Abstract
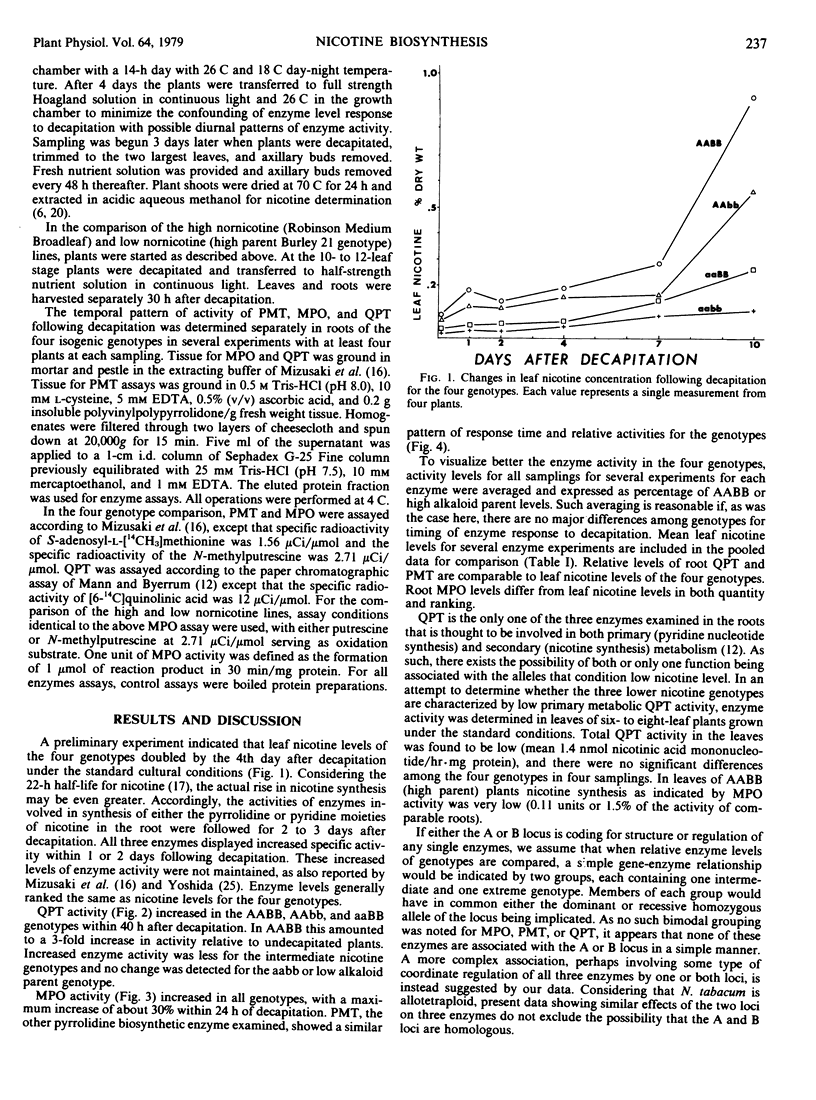
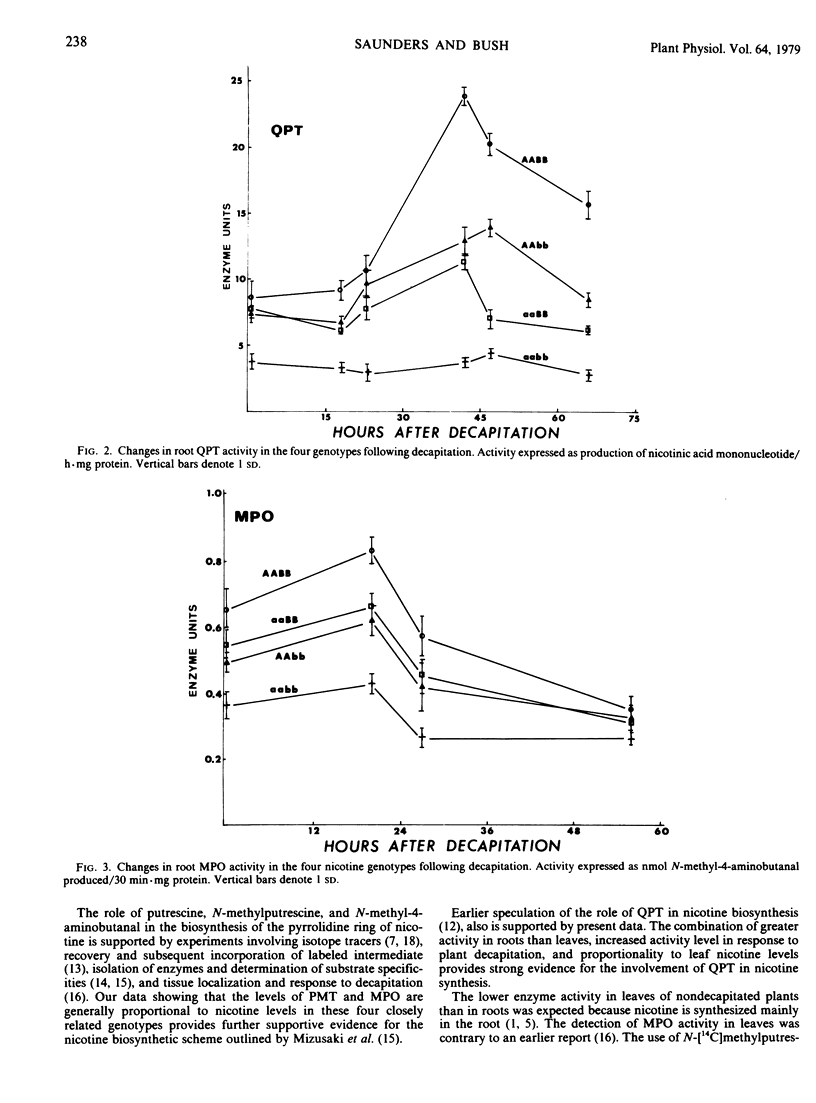
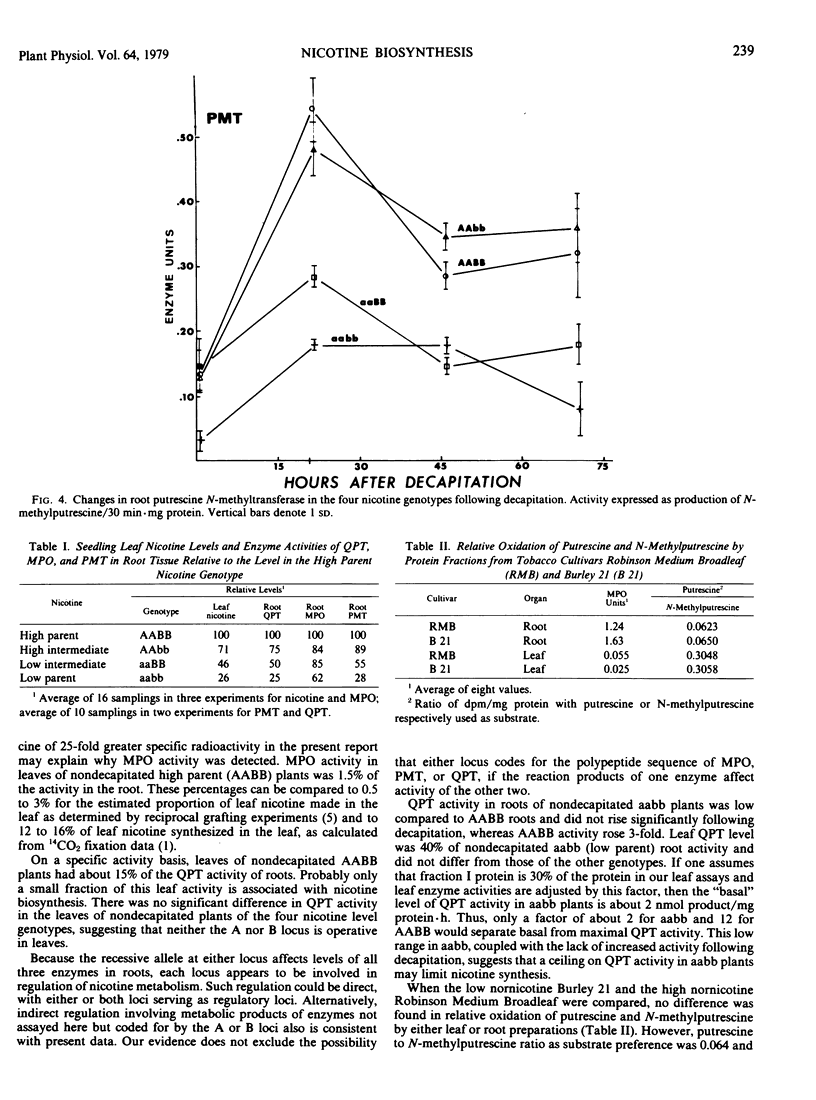
Young plants of five Nicotiana tabacum L. genotypes were examined for activity of nicotine biosynthetic enzymes. Genotypes near isogenic except at two loci each with two alleles controlling nicotine level were used in a comparison of the four homozygous allelic combinations producing high, high intermediate, low intermediate, and low nicotine levels in a “Burley 21” background. Putrescine N-methyltransferase (EC 2.1.1.53) and quinolinic acid phosphoribosyltransferase (EC 2.4.2.19) activities in root tissue of these four genotypes were proportional to leaf nicotine level, whereas N-methylputrescine oxidase activity in root tissue differed in proportion and ranking. Quinolinic acid phosphoribosyltransferase activities in leaf tissue were lower than in roots, but no differences were found among the four genotypes. The homozygous recessive alleles at either locus affect levels of all three enzyme activities examined in roots. Each locus seems to be involved in regulation of nicotine metabolism, but whether directly as a regulatory locus or indirectly through the metabolic product of a structural locus is not known.
