Abstract
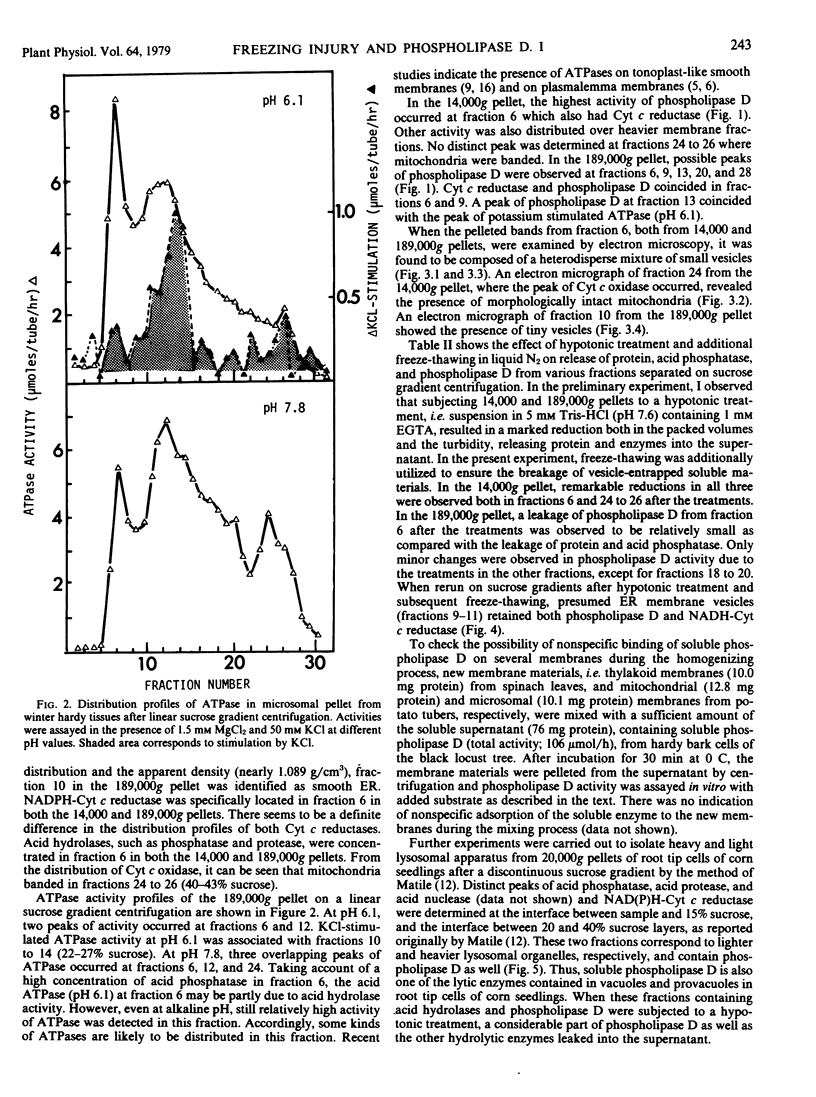
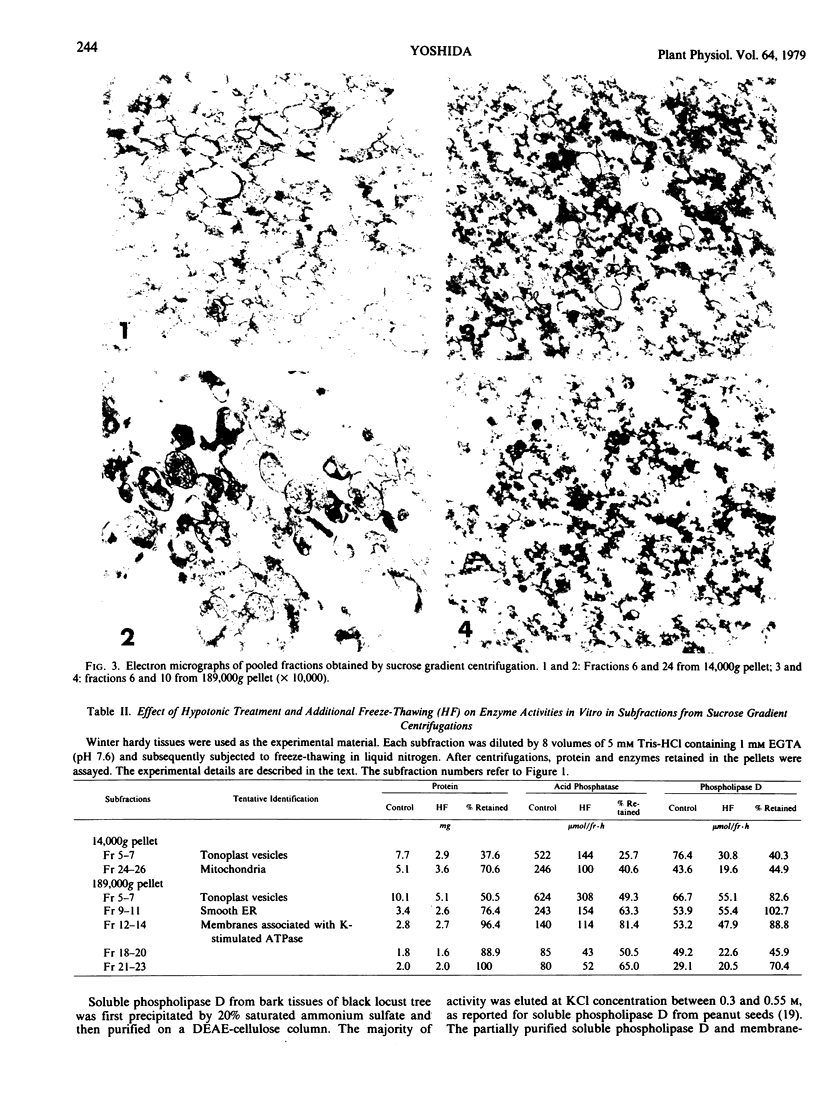
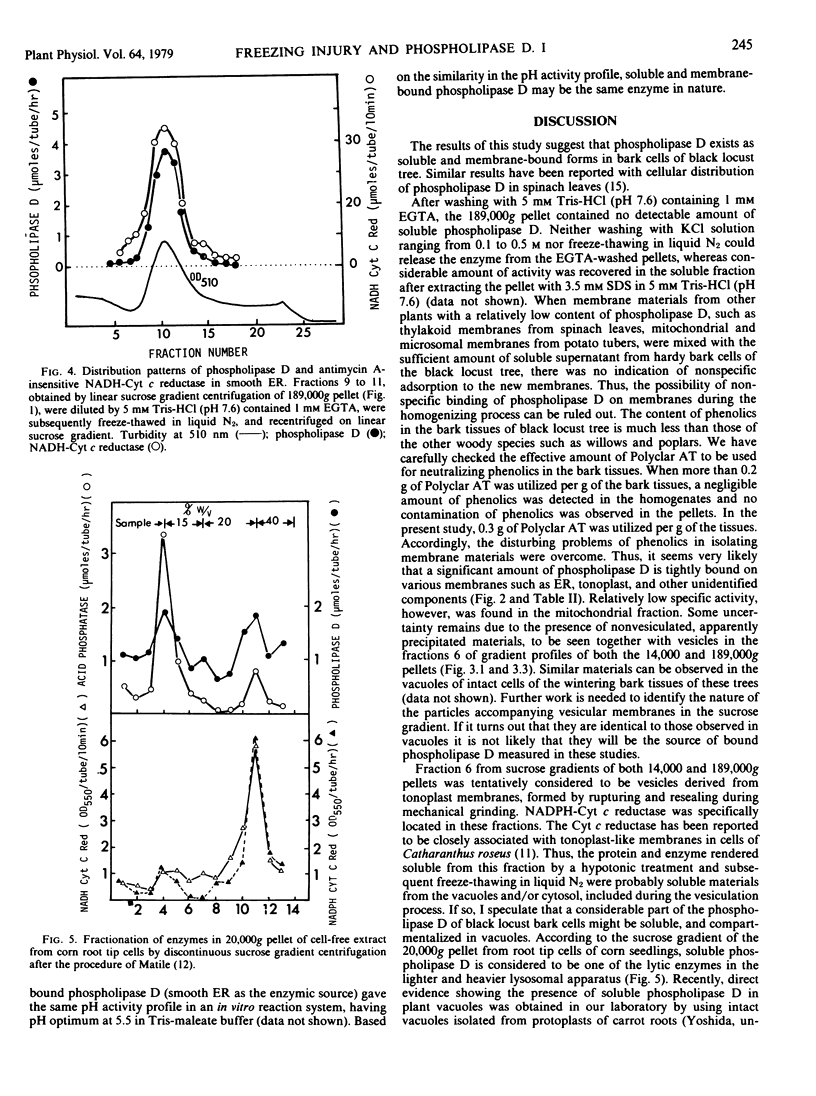
The subcellular localization of phospholipase D in homogenates of living bark tissues of the black locust tree (Robinia pseudoacacia L.) was examined and found in both soluble and particulate fractions. At least some of the soluble enzyme was considered to be compartmentalized in vacuoles. Considerable amounts of phospholipase D seemed to be tightly bound on several membranes such as endoplasmic reticulum, tonoplast, and a membrane associated with potassium-stimulated ATPase (pH 6.1). The mitochondrial fraction banding at the 40 to 43% (w/w) sucrose layer, however, had the lowest specific activity. The soluble and the particulate phospholipase D were considered to be similar in nature. It is possible that the particulate enzyme, as a part, may be derived from the coexisting nonvesiculated materials visualized in the electron micrograph of each membrane fraction. An involvement of the soluble or the presumed membrane-bound phospholipase D in phospholipid degradation in vivo during freezing at sublethal temperatures was discussed with special reference to freezing injury of plant cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIDSON F. M., LONG C. The structure of the naturally occurring phosphoglycerides. 