fiber potentials were obtained, stimulation frequency was increased to 10 Hz. All examinations recorded 68–100 sweeps (average was 99.7) of >20 muscle fiber action potentials (MFAPs). Jitter is the measurement of variation of the inter-potential interval. Jitter values were expressed as the mean consecutive difference (MCD), which is defined as the mean time interval between the triggered potential and the time-locked single muscle fiber action potential. Blockings are defined as the absence of a muscle fiber action potential after nerve stimulation. SFEMG was performed at baseline and repeated seven days after administration of antibodies.
Measurement of decrement of the compound muscle action potential (CMAP)
Decrement of CMAP was measured in the extensor digitorum brevis muscle upon stimulation of the peroneal nerve below the fibular head. Recording and stimulation was performed with surface electrodes. The reference electrode was placed distal to the active recording electrode at a distance of 3 to 4 cm. Using single stimuli of 0.1 ms duration and gradually increasing intensity, the current intensity was determined at which a maximal CMAP amplitude was reached. To detect a decremental response, ten stimuli with a 20 to 30% higher stimulus strength (supramaximal) were given at 3, 5 and 10 Hz. Test results had to be reproducible for at least three consecutive measurements and were considered positive when both the amplitude and the area of the negative peak op the CMAP showed a decrement of at least 10%. Average decrement values of individual animals were also analyzed statistically at the level of experimental groups.
Intercostal muscle biopsies
Biopsies were taken before and seven days after the first injection of antibody, under general anesthesia (induced with ketamine and maintained with endotracheal isoflurane/halothane/oxygen) and analgesia (Temgesic, 0.3 mg/mL buprenorfine base, Schering Plough B.V.), as originally described for human muscle56. Pieces of the external layer of parasternal intercostal muscle were prepared free from the internal layer by means of small non-traumatic forceps through an incision between the sixth and seventh rib. The biopsy was then taken carefully from rib-to-rib with pieces of periosteum attached. Subsequently, intercostal muscle biopsies were cut into fragments of 3 mm diameter. For electron microscopic analysis, biopsies submerged in fixation buffer (0.1 M phosphate buffer supplemented with 2.5% glutaraldehyde, pH 7.4) and stored at 4 °C for up to seven days. For immunofluorescent analysis, biopsies were frozen on melting isopentane and subsequently stored at −80 °C.
Electron microscopy
Glutaraldehyde-fixed biopsies were then postfixed with 1% osmiumtetroxide (in 0.1 M phosphate buffer, pH 7.4), dehydrated through a graded ethanol series and embedded in epoxy resin (Glycid ether 100, Serva). Endplates were located in toluidine blue-stained semi-thin sections. Ultra-thin sections from selected areas were contrasted with uranyl acetate and lead citrate and analyzed with a Philips CM 100 electron microscope. Quantitative morphometric analysis was performed as previously described57 and calculated as the postsynaptic membrane length divided by the presynaptic membrane length (folding index).
Postsynaptic widening of the primary or secondary cleft was scored blindly. The definitions for the ‘cleft widening score’ were: severely widened clefts (primary or secondary) = 0; widened clefts (primary or secondary) = 1, normal or very slightly widened clefts (primary or secondary) = 2, clearly intact postsynaptic membrane = 3. Four to twenty-five (median = ten) neuromuscular junctions were scored per monkey for postsynaptic widening. The percentage of widened postsynaptic clefts was calculated per monkey. In this calculation scores of 0 (severely widened clefts) and 1 (widened clefts) were regarded as proof of postsynaptic cleft widening, while scores of 2 (normal or very slightly widened clefts) and 3 (clearly intact postsynaptic membrane) were considered to have no cleft widening.
Immunofluorescence staining, microscopy and quantitative analysis
Biopsies were cryosectioned (10 µm) and stored at −80 °C. Sections were fixed with ice-cold acetone for 10 min. The membrane attack complex (MAC) C5b-9 was stained mouse mAb AE11 (1:50, Hycultbiotech, the Netherlands) for one hour. Immunofluorescent triple staining was performed for one hour with FITC-conjugated sheep anti-human IgG (the Binding Site; minimal cross-reaction with monkey immunoglobulins), and Alexa-647-conjugated alpha-bungarotoxin (1:300, Thermo Fischer Scientific, catalog number B35450) and a Alexa Fluor 594 conjugated goat anti-mouse IgG (H + L) secondary antibody (1:500, Thermo Fischer Scientific, catalog number A-11005) which had minimal cross-reaction to other immunoglobulins used in the staining procedure or present in the biopsies. Washes were performed with PBS containing 0.05% Triton-X100 (3 × 5 min). Sections were mounted with 80% glycerol in PBS. Fluorescent photomicrographs of endplate regions were acquired using µManager software 2.0 on an BX51WI spinning disk confocal fluorescence microscope (Olympus, Hamburg, Germany) with an Hamamatsu EM-CCD C9100 digital camera. Fluorescent intensities of endplates were analyzed using imageJ software (www.imagej.nih.gov/ij/) as described58–60. All staining procedures and fluorescent analysis were performed on coded samples by a blinded investigator.
