Abstract
Background
Several medicinal properties have been reported for plants in the genus Evolvulus, such as a brain tonic and antifungal from Evolvulus alsinoides, and a sedative and an anthelmintic from Evolvulus nummularius. Therefore, the correct identification of the source plants is critically important. The aim of this research was to investigate the micromorphology of two Evolvulus taxa used for herbal medicines compared with one worldwide ornamental species by using peeling, paraffin embedding, acetolysis, and SEM methods in order to support species identification.
Results
Our findings indicate that all taxa share several common features, such as a single layer of epidermis on both sides of leaf surfaces, sinuous anticlinal epidermal cell walls, anomocytic, paracytic or laterocytic stomata, and capitate glandular trichomes. Y-shaped hairs were found in two species but not in E. nummularius. Similarly, isobilateral mesophyll occurs in both E. alsinoides and Evolvulus glomeratus, but a dorsiventral mesophyll is present in E. nummularius. Stems consist of a single layer of epidermis, one to four chlorenchyma layers, one to seven layers of cortical cells and a bicollateral bundle with pith in the center. The seed coat epidermal cell shapes were irregular or polygonal with raised and undulated anticlinal boundaries, and folded or flattened to concave periclinal walls. Pollens of all taxa are monads, spheroidally shaped with 28–47 µm diameter, and 15-pantocolpate apertures type with microechinate ornamentation.
Conclusions
An identification key to species is constructed based on leaf anatomy and seed coat characters. This data can be used in other subjects such as pharmaceutical botany, organic chemistry, taxonomy and horticulture, in terms of species identification.
Keywords: Evolvulus alsinoides, Evolvulus nummularius, Evolvulus glomeratus, Leaf anatomy, Medicinal plant, Pollen, Seed coat
Background
The genus Evolvulus L. belongs to the family Convolvulaceae with two taxa (Evolvulus alsinoides (L.) L. and Evolvulus nummularius (L.) L.) that are used in Asian herbal medicine (Auddy et al. 2003; Chen 2007; Khare 2007; Ayyanar and Ignacimuthu 2011; Naikawadi et al. 2016), while Evolvulus glomeratus Nees & Martius subsp. grandiflorus (Parodi) Ooststr. is a worldwide ornamental plant (Staples 2010). Some medicinal properties have been reported from E. alsinoides and E. nummularius. Several substances were found in E. alsinoides, i.e., flavonols, flavonoids, alkaloids, cardiac glycosides, saponins, the alkanes pentatriacontane and triacontane, the phytosterol, β-sitosterol, phenolics, and tannins (Austin 2008; Naikawadi et al. 2016) and the plant is used as brain and memory tonic herb (Naikawadi et al. 2016), an anti-asthmatic, for treating uterine bleeding (Khare 2007), insanity, epilepsy and nervous debility (Auddy et al. 2003), for antibacterial, antifungal, and antiulcer properties (Austin 2008). The most common uses for medicinal applications are from India and surrounding regions (Manandhar 1985; Auddy et al. 2003; Khare 2007; Ayyanar and Ignacimuthu 2011; Naikawadi et al. 2016), however uses from some other countries in Southeastern Asia were found such as Taiwan (Chen 2007), Vietnam, Thailand (Austin 2008), and Philippines (Quisumbing 1978). In the New World people also use E. alsinoides, but there are fewer reports than the Old. Moreover, weak sedative and anthelmintic properties were reported in E. nummularius (Khare 2007; Ayyanar and Ignacimuthu 2011). Because of all these attributed properties, two Evolvulus species have been sold commonly via the internet as a powder used for brewing medicinal tea. Therefore, a proper identification is needed to correctly identify herbal ingredients used in remedies sold for home use.
Plant anatomy is one of the alternative ways to differentiate plant species (Stuessy 2009). Previous research on vegetative anatomy in Convolvulaceae has been reported to be useful for classification within several genera such as Argyreia Lour. (Sayeedud-Din 1953; Tayade and Patil 2012b; Staples et al. 2015), Calystegia R.Br. (Tayade and Patil 2012b), Cressa L. (Tayade and Patil 2012b), Evolvulus L. (Tayade and Patil 2012b; Harms 2014), Hewittia Wight & Arn. (Tayade and Patil 2012b), Hildebrantia Vatke (Tayade and Patil 2012b), Ipomoea L. (Sayeedud-Din 1953; Tayade and Patil 2012b), Merremia Dennst. (Pisuttimarn et al. 2013), and Quamoclit Mill., (Sayeedud-Din 1953) which is now treated as a synonym of Ipomoea (Staples 2010). However, only one species of Evolvulus, E. alsinoides, has been investigated (Metcalfe and Chalk 1950; Tayade and Patil 2012b). Metcalfe and Chalk (1950) provided some diagnostic characters for the genus, such as two-armed or Y-shaped hair, cruciferous stomata type and cotyledons with secretory cells.
Hallier (1893) separated Convolvulaceae into two informal, non-taxonomic groups based on pollen type; Echinoconieae (grains with spines) and Psiloconiae (grains without spines, appearing ‘smooth’). However, Erdtman (1952) used different terms to separate Convolvulaceae pollen into two types: Ipomoea type and other types. Meanwhile, pollen morphology of some groups of Convolvulaceae has been studied such as Convolvulaceae in southern new world (Tellería and Daners 2003), Bonamia Thouars (Lewis 1971), Convolvulus L. (Perveen et al. 1989; Menemen and Jury 2002), Cuscuta L. (Hamed 2005; Costea et al. 2008; Welsh et al. 2010; Dettke et al. 2014), Ipomoea (Hsiao and Kuoh 1995; Rajurkar et al. 2011; Robertson 1982), Odonellia K.R. Robertson (Robertson 1982) and Stylisma Raf. (Lewis 1971). A recent study of South American Convolvulaceae pollen classified Evolvulus pollen into microechinate-microgranulate pollen (Tellería and Daners 2003) and E. alsinoides has oval and tricolpate type (Singh and Dhakre 2010).