4. Action of cabbage-leaf phospholipase D on ovolecithin and related substances. Biochem J. 1958 Jul;69(3):458–466. doi: 10.1042/bj0690458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EINSET E., CLARK W. L. The enzymatically catalyzed release of choline from lecithin. J Biol Chem. 1958 Apr;231(2):703–715. [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- KATES M. Lecithinase systems in sugar beet, spinach, cabbage, and carrot. Can J Biochem Physiol. 1954 Sep;32(5):571–583. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin W., Wagner G. J., Siegelman H. W., Hind G. Membrane-bound ATPase of intact vacuoles and tonoplasts isolated from mature plant tissue. Biochim Biophys Acta. 1977 Feb 14;465(1):110–117. doi: 10.1016/0005-2736(77)90359-5. [DOI] [PubMed] [Google Scholar]
- Madyastha K. M., Ridgway J. E., Dwyer J. G., Coscia C. J. Subcellular localization of a cytochrome P-450-dependent monogenase in vesicles of the higher plant Catharanthus roseus. J Cell Biol. 1977 Feb;72(2):302–313. doi: 10.1083/jcb.72.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles R. H., Dawson R. M. The distribution of phospholipase D in developing and mature plants. Biochem J. 1969 May;112(5):787–794. doi: 10.1042/bj1120787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R. Is phospholipase D really an enzyme? A comparison of in situ and in vitro activities. Biochim Biophys Acta. 1976 Apr 22;431(1):86–95. doi: 10.1016/0005-2760(76)90262-9. [DOI] [PubMed] [Google Scholar]