No difference was observed between enzymic oxidation of putrescine and N-methylputrescine by leaf and root extracts of Burley 21 (a high nicotine, low nornicotine genotype) and a high nornicotine cultivar, “Robinson Medium Broadleaf.” Putrescine was utilized as a substrate to a greater extent than N-methylputrescine by leaf extracts compared with root extracts of both cultivars. It was concluded that genetic differences in levels of nicotine and nornicotine were not due to differences in enzymic oxidation of these two precursors during alkaloid biosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dawson R. F., Solt M. L. Estimated Contributions of Root and Shoot to the Nicotine Content of the Tobacco Plant. Plant Physiol. 1959 Nov;34(6):656–661. doi: 10.1104/pp.34.6.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D. F., Byerrum R. U. Activation of the de novo pathway for pyridine nucleotide biosynthesis prior to ricinine biosynthesis in castor beans. Plant Physiol. 1974 Apr;53(4):603–609. doi: 10.1104/pp.53.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusaki S., Kisaki T., Tamaki E. Phytochemical Studies on the Tobacco Alkaloids. XII. Identification of gamma-Methylaminobutyraldehyde and its Precursor Role in Nicotine Biosynthesis. Plant Physiol. 1968 Jan;43(1):93–98. doi: 10.1104/pp.43.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T. Metabolism and function of alkaloids in plants. Science. 1974 Apr 26;184(4135):430–435. doi: 10.1126/science.184.4135.430. [DOI] [PubMed] [Google Scholar]
