Abstract
Sensing reaction mechanism is crucial for enhancing the sensing performance of semiconductor-based sensing materials. Here we show a new strategy to enhancing sensing performance of SnO2 nanocrystals by increasing the density of unsaturated Sn atoms with dangling bonds at the SnO2 surface through hydrogenation. A concept of the surface unsaturated Sn atoms serving as active sites for the sensing reaction is proposed, and the sensing mechanism is described in detail at atomic and molecule level for the first time. Sensing properties of other metal oxide sensors and catalytic activity of other catalysts may be improved by using the hydrogenation strategy. The concept of the surface unsaturated metal atoms serving as active sites may be very useful for understanding the sensing and catalytic reaction mechanisms and designing advanced sensing sensors, catalysts and photoelectronic devices.
Introduction
As an important n-type metal-oxide semiconductor with a band gap of 3.6 eV at 300 K1, tin dioxide (SnO2) is well known for its numerous potential applications in field emission2–4, lithium ion batteries5–7, photocatalysis8, 9, dye-sensitizd solar cells10, 11, perovskite solar cells12–15, supercapacitors16, 17, light emitting devices18 and so on. On the other hand, SnO2 is considered to be one of the best known gas-sensing materials due to its remarkable receptivity variation in gaseous environment and excellent chemical stability19–22. Over the past decades, with the development of nanoscience and nanotechnology, SnO2 nanomaterials with controlled morphologies, including nanoring20, nanowires23, 24, nanobelts25, nanotubes26, 27, nanosheets28, hollow spheres29, 30, flower-like structure31, 32, nanopolyhedrons33, hierarchical nanoarchitectures34, 35, octahedra21, 22 and porous nanospheres36 have been employed to fabricate gas sensors for detection of inflammable and toxic gases and volatile organic compounds (VOC) to improve gas-sensing properties. It was found that sensitivity of the sensors based SnO2 nanostructured materials is increased by exposing high-energy facets21, 22 and increasing surface area26, 31, 36. Additionally, the sensitivity and selectivity of SnO2-based sensors can be significantly improved through doping with Cu, and Zn elements37, 38, decorating with Ag2O39, PdO40, Ag41 and Pd42 nanoparticles, and forming SnO2-ZnO heteronanostructures43.
Metal-oxide gas sensors like SnO2 operate on the basis of the change of the electrical resistance upon exposure to air or a test gas44. The variations of the resistances are brought about by the oxidation-reduction reaction of the adsorbed oxygen with the test gas taking place on the metal-oxide surface45, 46. For this reason, the sensing behaviors of metal oxides should be very sensitive to the surface adsorption oxygen ability of the sensing materials. The adsorption oxygen ability may be enhanced by increasing density of the unsaturated metallic atoms with dangling bonds on the surface of metal-oxide sensors through hydrogenation. However, such a strategy for increasing sensing properties has never been reported up to now.
Herein, we demonstrated the enhanced volatile-organic-compound sensors based on the hydrogenated SnO2 nanocrystals for the first time. The hydrogenated SnO2 nanocrystals displayed far higher response towards ethanol, methanol and triethylamine than SnO2 samples without hydrogenation, and the gas-sensing sensitivity was further increased with the hydrogenation time. The excellent gas-sensing performance arises from the increased density of the unsaturated Sn atoms with dangling bonds through hydrogenation, a concept of the unsaturated Sn atom serving as an active site for the sensing reaction is thus proposed, and a new sensing reaction mechanism is described in detail.
Results
Morphology and crystal structure of SnO2 samples. Figure 1a shows X-ray diffraction (XRD) pattern of the SnO2 samples without hydrogenation. In the XRD pattern, all diffraction peaks were attributed to the pure tetragonal phase with cell constants of a = b = 4.738 Å and c = 3.187 Å (Joint Committee on Powder Diffraction Standards No. 41-1445). Scanning electron microscope (SEM) image shown in Fig. 1b indicated that the SnO2 samples without hydrogenation consists of nanocrystals with irregular morphology and the sizes of 50–500 nm. The SnO2 nanocrystals were hydrogenated for 5, 10 and 15 h at 150 °C, and the as-obtained sample was labeled SnO2-H-5, SnO2-H-10 and SnO2-H-15, respectively. The three kinds of hydrogenated samples were characterized with FESEM and XRD, and the results are shown in Supporting Information Fig. 1. It can be seen that after H2 reduction the morphology and crystal structure of SnO2 nanocrystals remain unchanged. The as-obtained hydrogenated samples still consist of rutile SnO2 nanocrystals with various sizes.
Figure 1.