- Rungie J. M., Wiskich J. T. Salt-stimulated Adenosine Triphosphatase from Smooth Microsomes of Turnip. Plant Physiol. 1973 Jun;51(6):1064–1068. doi: 10.1104/pp.51.6.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- TOOKEY H. L., BALLS A. K. Plant phospholipase D. I. Studies on cottonseed and cabbage phospholipase D. J Biol Chem. 1956 Jan;218(1):213–224. [PubMed] [Google Scholar]
- Tzur R., Shapiro B. Purification of phospholipase D from peanuts. Biochim Biophys Acta. 1972 Oct 5;280(2):290–296. doi: 10.1016/0005-2760(72)90096-3. [DOI] [PubMed] [Google Scholar]
- Wilson R. F., Rinne R. W. Effect of freezing and cold storage on phospholipids in developing soybean cotyledons. Plant Physiol. 1976 Feb;57(2):270–273. doi: 10.1104/pp.57.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. F., Freer S., Benson A. A. Transphosphatidylation by phospholipase D. J Biol Chem. 1967 Feb 10;242(3):477–484. [PubMed] [Google Scholar]
- Yoshida S., Sakai A. Phospholipid degradation in frozen plant cells associated with freezing injury. Plant Physiol. 1974 Mar;53(3):509–511. doi: 10.1104/pp.53.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]