- Schütte H. R., Mothes K., Maier W. Methylputrescine as possible precursor of nicotine in Nicotiana rustica. Acta Biochim Pol. 1966;13(4):401–404. [PubMed] [Google Scholar]
- Stepka W., Dewey L. J. Conversion of nicotine to nornicotine in harvested tobacco: Fate of the methyl group. Plant Physiol. 1961 Sep;36(5):592–597. doi: 10.1104/pp.36.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso T. C., Jeffrey R. N. Studies on Tobacco Alkaloids. I. Changes in Nicotine and Nornicotine Content in Nicotiana. Plant Physiol. 1956 Nov;31(6):433–440. doi: 10.1104/pp.31.6.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso T. C., Jeffrey R. N. Studies on Tobacco Alkaloids. II. The Formation of Nicotine and Nornicotine in Tobacco Supplied with N. Plant Physiol. 1957 Mar;32(2):86–92. doi: 10.1104/pp.32.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADA E. Conversion of nicotine to nornicotine in cherry red tobacco during flue-curing. Arch Biochem Biophys. 1956 Jun;62(2):471–475. doi: 10.1016/0003-9861(56)90145-x. [DOI] [PubMed] [Google Scholar]
- Yang K. S., Gholson R. K., Waller G. R. Studies on nicotine biosynthesis. J Am Chem Soc. 1965 Sep 20;87(18):4184–4188. doi: 10.1021/ja01096a032. [DOI] [PubMed] [Google Scholar]