Seeds also offer useful taxonomic data. Convolvulaceae seeds provided some taxonomically significant characters to construct keys to some species in Egypt (Abdel Khalik and Osman 2007) and also some Ipomoea species (Rao and Leela 1993). The seed characters used to construct a key to species are shape, seed ornamentation, anticlinal boundaries and epidermal cell shape (Gunn 1969; Rao and Leela 1993; Abdel Khalik and Osman 2007). Seed morphology of Cuscuta and Ipomoea were studied by several researchers (Rao and Leela 1993; Abdel Khalik 2006; Hamed 2005; Abdel Khalik and Osman 2007).
In this study, leaf and stem micromorphological characters of three taxa in the genus Evolvulus are investigated and compared. In addition, seed and pollen morphology were investigated to support taxonomic identification because some medicinal preparations use the whole plant thus pollen and/or seeds could be present as well as leaves and stems. This approach maximizes the capacity for making an identification of any plant parts obtained from herbal medicaments. Furthermore, this research highlights the need for anatomical and micromorphological investigation of Evolvulus in tropical America, where about 100 species are known to occur (Junqueira and Simão-Bianchini 2006). So far as we know, there has been no intensive anatomical study done for the neotropical taxa of Evolvulus.
Methods
Sample collection and herbarium specimen preparation
Fresh materials were collected and voucher specimens were prepared as a standard method for plant taxonomy (Bridson and Forman 1999), and deposited at the Forest Herbarium (BKF) and Queen Sirikit Botanic Garden Herbarium (QBG) (Table 1).
Table 1.
List of specimens used in this study
| Plant species | Localities | Collector number | Herbarium |
|---|---|---|---|
| Evolvulus alsinoides var. decumbens | Thailand, Khon Kaen | KK & PT 12 | BKF, QBG |
| Thailand, Phetchaburi | KK & PT 13 | BKF, QBG | |
| E. glomeratus ssp. grandiflorus | Thailand, Bangkok | KK & PT 11 | BKF, QBG |
| Thailand, Khon Kaen | KK & PT 14 | BKF, QBG | |
| E. nummularius | Thailand, Bangkok | KK & PT 10 | BKF, QBG |
| Thailand, Khon Kaen | KK & PT 15 | BKF, QBG |
Anatomical study (peeling and paraffin methods)
Leaf surfaces from five leaves in each taxon were scraped by a razor blade, stained with 1 % Safranin-O and dehydrated by concentration series of ethanol. Leaf epidermal slides were mounted in DePeX mounting media. At least five leaves from each taxon were cut, dehydrated, infiltrated, and embedded in paraffin using a protocol modified from Johansen (1940). Sections were cut by sliding microtome (Leica SM2000R). Plant tissues were stained by Safranin-O and counterstained with Fast green and mounted in DePeX. Permanent slides were investigated and photographed under light microscopy using an Olympus BX43 with Olympus DP11 digital camera attached. The anatomical terminology used follows Metcalfe and Chalk (1950), Chen et al. (2008) and Pisuttimarn et al. (2013).
Pollen morphological study
Anthers from dried flower buds were treated by standard acetolysis method (Erdtman 1960). For light microscopy (LM), pollen was preserved in silicone oil. At least fifty grains of pollen were investigated randomly under LM. Pollen for scanning electron microscopy (SEM) was dried in the air, and coated with platinum and palladium (Pt + Pd) by ion sputter (Hitachi E102). Photographs were taken under SEM (Hitachi S-2500). Pollen terminology according to an illustrated handbook (Hesse et al. 2009) was used to describe pollen features.
Seed morphology
Mature seeds were fixed in 70 % ethanol and cleaned by sonicator. Samples were fixed on a stub, coated with platinum and palladium (Pt + Pd) by ion sputter (Hitachi E102) and investigated by SEM (Hitachi S-2500). Descriptions follow Abdel Khalik and Osman (2007) and Juan et al. (1999).
Results
Leaf and stem anatomy
Leaf epidermal cell shapes were polygonal with sinuous or straight to curved anticlinal walls (Fig. 1a–c, g–i). Anisocytic, anomocytic, and paracytic stomatal types were found on both leaf surfaces, especially on the abaxial side (Fig. 1g–i). Y-shaped hairs were present on both leaf surfaces of E. alsinoides collected from Khon Kaen and E. glomeratus, and the abaxial side of E. alsinoides from Phetchaburi (Fig. 1d, e, h–k). However, the hairs were absent in E. nummularius (Fig. 1c, f, i, l). Capitate glands were also present on both surfaces (Fig. 1e, f, j–l). The glands are composed of one small base cell, a single short stalk cell and two to four apical cells in a spheroidal or ellipsoid head (Fig. 1j, k).
Fig. 1.