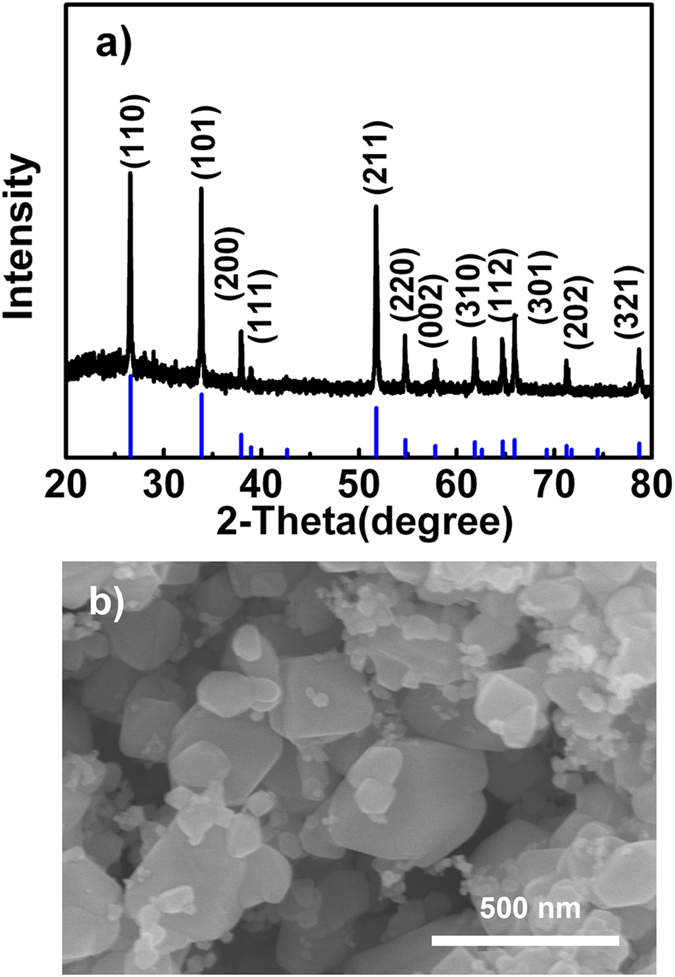
XRD and SEM characterizations of the SnO2 samples without hydrogenation. (a) XRD pattern, (b) SEM image.
Gas-sensing Performance. The transient response characteristics of the hydrogenated and non-hydrogenated SnO2 nanocrystals to different concentrations of ethanol, methanol or triethylamine are displayed in Supporting Information Fig. 2. When the VOC was injected, the electric resistance of four types of SnO2 nanocrystal sensors decreased suddenly, and then increased rapidly and recovered to their respective initial resistance after release of the VOC vapor. The resistance change of four kinds of SnO2 nanocrystal sensors is in accordance with the typical sensing property of the n-type semiconductor32. Figure 2a–c shows sensing response curves of the three types of SnO2 nanocrystal sensors to ethanol, methanol and triethylamine of different concentration, respectively. It can be clearly seen that the hydrogenated SnO2 nanocrystal sensors have higher response than the nanocrystals without hydrogenation for three kinds of VOC vapors. The response value of SnO2 sensors increases further with prolonging the hydrogenation time.
Figure 2.
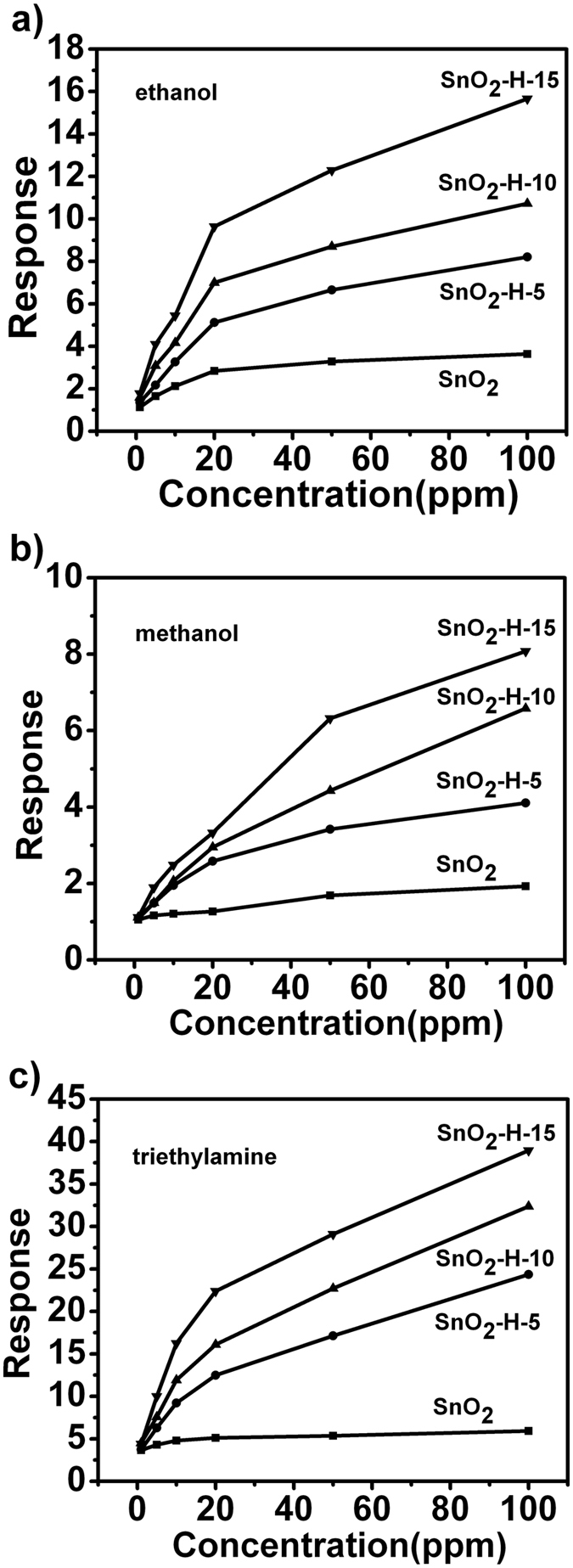
The response curves of the sensors based on the hydrogenated and non-hydrogenated SnO2 nanocrystals to different concentrations of VOCs with 50% of relative humidity at 350 °C.