Statistical analysis
Unless otherwise indicated, differences between treatment groups were tested for significance by a linear mixed model using SAS software (SAS Institute Inc., Cary, NC). In this model subject was included as a random effect and group and/or time as fixed effects. Compound symmetry was used as variance/covariance structure. The binary cleft widening data were analyzed by general estimating equations for longitudinal binary outcome data. P-values below 0.05 were considered significant.
Electronic supplementary material
Losen et al. supplementary material SREP-16-35335A
Acknowledgements
We are indebted to G. Braskamp (deceased), veterinarian physician, for taking intercostal biopsies. We thank L. van Geest, R. Schneider and J. Endert for expert technical assistance, N. Losic for expert statistical assistance and D. Wouters and M. Brouwer for measuring C4b/c levels.
Author Contributions
M.L. designed and performed experiments, analyzed data and co-wrote the manuscript. A.F.L. designed experiments, analyzed data and co-wrote the manuscript. V.H.v.K-M. performed SFEMG measurements, analyzed data and wrote parts of the manuscript. M.L.J. analyzed data, performed statistical analysis and wrote parts of the manuscript. K.G.H. organized and performed rhesus monkey in vivo experiments. F.J.B. designed experiments and analyzed data. T.V. developed the IgG4Δhinge concept. M.J. supervised rhesus monkey in vivo experiments. B.‘tH. set-up rhesus monkey in vivo experiments. M.M-D performed immunofluorescence staining and analyzed data. P.C.M. supervised the intercostal biopsy procedure and wrote parts of the manuscript. P.M-M. designed experiments and wrote parts of the manuscript. E.v.d.E. performed electron microscopy. J.S. designed experiments and wrote parts of the manuscript. M.H.d.B. designed experiments and wrote parts of the manuscript. P.W.H.I.P. designed experiments, analyzed data and wrote parts of the manuscript.
Competing Interests
These authors have a financial interest in Genmab: A.F.L., M.J., F.J.B., T.V., J.S. and P.W.H.I.P. have stock and/or warrants. M.L., P.M-M., J.S., M.H.d.B., and P.W.H.I.P own patent rights to the concept presented in this study. A.F.L., T.V., J.S., F.J.B., and P.W.H.I.P own patent rights to the IgG4Δhinge format presented in this study.
Footnotes
Mario Losen, Aran F. Labrijn and Vivianne H. van Kranen-Mastenbroek contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01019-5
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mario Losen, Email: m.losen@maastrichtuniversity.nl.
Aran F. Labrijn, Email: a.labrijn@genmab.com
References
- 1.Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976;26:1054–1059. doi: 10.1212/WNL.26.11.1054. [DOI] [PubMed] [Google Scholar]
- 2.Engel AG, Arahata K. The membrane attack complex of complement at the endplate in myasthenia gravis. Ann N Y Acad Sci. 1987;505:326–332. doi: 10.1111/j.1749-6632.1987.tb51301.x. [DOI] [PubMed] [Google Scholar]
- 3.Sahashi K, Engel AG, Lambert EH, Howard FM., Jr. Ultrastructural localization of the terminal and lytic ninth complement component (C9) at the motor end-plate in myasthenia gravis. J Neuropathol Exp Neurol. 1980;39:160–172. doi: 10.1097/00005072-198003000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Drachman DB, Angus CW, Adams RN, Michelson JD, Hoffman GJ. Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med. 1978;298:1116–1122. doi: 10.1056/NEJM197805182982004. [DOI] [PubMed] [Google Scholar]
- 5.Mitra AK, McCarthy MP, Stroud RM. Three-dimensional structure of the nicotinic acetylcholine receptor and location of the major associated 43-kD cytoskeletal protein, determined at 22 A by low dose electron microscopy and x-ray diffraction to 12.5 A. J Cell Biol. 1989;109:755–774. doi: 10.1083/jcb.109.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masuda T, et al. Antibodies against the main immunogenic region of the acetylcholine receptor correlate with disease severity in myasthenia gravis. J Neurol Neurosurg Psychiatry. 2012;83:935–940. doi: 10.1136/jnnp-2012-302705. [DOI] [PubMed] [Google Scholar]