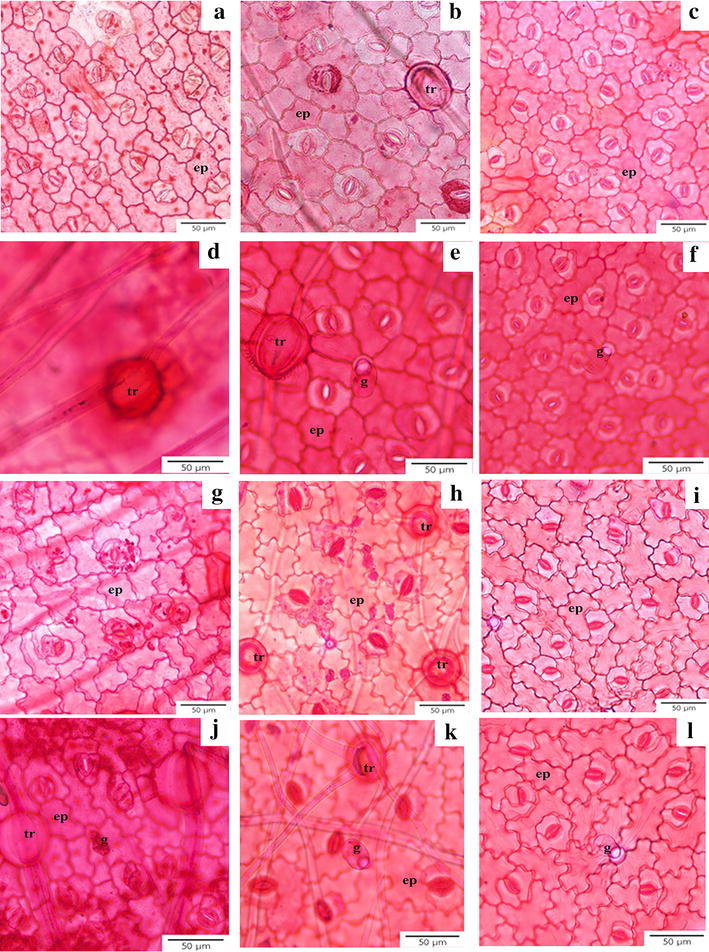
Epidermis under LM. Sinuous or straight to curved anticlinal wall and stomata occurring at the adaxial surface of E. alsinoides (a), E. glomeratus (b) and E. nummularius (c). Y-shaped hair on adaxial surface of E. alsinoides from Khon Kaen (d). Y-shaped hair and capitate gland of E. glomeratus (e), capitate gland on leaf surface of E. nummularius (f). Sinuous or straight to curved anticlinal walls on abaxial epidermal layer of E. alsinoides (g), E. glomeratus (h) and E. nummularius (i). Evolvulus alsinoides (j) and E. glomeratus (k) possess the Y-shaped hairs and capitate glands, but E. nummularius (l) has no hairs. ep epidermis, tr trichome, g capitate gland
Leaf
Blades in transverse section presented a single epidermal layer on both sides. Tanniniferous epidermal cells were detected only on the adaxial epidermis of E. alsinoides from Phetchaburi (Fig. 2a). Isobilateral mesophyll type was detected in E. alsinoides and E. glomeratus, while dorsiventral mesophyll type was restricted to E. nummularius (Fig. 2d–f). Leaf thickness is between 70–140 µm, except in E. glomeratus: its leaves are thicker than the others with approximately 200–240 µm thickness (Fig. 2d–f). Vascular bundles at the midrib of all species are bicollateral type consisting of the sclerenchyma arch (Fig. 2a–c). Leaf margins are rounded to slightly acute (Fig. 2g–i). Leaf characters of all species are summarized in Table 2.
Fig. 2.
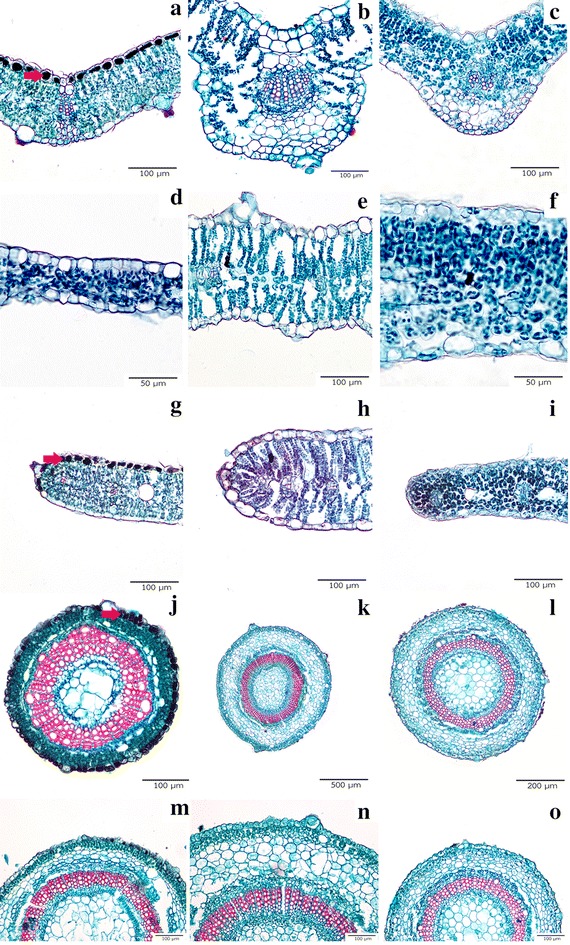
Transverse sections of leaf and stem. A single epidermal layer and bicollateral vascular bundle of E. alsinoides (a), E. glomeratus (b) and E. nummularius (c). Tanniniferous epidermal cell is unique in E. alsinoides from Petchaburi (a, g, j indicated by red arrows). Isobilateral mesophyll present in E. alsinoides (d) and E. glomeratus (e). Evolvulus nummularius showing dorsiventral mesophyll type (f). Rounded leaf margins of E. alsinoides (g), E. glomeratus (h) and E. nummularius (i). Outline of stem in E. alsinoides (j), E. glomeratus (k) and E. nummularius (l). Enlarged view to show the stem layers of E. alsinoides (m), E. glomeratus (n) and E. nummularius (o)
Table 2.