Moreover, to investigate stability of the sensor based on the SnO2-H-15, after the first measurement the hydrogenated SnO2 sensor was stored in air and kept working at 350 °C for subsequent sensing property tests. After the sensor fabrication and aging for 7 days, a series of tests were conducted with 100 ppm of ethanol. The result was shown in Supporting Information Fig. 3. It was found that response value of the hydrogenated SnO2 sensor to 100 ppm of ethanol is between 18.9 and 20.5 during the test of 31 days, revealing that the hydrogenated SnO2 sensor demonstrated good long-term stability.
Figure 3.
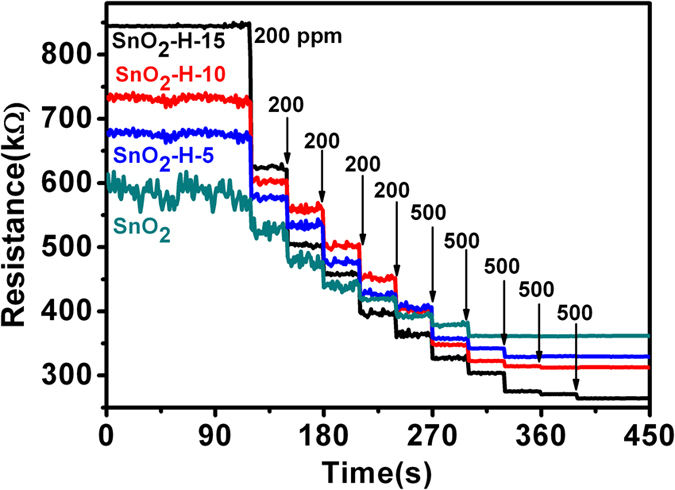
The resistance of the sensors based on the hydrogenated and non-hydrogenated SnO2 nanocrystals in air and in different concentrations of ethanol vapors with of 50% relative humidity at 350 °C. The concentration labeled in the figure is the ethanol concentration of injection each time.
Discussion
It is well accepted that the resistance change of the metal oxide semiconductor based sensors like SnO2 is based on the exchange of charges between the absorbed gaseous species and the surface of metal oxide sensing materials20–22, 44, 47, 48. In order to understand the role of hydrogenation in the increase of response of SnO2 nanocrystal sensor towards VOC, resistances of the four types of SnO2 nanocrystal sensors in air and in ethanol vapors of different concentrations were measured, and the results are displayed in Fig. 3. It was found that the hydrogenated SnO2 nanocrystal sensors have higher resistance values than SnO2 samples without hydrogenation, and the resistance change follows the order of SnO2-H-15 > SnO2-H-10 > SnO2-H-5 > SnO2. The resistance values of the four types of SnO2 sensors reduce with the injection of ethanol, and finally reach a constant value. The saturated ethanol concentrations for SnO2, SnO2-H-5, SnO2-H-10 and SnO2-H-15 are 2000, 2500, 3000 and 3500 ppm, respectively (Table 1). In the saturated ethanol vapor environment, the hydrogenated SnO2 nanocrystal sensors have lower electric resistances than the sensors based on the SnO2 samples without hydrogenation, and the resistance follows the order of SnO2 > SnO2-H-5 > SnO2-H-10 > SnO2-H-15.
Table 1.
Resistances in air (Ra) and in the saturated ethanol (Rsg) and the ethanol saturated concentrations of the hydrogenated and non-hydrogenated SnO2 samples.
| Samples | Ra (kΩ) | Rsg (kΩ) | The saturated concentration of ethanol (ppm) |
|---|---|---|---|
| SnO2 | 585.24 | 361.82 | 2000 |
| SnO2-H-5 | 675.98 | 329.30 | 2500 |
| SnO2-H-10 | 731.35 | 264.60 | 3000 |
| SnO2-H-15 | 844.30 | 244.52 | 3500 |
To determine the state of oxygen species on the surface of the hydrogenated and non-hydrogenated SnO2 samples, X-ray photoelectron spectroscopy (XPS) analysis was carried out. Figure 4a–b shows the survey spectra, Sn 2p5/2 and 2p3/2 spectra, respectively. The binding energy of Sn 2p5/2 and 2p3/2 is identified at 486.12 and 494.52 eV, respectively (Fig. 4b). Figure 4c–f shows the O 1s spectra from the four kinds of SnO2 samples. It was clearly observed that all O 1s XPS peaks can be decomposed into three Gaussian components centered at about 529, 531, and 533 eV, respectively. The three components are indexed to O2− ions in SnO2 lattice (OL), O2− ions in oxygen-deficient regions (Ov) and chemisorbed oxygen (OC) species and –OH groups, respectively46, 49. Intensities of OV and OC from the SnO2-H-5, SnO2-H-10 and SnO2-H-15 are higher than those from SnO2 samples without hydrogenation. The relative percentages of the OL and OV components as well as OC and –OH mixed components from the SnO2-H-5, SnO2-H-10, SnO2-H-15 and SnO2 are summarized in Supplementary Table 1. It is apparent that the relative percentages of the OV components as well as OC and –OH mixed components of the SnO2 nanocrystals can be increased through hydrogenation. Additionally, IR spectra of the four kinds of SnO2 nanocrystal samples were investigated and the results are shown in Fig. 5. The band at about 3500 cm−1 is attributed to the asymmetrical stretching vibration of O-H group. Apparently, the hydrogenated SnO2 nanocrystals had lower intensities of the O-H vibration model than SnO2 samples without hydrogenation, and the O-H vibration intensity reduced further with increasing hydrogenation time. Therefore, the hydrogenated SnO2 samples have higher relative percentage of OC components compared with SnO2 samples without hydrogenation, and the relative percentage of OC components further increases with the hydrogenation time. On basis of the experimental results, we concluded that the enhanced sensing property of the hydrogenated SnO2 VOC sensors may derive from the increase on the relative percentages of the OV and OC components and the decrease in the amount of O-H groups.