- 7.Graus YF, et al. Human anti-nicotinic acetylcholine receptor recombinant Fab fragments isolated from thymus-derived phage display libraries from myasthenia gravis patients reflect predominant specificities in serum and block the action of pathogenic serum antibodies. J Immunol. 1997;158:1919–1929. [PubMed] [Google Scholar]
- 8.Luo J, et al. Main immunogenic region structure promotes binding of conformation-dependent myasthenia gravis autoantibodies, nicotinic acetylcholine receptor conformation maturation, and agonist sensitivity. J Neurosci. 2009;29:13898–13908. doi: 10.1523/JNEUROSCI.2833-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li F, et al. Fusion protein of single-chain variable domain fragments for treatment of myasthenia gravis. Neural Regen Res. 2014;9:851–856. doi: 10.4103/1673-5374.131611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Losen M, et al. Treatment of myasthenia gravis by preventing acetylcholine receptor modulation. Ann N Y Acad Sci. 2008;1132:174–179. doi: 10.1196/annals.1405.034. [DOI] [PubMed] [Google Scholar]
- 11.van der Neut Kolfschoten M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 12.Toyka KV, et al. Passively transferred myasthenia gravis: protection of mouse endplates by Fab fragments from human myasthenic IgG. J Neurol Neurosurg Psychiatry. 1980;43:836–842. doi: 10.1136/jnnp.43.9.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labrijn AF, et al. Species-Specific Determinants in the IgG CH3 Domain Enable Fab-Arm Exchange by Affecting the Noncovalent CH3-CH3 Interaction Strength. J Immunol. 2011;187:3238–3246. doi: 10.4049/jimmunol.1003336. [DOI] [PubMed] [Google Scholar]
- 14.van der Zee JS, van Swieten P, Aalberse RC. Serologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J Immunol. 1986;137:3566–3571. [PubMed] [Google Scholar]
- 15.Labrijn AF, et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol. 2009;27:767–771. doi: 10.1038/nbt.1553. [DOI] [PubMed] [Google Scholar]
- 16.Armour KL, van de Winkel JG, Williamson LM, Clark MR. Differential binding to human FcgammaRIIa and FcgammaRIIb receptors by human IgG wildtype and mutant antibodies. Mol Immunol. 2003;40:585–593. doi: 10.1016/j.molimm.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Niwa R, et al. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods. 2005;306:151–160. doi: 10.1016/j.jim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Parren PW, et al. On the interaction of IgG subclasses with the low affinity Fc gamma RIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J Clin Invest. 1992;90:1537–1546. doi: 10.1172/JCI116022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayam Trouth A, Dabi A, Solieman N, Kurukumbi M, Kalyanam J. Myasthenia gravis: a review. Autoimmune Dis. 2012;2012:874680–10. doi: 10.1155/2012/874680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toro-Goyco E, Cora EM, Kessler MJ, Martinez-Carrion M. Induction of experimental myasthenia gravis in rhesus monkeys: a model for the study of the human disease. P R Health Sci J. 1986;5:13–18. [PubMed] [Google Scholar]
- 21.Tarrab-Hazdai R, Aharonov A, Silman I, Fuchs S, Abramsky O. Experimental autoimmune myasthenia induced in monkeys by purified acetylcholine receptor. Nature. 1975;256:128–130. doi: 10.1038/256128a0. [DOI] [PubMed] [Google Scholar]
- 22.Aase A, Sandlie I, Norderhaug L, Brekke OH, Michaelsen TE. The extended hinge region of IgG3 is not required for high phagocytic capacity mediated by Fc gamma receptors, but the heavy chains must be disulfide bonded. Eur J Immunol. 1993;23:1546–1551. doi: 10.1002/eji.1830230723. [DOI] [PubMed] [Google Scholar]
- 23.Klein M, et al. Expression of biological effector functions by immunoglobulin G molecules lacking the hinge region. Proc Natl Acad Sci USA. 1981;78:524–528. doi: 10.1073/pnas.78.1.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose RJ, et al. Quantitative analysis of the interaction strength and dynamics of human IgG4 half-molecules by native mass spectrometry. Structure. 2011;19:1274–1282. doi: 10.1016/j.str.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Angal S, et al. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol Immunol. 1993;30:105–108. doi: 10.1016/0161-5890(93)90432-B. [DOI] [PubMed] [Google Scholar]