Comparison of the leaf anatomy in three taxa
| Plant taxa | E. alsinoides var. decumbens (KK & PT 12) | E. alsinoides var. decumbens (KK & PT 13) | E. glomeratus ssp. grandiflorus | E. nummularius |
|---|---|---|---|---|
| Characters | ||||
| Leaf epidermis | ||||
| Adaxial surface | ||||
| Cell shape | Polygonal | Polygonal | Polygonal | Polygonal |
| Anticlinal cell wall | Sinuous or straight to curved | Sinuous or straight to curved | Sinuous or straight to curved | Sinuous or straight to curved |
| Stomatal type | Anisocytic, anomocytic and paracytic | Anisocytic, anomocytic and paracytic | Anisocytic, anomocytic and paracytic | Anisocytic, anomocytic and paracytic |
| Y-shaped hairs | Present | Absent | Present | Absent |
| Capitate gland | Present | Present | Present | Present |
| Abaxial surface | ||||
| Cell shape | Polygonal | Polygonal | Polygonal | Polygonal |
| Anticlinal cell wall | Sinuous or straight to curved | Sinuous or straight to curved | Sinuous or straight to curved | Sinuous or straight to curved |
| Stomatal type | Anisocytic, anomocytic and paracytic | Anisocytic, anomocytic and paracytic | Anisocytic, anomocytic and paracytic | Anisocytic, anomocytic and paracytic |
| Y-shaped hairs | Present | Present | Present | Absent |
| Capitate gland | Present | Present | Present | Present |
| Leaf transverse section | ||||
| Epidermal layer | Single layer | Single layer | Single layer | Single layer |
| Parenchyma at midrib | 2‒4 layers | 2‒4 layers | 4‒5 layers | 3‒7 layers |
| Mesophyll | Isobilateral | Isobilateral | Isobilateral | Dorsiventral |
| Midrib vascular bundle | Bicollateral type | Bicollateral type | Bicollateral type | Bicollateral type |
| Leaf thickness (µm) | 70‒100 | 70‒100 | 200‒240 | 120‒140 |
| Leaf margins | Rounded to slightly acute | Rounded to slightly acute | Rounded to slightly acute | Rounded to slightly acute |
| Stem transverse section | ||||
| Epidermal layer | Single layer | Single layer | Single layer | Single layer |
| Chlorenchyma layer | 1‒3 layers | 2‒4 layers | 2‒4 layers | 2‒4 layers |
| Cortex | 1‒3 layers | 3‒5 layers | 5‒7 layers | 5‒7 layers |
| Vascular bundle | Bicollateral type | Bicollateral type | Bicollateral type | Bicollateral type |
| Pith | Present | Present | Present | Present |
Stems
As seen in transverse sections stems in all species are composed of a single epidermis with one to four layers of chlorenchyma cells. Evolvulus alsinoides is the only species that possesses the tanniferous cells in the epidermis. Cortex was restricted by one to seven parenchyma cell layers. Vascular tissues are the bicollateral bundle type with cylindrical xylem (Fig. 2j–o). Pith was observed at the center. All stem anatomical characters are summarized (Table 2).
Pollen morphology
All species share some common pollen characters i.e., monads, medium size and 15-pantocolpate apertures (5 polar × 5 equatorial × 5 polar arrangement of the colpi) (Fig. 3a–i). The length of polar axis (P) is between 27–45 µm, whereas 28–47 µm in equatorial axis (E). Therefore, the P/E ratio is calculated to be a spheroidal shape. Evolvulus alsinoides and E. nummularius have similar size; 27–33 µm in polar axis and 28–33 µm in equatorial axis: however E. glomeratus differs from the other two species by having 39–45 µm polar axis and 41–47 µm equatorial length. Pantocolpate apertures and microechinate ornamentation were recorded in all species (Fig. 3j–l) (Table 3).
Fig. 3.
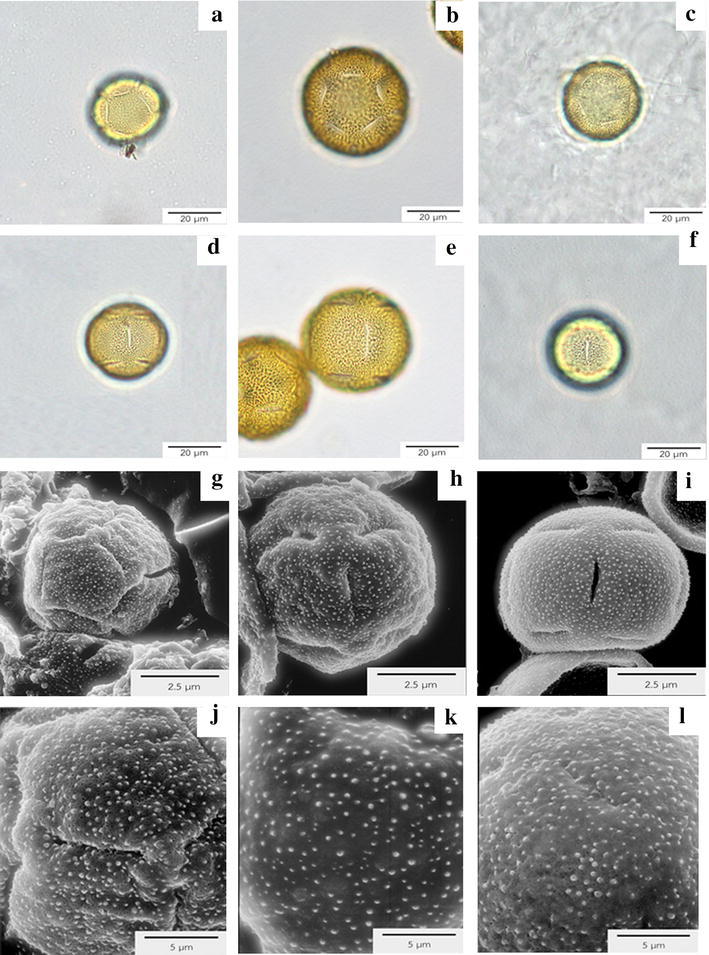
Pollen under light microscopy (LM) and scanning electron microscopy (SEM). a–c Polar view under LM of E. alsinoides (a), E. glomeratus (b) and E. nummularius (c), which demonstrates the monad pollen unit and 5-pantocolpate apertures. d–f Monad pollen under LM with 5-pantocolpate apertures at equatorial view of E. alsinoides (d), E. glomeratus (e) and E. nummularius (f). g–i SEM of E. alsinoides (g) in polar view, showing 5-pantocolpate apertures. Polar view under SEM of E. glomeratus (h), equatorial view of E. nummularius (i) presenting 5-pantocolpate apertures. j–l Microechinate ornamentation of E. alsinoides (j), E. glomeratus (k) and E. nummularius (l)
Table 3.