Figure 4.
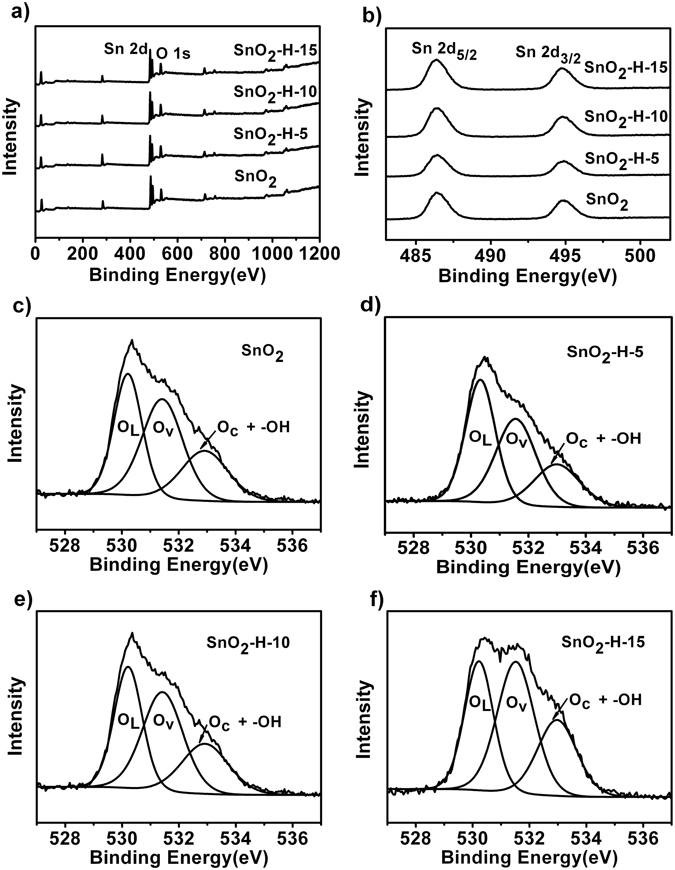
XPS Characterizations of the hydrogenated and non-hydrogenated SnO2 nanocrystals. (a) The survey spectra. (b) Sn 2d5/2, 2d3/2 and (c–f) O 1 s spectra of (c) SnO2, (d) SnO2-H-5, (e) SnO2-H-10 and (f) SnO2-H-15.
Figure 5.
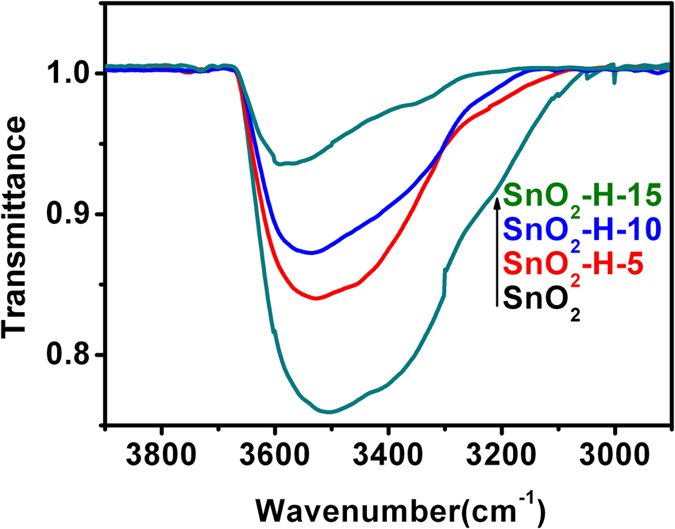
IR spectra of the hydrogenated and non-hydrogenated SnO2 nanocrystals.
When SnO2 nanocrystals were hydrogenated, the O-H groups and O ions at the surface reacted with H2 to form H2O, and thus more the unsaturated Sn atoms with dangling bonds were formed at the SnO2 surface, as a result, density of unsaturated Sn atoms with dangling bonds increases at the surface, as shown in Fig. 6a. Therefore, we considered that the unsaturated Sn atoms with dangling bonds at the surface may play a pivotal role in the enhancement of gas-sensing property. The surface unsaturated Sn atoms with dangling bonds may serve as an active site for the sensing reaction.
Figure 6.