- 26.Bloom JW, Madanat MS, Marriott D, Wong T, Chan SY. Intrachain disulfide bond in the core hinge region of human IgG4. Protein Sci. 1997;6:407–415. doi: 10.1002/pro.5560060217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuurman J, Perdok GJ, Gorter AD, Aalberse RC. The inter-heavy chain disulfide bonds of IgG4 are in equilibrium with intra-chain disulfide bonds. Mol Immunol. 2001;38:1–8. doi: 10.1016/S0161-5890(01)00050-5. [DOI] [PubMed] [Google Scholar]
- 28.Teeling JL, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1793–1800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- 29.Kim JK, Tsen MF, Ghetie V, Ward ES. Evidence that the hinge region plays a role in maintaining serum levels of the murine IgG1 molecule. Mol Immunol. 1995;32:467–475. doi: 10.1016/0161-5890(95)00019-B. [DOI] [PubMed] [Google Scholar]
- 30.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 31.Kim JK, Tsen MF, Ghetie V, Ward ES. Catabolism of the murine IgG1 molecule: evidence that both CH2-CH3 domain interfaces are required for persistence of IgG1 in the circulation of mice. Scand J Immunol. 1994;40:457–465. doi: 10.1111/j.1365-3083.1994.tb03488.x. [DOI] [PubMed] [Google Scholar]
- 32.Petkova SB, et al. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol. 2006;18:1759–1769. doi: 10.1093/intimm/dxl110. [DOI] [PubMed] [Google Scholar]
- 33.Heinemann S, Bevan S, Kullberg R, Lindstrom J, Rice J. Modulation of acetylcholine receptor by antibody against the receptor. Proc Natl Acad Sci USA. 1977;74:3090–3094. doi: 10.1073/pnas.74.7.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kao I, Drachman DB. Myasthenic immunoglobulin accelerates acetylcholine receptor degradation. Science. 1977;196:527–529. doi: 10.1126/science.850793. [DOI] [PubMed] [Google Scholar]
- 35.Loutrari H, Kokla A, Tzartos SJ. Passive transfer of experimental myasthenia gravis via antigenic modulation of acetylcholine receptor. Eur J Immunol. 1992;22:2449–2452. doi: 10.1002/eji.1830220939. [DOI] [PubMed] [Google Scholar]
- 36.Bromberg MB, Scott DM. Single fiber EMG reference values: reformatted in tabular form. AD HOC Committee of the AAEM Single Fiber Special Interest Group. Muscle Nerve. 1994;17:820–821. doi: 10.1002/mus.880170720. [DOI] [PubMed] [Google Scholar]
- 37.Kokubun N, et al. Reference values for voluntary and stimulated single-fibre EMG using concentric needle electrodes: A multicentre prospective study. Clin Neurophysiol. 2012;123:613–620. doi: 10.1016/j.clinph.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 38.Abraham A, et al. Electrophysiological testing is correlated with myasthenia gravis severity. Muscle Nerve. 2016 doi: 10.1002/mus.25539. [DOI] [PubMed] [Google Scholar]
- 39.Gaither AM, et al. Videotaped behavior as a predictor of clinical outcome in rhesus macaques (Macaca mulatta) Comp Med. 2014;64:193–199. [PMC free article] [PubMed] [Google Scholar]
- 40.Desmedt JE, Borenstein S. Diagnosis of myasthenia gravis by nerve stimulation. Ann N Y Acad Sci. 1976;274:174–188. doi: 10.1111/j.1749-6632.1976.tb47684.x. [DOI] [PubMed] [Google Scholar]
- 41.Abraham A, et al. Repetitive nerve stimulation cutoff values for the diagnosis of myasthenia gravis. Muscle Nerve. 2017;55:166–170. doi: 10.1002/mus.25214. [DOI] [PubMed] [Google Scholar]
- 42.Schulz E, Benker G, Bethauser H, Stempka L, Hufner M. An autoimmuno-dominant thyroglobulin epitope characterized by a monoclonal antibody. J Endocrinol Invest. 1992;15:25–30. doi: 10.1007/BF03348649. [DOI] [PubMed] [Google Scholar]
- 43.Warren KG, Catz I, Steinman L. Fine specificity of the antibody response to myelin basic protein in the central nervous system in multiple sclerosis: the minimal B-cell epitope and a model of its features. Proc Natl Acad Sci USA. 1995;92:11061–11065. doi: 10.1073/pnas.92.24.11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tradtrantip L, et al. Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann Neurol. 2012;71:314–322. doi: 10.1002/ana.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rispens T, Ooijevaar-de Heer P, Bende O, Aalberse RC. Mechanism of Immunoglobulin G4 Fab-arm Exchange. J Am Chem Soc. 2011;133:10302–10311. doi: 10.1021/ja203638y. [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson IC, et al. Monovalent IgG4 molecules: Immunoglobulin Fc mutations that result in a monomeric structure. MAbs. 2013;5:406–417. doi: 10.4161/mabs.23941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin H, et al. MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res. 2008;68:4360–4368. doi: 10.1158/0008-5472.CAN-07-5960. [DOI] [PubMed] [Google Scholar]