Pollen and seed morphological characters in all taxa
| Plant taxa | E. alsinoides var. decumbens (KK & PT 12) | E. alsinoides var. decumbens (KK & PT 13) | E. glomeratus ssp. grandiflorus | E. nummularius |
|---|---|---|---|---|
| Characters | ||||
| Pollen morphology | ||||
| Pollen unit | Monad | Monad | Monad | Monad |
| Size polar axis (P) (µm) | 29–32 | 27–33 | 39–45 | 28–33 |
| Size equatorial axis (E) (µm) | 28–31 | 29–33 | 41–47 | 28–33 |
| Shape | Spheroidal | Spheroidal | Spheroidal | Spheroidal |
| Aperture | 15-pantocolpate | 15-pantocolpate | 15-pantocolpate | 15-pantocolpate |
| Ornamentation | Microechinate | Microechinate | Microechinate | Microechinate |
| Seed morphology | ||||
| Shape | Ovoid | Ovoid | No material available | Broadly elliptic |
| Size | 1–1.5 × 1–1.5 | 1–1.5 × 1–1.5 | No material available | 1.5–2 × 1–1.5 |
| Color | Yellow/orange/red/brown | Yellow/orange/red/brown | No material available | Yellow/brown |
| Surface | Glabrous | Glabrous | No material available | Glabrous |
| Epidermal cell shape | Irregular, polygonal | Irregular, polygonal | No material available | Irregular, polygonal |
| Anticlinal boundaries | Undulate | Undulate | No material available | Undulate |
| Periclinal cell wall | Folded | Folded | No material available | Flattened to concave |
Seed morphology
Seeds of two species studied (the seeds of E. glomeratus were not available) varied from 1–2 mm in length and 1–1.5 mm in width with yellow, orange, red or brown seed coat (Table 3). The seeds are glabrous. Seed shape was trigonous-ovoid with glabrous surface. Epidermal cell shapes were irregular or polygonal in both species with undulate and raised anticlinal boundaries. Periclinal cell walls were flattened to concave in E. nummularius and folded in E. alsinoides. All seed features are summarized in Table 3 (Fig. 4).
Fig. 4.
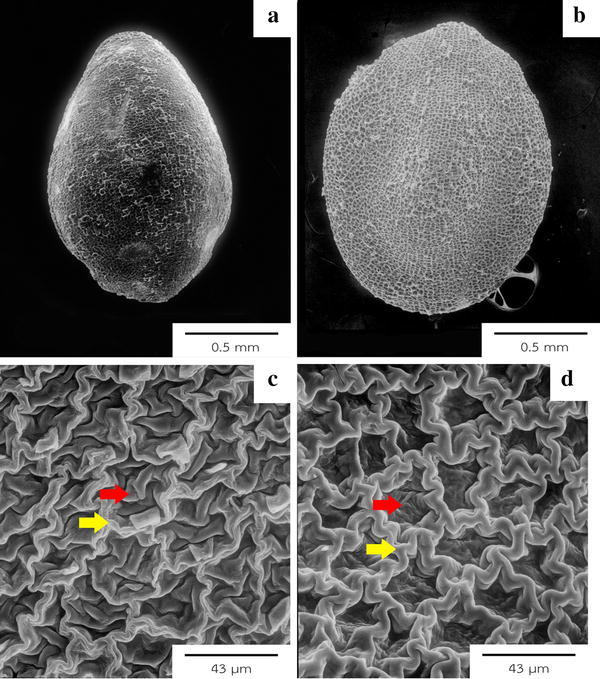
Seed morphology from SEM. Glabrous and ovoid shaped seed of E. alsinoides (a) from Petchaburi, broadly ellipsoid seed with glabrous surface in E. nummularius (b), close up of E. alsinoides (c) from Khon Kaen, seed testa showing undulate anticlinal boundaries (yellow arrows) and folded periclinal cell wall (red arrows). Evolvulus nummularius (d) displayed undulate anticlinal boundaries with flat to concave periclinal cell wall
Discussion
This study provides for the first time comprehensive micro-morphological information for three Evolvulus taxa. Leaf and stem anatomical characters basically coincide with the descriptions of other Convolvulaceae species previously studied by Metcalfe and Chalk (1950).
General diagnostic characters for Evolvulus leaves on surface view are the sinuous or straight to curved anticlinal walls, anisocytic, anomocytic and paracytic stomatal types, the presence of Y-shaped hairs, and capitate trichomes. The presence of the anisocytic (or cruciferous) stomatal type corresponds to the previous report by Metcalfe and Chalk (1950). Most species have Y-shaped hairs on both sides of the leaves, except in E. nummularius. Furthermore, E. alsinoides from Phetchaburi has Y-shaped hairs on the abaxial surface only. The occurrence of Y-shaped hairs on both leaf surfaces was similar to the discovery of Metcalfe and Chalk (1950), and also matched with the study of Harms (2014) which recorded Y-shaped hairs in three Evolvulus species found in Texas and New Mexico. The function of Y-shaped hairs might be functionally related to reduction in water loss for the plant because E. alsinoides is always found on limestone in a habitat which has intense insolation accompanied by low humidity and dry soils. These non-glandular trichomes cover up the guard cells in order to protect the plant from dessication. Metcalfe and Chalk (1950) mentioned the secretory cells found in the cotyledons of many genera including Evolvulus, but did not specify the type of cell. Capitate glandular trichomes were found in all three Evolvulus taxa, and here we describe this character in detail for the first time.