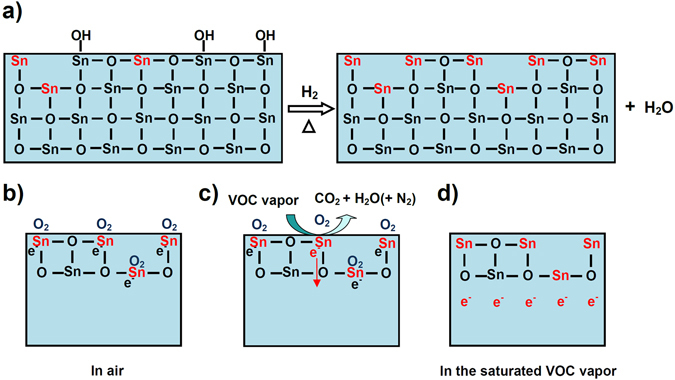
Schematic diagrams of sensing mechanism. (a) Hydrogenation reaction of SnO2 nanocrystals. (b–d) Sensing reaction mechanism of the hydrogenated SnO2 nanocrystals at atomic and molecule level. The black e− and red e− represent the absorbed electrons by O2 and free electrons, respectively.
As we know, SnO2 is a typical n-type semiconductor sensing material with oxygen vacancies, in which electrons participate in the electric conduction process50, 51. Like ZnO52, TiO2 53 and Fe2O3 54, the loss of oxygen in SnO2 create non-contributing (extra) electrons. As shown in Fig. 6a, the presence of oxygen vacancies necessarily led to the production of the unsaturated Sn atoms with dangling bonds, and thus we consider that the Sn atoms with dangling bonds can provide free electrons. In air, the unsaturated Sn atoms with dangling bonds at the surface of SnO2 sensing material have reducing capacity and adsorb oxygen molecules due to the deficiency of oxygen. The adsorbed oxygen molecules have good oxidation capacity and can draw free electrons in SnO2 sensing material, the electrons captured by the adsorbed oxygen can not participate in the electric conduction process. As a result, the number of free electrons within SnO2 decreases, and the SnO2 sensing material thus shows a high resistance state, as shown in Fig. 6b. The density of unsaturated Sn atoms with dangling bonds increase at the SnO2 surface is increased through hydrogenation (Fig. 6a), and thus the amounts of the adsorbed oxygen and the electrons captured by the adsorbed oxygen are enhanced. Therefore, in comparison with SnO2 samples without hydrogenation, the hydrogenated SnO2 samples have less free electrons and show higher resistance. When the SnO2 sensor is exposed to a VOC vapor, the VOC gas molecules are oxidized into CO2 and H2O (H2O + N2) by surface-adsorbed oxygen molecules, and thus the adsorbed oxygen was removed47, 48. The electrons captured by the adsorbed oxygen molecules are released into SnO2, the number of free electrons in SnO2 increases, and thus resistance value reduces, as shown in Fig. 6c. In the saturated VOC vapor, all the adsorbed oxygen molecules are removed, all the electrons captured by the adsorbed oxygen became into free electrons, and thus the electric resistance value is constant and the smallest, as shown in Fig. 6d. A total of electron in SnO2 sensing materials can be increased with an increase on the density of unsaturated Sn atoms with dangling bonds at the SnO2 surface because that the Sn atoms with dangling bonds can provide extra electrons. Therefore, in the saturated ethanol, the hydrogenated SnO2 nanocrystals with higher densities of unsaturated Sn atoms with dangling bonds have more free electrons and lower electric resistances than SnO2 samples without hydrogenation. Moreover, based on the experimental results in Fig. 3, we considered that the unsaturated Sn atoms with dangling bonds at SnO2 surface can catalyze the reaction of the chemisorbed oxygen with the VOC molecules. The hydrogenated SnO2 nanocrystal sensors have higher density of sensing reaction active sites (the unsaturated Sn atoms with dangling bonds) than SnO2 samples without hydrogenation, and thus demonstrate higher response towards the VOC vapors.
In summary, the hydrogenated SnO2 nanocrystals exhibit superior gas-sensing performance, compared with SnO2 samples without hydrogenation. The enhanced sensing performances originate from the increased density of the unsaturated Sn atoms with dangling bonds at the SnO2 surface through hydrogenation. The unsaturated Sn atoms with dangling bonds are regarded as active sites of the sensing reaction, and the sensing mechanism is firstly elaborated at atomic and molecule level. The hydrogenation may be a general strategy for improving sensing performances of metal oxide sensors and catalytic activities of catalysts. The concept of the unsaturated metal atoms with dangling bonds serving as the reaction active sites not only can deepen understanding of the sensing and catalytic reaction mechanisms, but also provides now insights into the design and fabrication of highly efficient sensing materials, catalysts and photoelectronic devices.
Methods
Preparation of samples
SnO2 nanocrystals were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). SnO2 nanocrystals were hydrogenated: 100 mg of SnO2 nanocrystals were heated in a horizontal furnace and maintained at 150 °C for 5, 10 or 15 h under a H2 gas flow to obtain hydrogenated SnO2 nanocrystals.
Characterization of SnO2 samples
The crystal structure of the hydrogenated and non-hydrogenated SnO2 nanocrystals were characterized by X-ray diffraction (Haoyuan DX-2700, Dandong, China) using Cu Kα1 radiation with 2θ ranging from 20° to 80°. The morphology was analyzed by field-emission scanning electron microscope (Hitachi SU8020, Japan) with an acceleration voltage of 20 kV. The surface compositions were determined on an X-ray photoelectron spectroscope (Kratos Axis ultra, Japan) and on an infrared (IR) spectrometer (Bruker Tensor 27, Germany) by mixing 0.001 g of sample with 0.100 g of KBr and pressing into tablet.