- 48.Labrijn AF, et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci USA. 2013;110:5145–5150. doi: 10.1073/pnas.1220145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vrolix K, Fraussen J, Losen M, Stevens J, Lazaridis K, Molenaar PC, et al. Clonal heterogeneity of thymic B cells from early-onset myasthenia gravis patients with antibodies against the acetylcholine receptor. J Autoimmu. 2014;52:101–112. doi: 10.1016/j.jaut.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Farrar J, Portolano S, Willcox N, Vincent A, Jacobson L, Newsom-Davis J, et al. Diverse Fab specific for acetylcholine receptor epitopes from a myasthenia gravis thymus combinatorial library. Int Immunol. 1997;9:1311–1318. doi: 10.1093/intimm/9.9.1311. [DOI] [PubMed] [Google Scholar]
- 51.Fostieri E, Tzartos SJ, Berrih-Aknin S, Beeson D, Mamalaki A. Isolation of potent human Fab fragments against a novel highly immunogenic region on human muscle acetylcholine receptor which protect the receptor from myasthenic autoantibodies. Eur J Immunol. 2005;35:632–643. doi: 10.1002/eji.200425671. [DOI] [PubMed] [Google Scholar]
- 52.Matthews I, Sims G, Ledwidge S, Stott D, Beeson D, Willcox N, et al. Antibodies to acetylcholine receptor in parous women with myasthenia: evidence for immunization by fetal antigen. Lab Invest. 2002;82:1407–1417. doi: 10.1097/01.LAB.0000032379.63784.9C. [DOI] [PubMed] [Google Scholar]
- 53.Vincent A. Autoimmune channelopathies: new antibody-mediated disorders of the central nervous system. F1000 Biol Rep. 2009;1:61. doi: 10.3410/B1-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stratton MR, et al. Characterization of the human cell line TE671. Carcinogenesis. 1989;10:899–905. doi: 10.1093/carcin/10.5.899. [DOI] [PubMed] [Google Scholar]
- 55.Abbink JJ, et al. Quantification of functional and inactivated alpha 2-macroglobulin in sepsis. Thromb Haemost. 1991;65:32–39. [PubMed] [Google Scholar]
- 56.Jennekens FG, et al. Deficiency of acetylcholine receptors in a case of end-plate acetylcholinesterase deficiency: a histochemical investigation. Muscle Nerve. 1992;15:63–72. doi: 10.1002/mus.880150112. [DOI] [PubMed] [Google Scholar]
- 57.Gomez AM, et al. Proteasome inhibition with bortezomib depletes plasma cells and autoantibodies in experimental autoimmune myasthenia gravis. J Immunol. 2011;186:2503–2513. doi: 10.4049/jimmunol.1002539. [DOI] [PubMed] [Google Scholar]
- 58.Gomez AM, et al. Silencing of Dok-7 in Adult Rat Muscle Increases Susceptibility to Passive Transfer Myasthenia Gravis. Am J Pathol. 2016;186:2559–2568. doi: 10.1016/j.ajpath.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 59.Losen M, et al. Standardization of the experimental autoimmune myasthenia gravis (EAMG) model by immunization of rats with Torpedo californica acetylcholine receptors—Recommendations for methods and experimental designs. Exp Neurol. 2015;270:18–28. doi: 10.1016/j.expneurol.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tse, N. et al. The neuromuscular junction: measuring synapse size, fragmentation and changes in synaptic protein density using confocal fluorescence microscopy. J Vis Exp 52220, doi:10.3791/52220 (2014). [DOI] [PMC free article] [PubMed]
- 61.Corper AL, et al. Structure of human IgM rheumatoid factor Fab bound to its autoantigen IgG Fc reveals a novel topology of antibody-antigen interaction. Nat Struct Biol. 1997;4:374–381. doi: 10.1038/nsb0597-374. [DOI] [PubMed] [Google Scholar]
- 62.Guddat LW, Herron JN, Edmundson AB. Three-dimensional structure of a human immunoglobulin with a hinge deletion. Proc Natl Acad Sci USA. 1993;90:4271–4275. doi: 10.1073/pnas.90.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saphire EO, et al. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science. 2001;293:1155–1159. doi: 10.1126/science.1061692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Losen et al. supplementary material SREP-16-35335A