The characters of leaf transverse sections useful for species separation are as follows: the presence of tanniniferous epidermal cells; type of palisade mesophyll and leaf thickness. Evolvulus alsinoides from Phetchaburi is the only plant sample that presented a storage substance on the adaxial epidermal cells, coincident with Metcalfe and Chalk (1950) treatment. Thai Evolvulus taxa showed both isobilateral and dorsiventral mesophyll types. Isobilateral type was found in E. alsinoides and E. glomeratus, while the dorsiventral type occurs in E. nummularius. Tayade and Patil (2012b) described a dorsiventral mesophyll for E. alsinoides from India, which is not congruent with our findings. The reason might be the differences between two populations of E. alsinoides from India and Thailand in terms of variability of anatomical features. It is also possible that Tayade and Patil (2012b) had another species entirely: there were no voucher specimens cited for the plants they studied, and thus we cannot be certain that our results are comparable with their findings.
According to the monograph by van Ooststroom (1934), E. alsinoides was separated into 15 varieties. It is possible that E. alsinoides collected from Phetchaburi might not be the same variety as the plant from Khon Kaen province as suggested by the differences of Y-shaped hairs as well as the storage epidermal cells and the mesophyll arrangement. Another possibility would be the environmental differences in two populations of E. alsinoides because E. alsinoides (KK & PT 12) came from limestone area but E. alsinoides (KK & PT 13) was collected from weedy place. The occurrence of Y-shaped hairs and dorsiventral mesophyll can clearly be used to separate E. nummularius from the others, while the other two species can be identified by leaf thickness. Evolvulus alsinoides has thinner leaves (70–100 µm) than E. glomeratus (200–240 µm). With sufficient fertilizer and water, cultivated plants are usually bigger than wild plants of the same species (Lau and Stephenson 1994) almost in all aspects as shown in leaf thickness from this study. Thus, a key to the species provided here has to be used with caution because the anatomical characters might be different from wild species. Presence of Y-shaped hair was also useful to distinguish the species of Evolvulus as was proposed recently by Harms (2014).
Stem anatomical characters are similar in all species; however, the stem diameter in E. glomeratus is bigger than the other two species. The presence of tanniferous and chlorenchyma cells in the stem has never been described previously. A bicollateral bundle is the typical type for the family Convolvulaceae as was formerly reported by Metcalfe and Chalk (1950) and Tayade and Patil (2012a).
Pollen characters of all taxa are quite similar in all aspects; therefore pollen morphology has low taxonomic value to identify Evolvulus taxa. The only one quantitative datum that can be used to separate E. glomeratus from other taxa was the pollen size. Evolvulus glomeratus possessed the largest pollen with 39–45 µm in polar axis (length) and 41–47 µm in equatorial axis (width). Pollen ornamentation and apertures are consistent with the investigation of Tellaría and Daners (2003) which classified Evolvulus pollen into Type II with microechinate exine. In their study, three species of Evolvulus were examined i.e., E. arizonicus A. Gray, E. glomeratus Nees & Mart. and E. sericeus Sw., therefore this investigation confirms the exine pattern of Evolvulus by having studied two more species in the genus. However, this current study disagrees with the reproductive biological examination of medicinal plant by Singh and Dhakre (2010) which recorded E. alsinoides pollen as tricolpate apertures and reticulate ornamentation. The photos of live plants shown in their paper looked quite different from E. alsinoides collected from Thailand. Sarma et al. (2007) have suggested that the pollinator of E. nummularius in India is a Graceful Awl snail (Lamellaxis gracile) on rainy days, while a honey bee (Apis cerana indica) is the pollinator on a sunny day. However, E. nummularius is not a native species in India. Therefore, the pollination relationships may not be typical for the native habitat in South America. Singh and Dhakre (2010) mentioned the honey bee as one of nine insect pollinators of E. alsinoides. From plant morphology, a snail should not be a pollinator for E. alsinoides and E. glomeratus because their flower position is not located close to the soil surface as it is for E. nummularius. Therefore, the honey bee and flies might be the pollinators of E. alsinoides and E. glomeratus.
Seed characters have taxonomic significance and can be used to construct a key to species. Periclinal walls differ between two taxa: folded in E. alsinoides and flat to concave in E. nummularius. The testal periclinal cell wall corresponded to that described by Abdel Khalik and Osman (2007).
Conclusions
From leaf anatomical characters, there are some taxonomic aspects to distinguish these three Evolvulus taxa, therefore a key to taxa based on leaf anatomy was constructed.
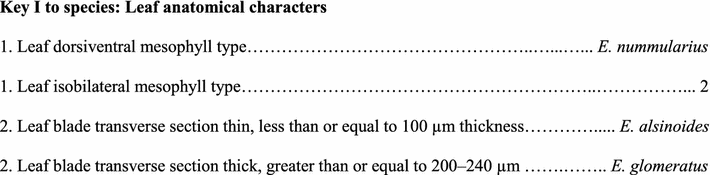 Seed characters have taxonomic significance and can be used to construct a key to species.
Seed characters have taxonomic significance and can be used to construct a key to species.