Gas-sensing measurements
Measurements on gas sensitivity of SnO2 samples were performed using a WS-30A system (Weisheng Instruments Co., Zhengzhou, China). In a typical test, a sensor was fabricated by coating a certain amount of SnO2 paste (consisting of SnO2 nanocrystals and the terpineol solvent) onto a ceramic tube with Au electrodes and Pt conducting wires. A Ni-Cr filament was inserted in the tube as a heater element to provide the operation temperature from 200 to 400 °C. To improve the device’s stability, the as-prepared SnO2 sensors were aged at 350 °C for 7 days before testing. Measurement of gas-sensing property has been described in the reference32. The response of the SnO2 sensor is defined as the ratio Ra/Rg, where Ra and Rg are the resistances of the sensor in air and in the test gas at the operation temperature of about 350 °C, respectively.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos 21073116 and 21501116), and the Fundamental Research Funds for the Central Universities (GK 201601003).
Author Contributions
Y.K.Y. and H.Q.Y. are responsible for all phases of the research. Y.K.Y. prepared the samples and performed the characterizations of XRD, FESEM and XPS and the gas-sensing property measurement. Y.W., M.W., and J.F. L. assisted with the IR characterization. C.J.P., B.L., H.Z. and S.Z.L. helped with the data analysis. Y.K.Y. wrote the initial draft, H.Q.Y. revised the final draft.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-00891-5
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu LL, Hong MH, Ho GW. Hierarchical assembly of SnO2/ZnO nanostructures for enhanced photocatalytic performance. Sci. Rep. 2015;5:11609. doi: 10.1038/srep11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He H, et al. Beaklike SnO2 nanorods with strong photoluminescent and field-emission properties. Small. 2006;2:116–120. doi: 10.1002/smll.200500210. [DOI] [PubMed] [Google Scholar]
- 3.Ding JJ, et al. Enhancement of field emission and photoluminescence properties of graphene-SnO2 composite nanostructures. ACS Appl. Mater. Interfaces. 2011;3:4299–4305. doi: 10.1021/am2009635. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, et al. Low-temperature vapor–solid growth and excellent field emission performance of highly oriented SnO2 nanorod arrays. Acta Mater. 2011;59:1291–1299. doi: 10.1016/j.actamat.2010.10.061. [DOI] [Google Scholar]
- 5.Wang Y, et al. Designed hybrid nanostructure with catalytic effect: beyond the theoretical capacity of SnO2 anode material for lithium ion batteries. Sci. Rep. 2015;5:9164. doi: 10.1038/srep09164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park MS, et al. Preparation and electrochemical properties of SnO2 nanowires for application in lithium-ion batteries. Angew. Chem. Int. Ed. 2007;46:750–753. doi: 10.1002/anie.200603309. [DOI] [PubMed] [Google Scholar]
- 7.Wu HB, Chen JS, Lou XW, Hng HH. Synthesis of SnO2 hierarchical structures assembled from nanosheets and their lithium storage properties. J. Phys. Chem. C. 2011;115:24605–24610. doi: 10.1021/jp208158m. [DOI] [Google Scholar]
- 8.Huang X, et al. Type-II ZnO nanorod-SnO2 nanoparticle heterostructures: characterization of structural, optical and photocatalytic properties. Nanoscale. 2013;5:3828–3833. doi: 10.1039/c3nr34327h. [DOI] [PubMed] [Google Scholar]
- 9.Li P, et al. Iodinated SnO2 quantum dots: A facile and efficient approach to increase solar absorption for visible-light photocatalysis. J. Phys. Chem. C. 2016;120:9253–9262. doi: 10.1021/acs.jpcc.6b01530. [DOI] [Google Scholar]
- 10.Dong ZH, et al. Quintuple-shelled SnO2 hollow microspheres with superior light scattering for high-performance dye-sensitized solar cells. Adv. Mater. 2014;26:905–909. doi: 10.1002/adma.201304010. [DOI] [PubMed] [Google Scholar]
- 11.Ahn SH, Kim DJ, Chi WS, Kim JH. One-dimensional hierarchical nanostructures of TiO2 nanosheets on SnO2 nanotubes for high efficiency solid-state dye-sensitized solar cells. Adv. Mater. 2013;25:4893–4897. doi: 10.1002/adma.201302226. [DOI] [PubMed] [Google Scholar]
- 12.Song JX, et al. Low-temperature SnO2-based electron selective contact for efficient and stable perovskite solar cells. J. Mater. Chem. A. 2015;3:10837–10844. doi: 10.1039/C5TA01207D. [DOI] [Google Scholar]
- 13.Ke WJ, et al. Low-temperature solution-processed tin oxide as an alternative electron transporting layer for efficient perovskite solar cells. J. Am. Chem. Soc. 2015;137:6730–6733. doi: 10.1021/jacs.5b01994. [DOI] [PubMed] [Google Scholar]