 This data serves as a base line to be used in other subjects such as taxonomy, horticulture, pharmaceutical botany and organic chemistry in terms of species identification, ornamental plant improvement and medicinal plant identification, respectively.
This data serves as a base line to be used in other subjects such as taxonomy, horticulture, pharmaceutical botany and organic chemistry in terms of species identification, ornamental plant improvement and medicinal plant identification, respectively.
Authors’ contributions
KK collected the plants, analyzed the data and drafted the manuscript. GWS planned the study, guided a taxonomic identification, provided important taxonomic literatures, revised the manuscript and polished the English. SCS guided lab work, revised the manuscript. PT suggested the study topic, collected the plants, planned the study, guided all lab work, revised and developed the manuscript, prepared the manuscript for submission. All authors read and approved the final manuscript.
Acknowledgements
The first author would like to thank the Science Achievement Scholarship of Thailand (SAST) and all members of the Plant Anatomy Laboratory, Department of Plant Science, Mahidol University. Special thanks to Mr. Sittinut Soonthornkalump for his kindness in allowing the authors to collect the samples from his land. Anja Buijsen, Botanical Library, Naturalis Biodiversity Centre, provided literature critical for this study. Traiperm wishes to acknowledge the TRF-RSA5880022 and the Faculty of Science, Mahidol University, for supporting this research.
Competing interests
The authors declare that they have no competing interests.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1186/s40529-016-0144-8.
Contributor Information
Kanapol Ketjarun, Email: kanapol99@gmail.com.
George W. Staples, Email: geozsg@gmail.com
Sasivimon C. Swangpol, Email: sasivimon.swa@mahidol.edu
Paweena Traiperm, Email: paweena.tra@mahidol.edu.
References
- Abdel Khalik KN. Seed morphology of Cuscuta L. (Convolvulaceae) in Egypt and its systematic significance. Feddes Repert. 2006;117:217–224. doi: 10.1002/fedr.200511100. [DOI] [Google Scholar]
- Abdel Khalik KN, Osman AK. Seed morphology of some species of Convolvulaceae from Egypt (identification of species and systematic significance) Feddes Repert. 2007;118:24–37. doi: 10.1002/fedr.200711123. [DOI] [Google Scholar]
- Auddy B, Ferreira M, Blasina F, Lafon L, Arredondo F, Dajas F, Tripathi PC, Seal T, Mukherjee B. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J Ethnopharmacol. 2003;84:131–138. doi: 10.1016/S0378-8741(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Austin DF. Evolvulus alsinoides (Convolvulaceae): an American herb in the old world. J Ethnopharmacol. 2008;117:185–198. doi: 10.1016/j.jep.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Ayyanar M, Ignacimuthu S. Ethnobotanical survey of medicinal plants commonly used by Kani tribals in Tirunelveli hills of Western Ghats, India. J Ethnopharmacol. 2011;134:851–864. doi: 10.1016/j.jep.2011.01.029. [DOI] [PubMed] [Google Scholar]
- Bridson D, Forman L. The herbarium handbook. 3. London: Royal Botanic Gardens, Kew; 1999. [Google Scholar]
- Chen HW (2007) Pharmacognostical studies on Evolvulus species in Taiwan: (Evolvulus alsinoides and Evolvulus glomeratus). Master Class Thesis, China Medical University
- Chen JH, Hang S, Yang YP. Comparative morphology of leaf epidermis of Salix (Salicaceae) with special emphasis on sections Lindleyanae and Retusae. Bot J Linn Soc. 2008;157:311–322. doi: 10.1111/j.1095-8339.2008.00809.x. [DOI] [Google Scholar]
- Costea M, Ruiz IG, Welsh M. A new species of Cuscuta (Convolvulaceae) from Michoacán, Mexico. Brittonia. 2008;60:235–239. doi: 10.1007/s12228-008-9017-0. [DOI] [Google Scholar]
- Dettke GA, Waechter JL, Miotto STS. Cuscuta taimensis (Convolvulaceae, Cuscuteae), a new species from South America. Brittonia. 2014;66:269–273. doi: 10.1007/s12228-014-9329-1. [DOI] [Google Scholar]
- Erdtman G (1952) Pollen morphology and plant taxonomy. Angiosperms, 1st edn. Almqvist & Wiksell, Stockholm
- Erdtman G. The acetolysis method, a revised description. Svensk Botanisk Tidskrift. 1960;54:561–564. [Google Scholar]
- Gunn CR. Seeds of the United States noxious and common weeds in the Convolvulaceae, excluding the genus Cuscuta. Proc Assoc Off Seed Anal North Am. 1969;59:101–115. [Google Scholar]
- Hallier H. Versuch einer natürlichen Gliederung der Convolvulaceen auf morphologischer und anatomischer Grundlage. Botanische Jahrbücher für Systematik. 1893;16:453–591. [Google Scholar]
- Hamed KA. Pollen and seed characters of certain Cuscuta species growing in Egypt with a reference to a taxonomic treatment of the genus. Int J Agr Biol. 2005;7:325–332. [Google Scholar]
- Harms RT. A new species of Evolvulus (Convolvulaceae) from the high plains of the Texas/New Mexico border. Phytoneuron. 2014;20:1–20. [Google Scholar]