- 14.Zhu ZL, et al. Mesoporous SnO2 single crystals as an effective electron collector for perovskite solar cells. Phys. Chem. Chem. Phys. 2015;17:18265–18268. doi: 10.1039/C5CP01534K. [DOI] [PubMed] [Google Scholar]
- 15.Dong QS, et al. Insight into perovskite solar cells based on SnO2 compact electron-selective layer. J. Phys. Chem. C. 2015;119:10212–10217. doi: 10.1021/acs.jpcc.5b00541. [DOI] [Google Scholar]
- 16.Zhang YD, et al. Growth of 3D SnO2 nanosheets on carbon cloth as a binder-free electrode for supercapacitors. J. Mater. Chem. A. 2015;3:15057–15067. doi: 10.1039/C5TA02479J. [DOI] [Google Scholar]
- 17.Li ZJ, Chang TQ, Yun GQ, Guo J, Yang BC. 2D tin dioxide nanoplatelets decorated graphene with enhanced performance supercapacitor. J. Alloys Compd. 2014;586:353–359. doi: 10.1016/j.jallcom.2013.10.037. [DOI] [Google Scholar]
- 18.Brovelli S, et al. Fully inorganic oxide-in-oxide ultraviolet nanocrystal light emitting devices. Nat. Commun. 2012;3:690. doi: 10.1038/ncomms1683. [DOI] [PubMed] [Google Scholar]
- 19.Kuang Q, Lao CS, Wang ZL, Xie ZX, Zheng LS. High-sensitivity humidity sensor based on a single SnO2 nanowire. J. Am. Chem. Soc. 2007;129:6070–6071. doi: 10.1021/ja070788m. [DOI] [PubMed] [Google Scholar]
- 20.Li SH, et al. Highly sensitive gas sensor based on SnO2 nanorings for detection of isopropanol. J. Alloys Compd. 2016;688:712–717. doi: 10.1016/j.jallcom.2016.07.248. [DOI] [Google Scholar]
- 21.Han XG, et al. Synthesis of tin dioxide octahedral nanoparticles with exposed high-energy {221} facets and enhanced gas-sensing properties. Angew. Chem. Int. Ed. 2009;48:9180–9183. doi: 10.1002/anie.200903926. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, et al. Controlled synthesis and enhanced catalytic and gas-sensing properties of tin dioxide nanoparticles with exposed high-energy facets. Chem. Eur. J. 2012;18:2283–2289. doi: 10.1002/chem.201103280. [DOI] [PubMed] [Google Scholar]
- 23.Kolmakov A, Zhang YX, Cheng GS, Moskovits M. Detection of CO and O2 using tin oxide nanowire sensors. Adv. Mater. 2003;12:997–1000. doi: 10.1002/adma.200304889. [DOI] [Google Scholar]
- 24.Wang YL, Jiang XC, Xia YN. A solution-phase, precursor route to polycrystalline SnO2 nanowires that can be used for gas sensing under ambient conditions. J. Am. Chem. Soc. 2003;125:16176–16177. doi: 10.1021/ja037743f. [DOI] [PubMed] [Google Scholar]
- 25.Fields LL, Zheng JP, Cheng Y, Xiong P. Room-temperature low-power hydrogen sensor based on a single tin dioxide nanobelt. Appl. Phys. Lett. 2006;88:263102. doi: 10.1063/1.2217710. [DOI] [Google Scholar]
- 26.Zhang J, Guo J, Xu HY, Cao BQ. Reactive-template fabrication of porous SnO2 nanotubes and their remarkable gas-sensing performance. ACS Appl. Mater. Interfaces. 2013;5:7893–7898. doi: 10.1021/am4019884. [DOI] [PubMed] [Google Scholar]
- 27.Shi L, Lin HL. Preparation of band gap tunable SnO2 nanotubes and their ethanol sensing properties. Langmuir. 2011;27:3977–3981. doi: 10.1021/la104529h. [DOI] [PubMed] [Google Scholar]
- 28.Sun P, et al. Porous SnO2 hierarchical nanosheets: hydrothermal preparation, growth mechanism, and gas sensing properties. CrystEngComm. 2011;13:3718–3724. doi: 10.1039/c1ce05073g. [DOI] [Google Scholar]
- 29.Gyger F, Hübner M, Feldmann C, Barsan N, Weimar U. Nanoscale SnO2 hollow spheres and their application as a gas-sensing material. Chem. Mater. 2010;22:4821–4827. doi: 10.1021/cm1011235. [DOI] [Google Scholar]
- 30.Zhang J, et al. Polypyrrole-coated SnO2 hollow spheres and their application for ammonia sensor. J. Phys. Chem. C. 2009;113:1662–1665. doi: 10.1021/jp8096633. [DOI] [Google Scholar]
- 31.Wang H, et al. Preparation of flower-like SnO2 nanostructures and their applications in gas-sensing and lithium storage. Cryst. Growth Des. 2011;11:2942–2947. doi: 10.1021/cg2001255. [DOI] [Google Scholar]
- 32.Liu B, Zhang LH, Zhao H, Chen Y, Yang HQ. Synthesis and sensing properties of spherical flowerlike architectures assembled with SnO2 submicron rods. Sens. Actuators, B. 2012;173:643–651. doi: 10.1016/j.snb.2012.07.084. [DOI] [Google Scholar]
- 33.Chen D, Xu J, Xie Z, Shen GZ. Nanowires assembled SnO2 nanopolyhedrons with enhanced gas sensing properties. ACS Appl. Mater. Interfaces. 2011;3:2112–2117. doi: 10.1021/am2003312. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, et al. Hierarchical SnO2 nanostructures made of intermingled ultrathin nanosheets for environmental remediation, smart gas sensor, and supercapacitor applications. ACS Appl. Mater. Interfaces. 2014;6:2174–2184. doi: 10.1021/am405301v. [DOI] [PubMed] [Google Scholar]