- Hesse M, Halbritter H, Zetter R, Weber M, Buchner R, Frosch-Radivo A, Ulrich S. Pollen terminology an illustrated handbook. 1. Berlin: Springer-Verlag/Wein; 2009. [Google Scholar]
- Hsiao L, Kuoh C. Pollen morphology of the Ipomoea (Convolvulaceae) in Taiwan. Taiwania. 1995;40:299–316. [Google Scholar]
- Johansen DA. Plant microtechnique. 1. New York: McGraw-Hill Book Co.; 1940. [Google Scholar]
- Juan R, Pastor J, Fernandez I. Morphological and anatomical studies of Linaria species from south-west Spain: seeds. Ann Bot. 1999;84:11–19. doi: 10.1006/anbo.1999.0879. [DOI] [Google Scholar]
- Junqueira MER, Simão-Bianchini R. O gênero Evolvulus L. (Convolvulaceae) no município de Morro do Chapéu, BA, Brasil. Acta Botanica Brasilica. 2006;20:157–172. doi: 10.1590/S0102-33062006000100015. [DOI] [Google Scholar]
- Khare CP. Indian medicinal plants an illustrated dictionary. 1. Heidelberg: Springer Science + Business Media; 2007. [Google Scholar]
- Lau TC, Stephenson AG. Effects of soil phosphorus on pollen production, pollen size, pollen phosphorus content, and the ability to sire seeds in Cucurbita pepo (Cucurbitaceae) Sex Plant Reprod. 1994;7:215–220. doi: 10.1007/BF00232740. [DOI] [Google Scholar]
- Lewis WH. Pollen differences between Stylisma and Bonamia (Convolvulaceae) Brittonia. 1971;23:331–334. doi: 10.2307/2805701. [DOI] [Google Scholar]
- Manandhar NP. Ethnobotanical notes on certain medicinal plants used by Tharus of Dang-Deokhuri District, Nepal. Int J Crude Drug Res. 1985;23:153–159. doi: 10.3109/13880208509069022. [DOI] [Google Scholar]
- Menemen Y, Jury SL. Pollen studies on some species of the genus Convolvulus L. (Convolvulaceae) from Morocco. Turk J Bot. 2002;26:141–148. [Google Scholar]
- Metcalfe CR, Chalk L. Convolvulaceae. In: Metcalfe CR, Chalk L, editors. Anatomy of the dicotyledons. 1. Oxford: Clarendon Press; 1950. pp. 954–964. [Google Scholar]
- Naikawadi VB, Ahire ML, Lahiri A, Nikam TD. In vitro propagation and cell cultures of memory tonic herb Evolvulus alsinoides: a best source for elicited production of scopoletin. Appl Microbiol Biotechnol. 2016;100:3463–3476. doi: 10.1007/s00253-015-7153-5. [DOI] [PubMed] [Google Scholar]
- Perveen A, Al-Alawi AH, Husain SZ. A contribution to the palynological survey of the genus Convolvulus from South West Asia and Arabian Peninsula. Pak J Bot. 1989;21:197–209. [Google Scholar]
- Pisuttimarn P, Traiperm P, Pornpongrungrueng P (2013) Comparative anatomy of Merremia section Xanthips (Convolvulaceae) in Thailand (in Thai). In: Proceeding of NGRC 29th The National Graduate Research Conference, Chiang Rai, Thailand, 24–25 October 2013
- Quisumbing EA. Medicinal plants of the Philippines. Philippines: Katha Publishing Co.; 1978. [Google Scholar]
- Rajurkar AV, Tidke JA, Patil GV. Studies on pollen morphology of Ipomoea species (Convolvulaceae) Res Plant Biol. 2011;1:41–47. [Google Scholar]
- Rao SR, Leela M. Seed morphology (LM and SEM) in some Ipomoea L. (Convolvulaceae. Feddes Repert. 1993;104:209–213. doi: 10.1002/fedr.19931040307. [DOI] [Google Scholar]
- Robertson KR. Odonellia, a new genus of Convolvulaceae from tropical America. Brittonia. 1982;34:417–423. doi: 10.2307/2806498. [DOI] [Google Scholar]
- Sarma K, Tandon R, Shivanna KR, Mohan Ram HY. Snail-pollination in Volvulopsis nummularium. Curr Sci. 2007;93:826–831. [Google Scholar]
- Sayeedud-Din M. Observations on the anatomy of some of the Convolvulaceæ. Proc Plant Sci. 1953;37:106–109. [Google Scholar]
- Singh KP, Dhakre G. Reproductive biology of Evolvulus alsinoides L. (medicinal herb) Int J Bot. 2010;6:304–309. doi: 10.3923/ijb.2010.304.309. [DOI] [Google Scholar]
- Staples G. Convolvulaceae. In: Santisuk T, Larsen K, editors. Flora of Thailand, vol 10(3) Bangkok: Prachacohon; 2010. pp. 330–468. [Google Scholar]
- Staples G, Traiperm P, Chow J. Another New Thai Argyreia Species (Convolvulaceae) Phytotaxa. 2015;204:223–229. doi: 10.11646/phytotaxa.204.3.5. [DOI] [Google Scholar]
- Stuessy TF. Plant taxonomy the systematic evaluation of comparative data. 2. New York: Columbia University Press; 2009. [Google Scholar]
- Tayade SK, Patil DA. Anatomical studies of stem in some Convolvulaceae. Lisper. 2012;1:42–46. [Google Scholar]
- Tayade SK, Patil DA. Leaf anatomical studies in some species of Convolvulaceae. Life Sci Leafl. 2012;3:64–74. [Google Scholar]
- Tellería MC, Daners G. Pollen types in Southern New World Convolvulaceae and their taxonomic significance. Plant Syst Evol. 2003;243:99–118. doi: 10.1007/s00606-003-0069-z. [DOI] [Google Scholar]
- van Ooststroom SJ. A monograph of the genus Evolvulus. Mededeelingen van het Botanisch Museum en Herbarium van de Rijks Universiteit te Utrecht. 1934;14:1–267. [Google Scholar]
- Welsh M, Stefanović S, Costea M. Pollen evolution and its taxonomic significance in Cuscuta (dodders, Convolvulaceae) Plant Syst Evol. 2010;285:83–101. doi: 10.1007/s00606-009-0259-4. [DOI] [Google Scholar]