- 35.Jiang LY, Wu XL, Guo YG, Wan LJ. SnO2-based hierarchical nanomicrostructures: facile synthesis and their applications in gas sensors and lithium-ion batteries. J. Phys. Chem. C. 2009;113:14213–14219. doi: 10.1021/jp904209k. [DOI] [Google Scholar]
- 36.Manjula P, Boppella R, Manorama SV. A facile and green approach for the controlled synthesis of porous SnO2 nanospheres: application as an efficient photocatalyst and an excellent gas sensing material. ACS Appl. Mater. Interfaces. 2012;4:6252–6260. doi: 10.1021/am301840s. [DOI] [PubMed] [Google Scholar]
- 37.Jin WX, et al. One-step synthesis and highly gas-sensing properties of hierarchical Cu-doped SnO2 nanoflowers. Sens. Actuators, B. 2015;213:171–180. doi: 10.1016/j.snb.2015.02.075. [DOI] [Google Scholar]
- 38.Zhao QQ, et al. Morphology-modulation of SnO2 hierarchical architectures by Zn doping for glycol gas sensing and photocatalytic applications. Sci. Rep. 2015;5:7874. doi: 10.1038/srep07874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, et al. Templating synthesis of SnO2 nanotubes loaded with Ag2O nanoparticles and their enhanced gas sensing properties. Adv. Funct. Mater. 2011;21:2049–2056. doi: 10.1002/adfm.201002701. [DOI] [Google Scholar]
- 40.Zhu YF, et al. A facile synthesis of PdO-decorated SnO2 nanocomposites with open porous hierarchical architectures for gas sensors. J. Am. Ceram. Soc. 2016;99:3770–3774. doi: 10.1111/jace.14378. [DOI] [Google Scholar]
- 41.Hwang IS, et al. Facile control of C2H5OH sensing characteristics by decorating discrete Ag nanoclusters on SnO2 nanowire networks. ACS Appl. Mater. Interfaces. 2011;3:3140–3145. doi: 10.1021/am200647f. [DOI] [PubMed] [Google Scholar]
- 42.Yang DJ, Kamienchick I, Youn YD, Rothschild A, Kim ID. Ultrasensitive and highly selective gas sensors based on electrospun SnO2 nanofibers modified by Pd loading. Adv. Funct. Mater. 2010;20:4258–4264. doi: 10.1002/adfm.201001251. [DOI] [Google Scholar]
- 43.Fu DY, Zhu CL, Zhang XT, Li CY, Chen YJ. Two-dimensional net-like SnO2/ZnO heteronanostructures for high-performance H2S gas sensor. J. Mater. Chem. A. 2016;4:1390–1398. doi: 10.1039/C5TA09190J. [DOI] [Google Scholar]
- 44.Morrison SR. Semiconductor gas sensors. Sens. Actuators. 1982;2:329–341. doi: 10.1016/0250-6874(81)80054-6. [DOI] [Google Scholar]
- 45.Gardon M, Guilemany JM. A review on fabrication, sensing mechanisms and performance of metal oxide gas sensors. J. Mater. Sci: Mater. Electron. 2013;24:1410–1421. [Google Scholar]
- 46.Han XG, et al. Controlling morphologies and tuning the related properties of nano/microstructured ZnO crystallites. J. Phys. Chem. C. 2009;113:584–589. doi: 10.1021/jp808233e. [DOI] [Google Scholar]
- 47.Liu B, et al. Synthesis and enhanced gas-sensing properties of ultralong NiO nanowires assembled with NiO nanocrystals. Sens. Actuators, B. 2011;156:251–262. doi: 10.1016/j.snb.2011.04.028. [DOI] [Google Scholar]
- 48.Li L, et al. Hydrothermal synthesis and gas sensing properties of single-crystalline ultralong ZnO nanowires. Appl. Phys. A. 2010;98:635–641. doi: 10.1007/s00339-009-5457-y. [DOI] [Google Scholar]
- 49.Li CC, Yin XM, Li QH, Wang TH. Enhanced gas sensing properties of ZnO/SnO2 hierarchical architectures by glucose-induced attachment. CrystEngComm. 2011;13:1557–1563. doi: 10.1039/C0CE00244E. [DOI] [Google Scholar]
- 50.Wang Y, Zhang H, Sun XH. Electrospun nanowebs of NiO/SnO2 p-n heterojunctions for enhanced gas sensing. Appl. Surf. Sci. 2016;389:514–520. doi: 10.1016/j.apsusc.2016.07.073. [DOI] [Google Scholar]
- 51.Law M, Kind H, Messer B, Kim F, Yang PD. Photochemical sensing of NO2 with SnO2 nanoribbon nanosensors at room temperature. Angew. Chem. Int. Ed. 2002;41:2405–2408. doi: 10.1002/1521-3773(20020703)41:13<2405::AID-ANIE2405>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 52.Kim HS, et al. Effects of oxygen concentration on the electrical properties of ZnO films. Cream. Int. 2008;34:1097–1101. doi: 10.1016/j.ceramint.2007.09.105. [DOI] [Google Scholar]
- 53.Pan XY, Yang MQ, Fu XZ, Zhang N, Xu YJ. Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications. Nanoscale. 2013;5:3601–3614. doi: 10.1039/c3nr00476g. [DOI] [PubMed] [Google Scholar]
- 54.Gurlo A, Sahm M, Opera A, Barsan N, Weimar U. A p- to n-transition on α-Fe2O3-based thick film sensors studied by conductance and work function change measurements. Sens. Actuators, B. 2004;102:291–298. doi: 10.1016/j.snb.2004.04.075. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


