Abstract
Small heat shock proteins (sHSPs) play important roles in responses to heat stress. However, the functions of sHSPs in tea plants (Camellia sinensis) remain uncharacterized. A novel sHSP gene, designated CsHSP17.2, was isolated from tea plants. Subcellular localization analyses indicated that the CsHSP17.2 protein was present in the cytosol and the nucleus. CsHSP17.2 expression was significantly up-regulated by heat stress but was unaffected by low temperature. The CsHSP17.2 transcript levels increased following salt and polyethylene glycol 6000 treatments but decreased in the presence of abscisic acid. The molecular chaperone activity of CsHSP17.2 was demonstrated in vitro. Transgenic Escherichia coli and Pichia pastoris expressing CsHSP17.2 exhibited enhanced thermotolerance. The transgenic Arabidopsis thaliana exhibited higher maximum photochemical efficiencies, greater soluble protein proline contents, higher germination rates and higher hypocotyl elongation length than the wild-type controls. The expression levels of several HS-responsive genes increased in transgenic A. thaliana plants. Additionally, the CsHSP17.2 promoter is highly responsive to high-temperature stress in A. thaliana. Our results suggest that CsHSP17.2 may act as a molecular chaperone to mediate heat tolerance by maintaining maximum photochemical efficiency and protein synthesis, enhancing the scavenging of reactive oxygen species and inducing the expression of HS-responsive genes.
Introduction
Heat shock (HS) is induced by a higher temperature, which is approximately 10–15 °C above the optimal growth temperature1, 2. After exposure to HS, prokaryotic and eukaryotic cells produce a group of heat shock proteins (HSPs)2, 3. The HSPs can be divided into the following five categories based on their approximate molecular weights: HSP100, HSP90, HSP70, HSP60, and small HSPs (sHSPs). The sHSPs are the most prevalent in plants, and their importance is implied by their unusual abundance and diversity4. Six classes of sHSPs have been identified based on their amino acid sequence similarities, immunological cross-reactivities, and intracellular localizations4, 5. Members of Classes I and II are localized in the cytosol and the nucleus. Classes III, IV, V, and VI consist of sHSPs present in the chloroplast, endoplasmic reticulum, mitochondrion, and membrane, respectively6, 7.
Molecular chaperones are responsible for protein folding, assembly, translocation and degradation in many normal cellular processes. They also stabilize proteins and membranes, and assist in protein refolding under stress conditions4. Previous studies revealed that a wide range of sHSPs have molecular chaperone activities in vitro and in vivo 8, 9. Analyses of sHSP transcript levels indicated that some sHSPs were undetectable in the absence of stress, but rapidly accumulated following exposure to HS10, 11. In addition to HS, the expression of sHSP genes can be induced by other abiotic stress, including salinity12, low temperature13 and drought14. Furthermore, some plant sHSPs are only produced in specific tissues or during particular developmental stages10, 15. Previous studies have demonstrated that heterologous expression of sHSP genes confers abiotic stress tolerance in transgenic A. thaliana 16, rice17, tomato18, potato19, and tobacco20, suggesting that sHSPs are involved in stress tolerance. Ruibal et al.21 found that a functional sHSP PpHsp16.4 was essential for recovery from heat, salt and osmotic stress in Physcomitrella patens. However, overexpression of AsHSP17, a new sHSP gene from creeping bentgrass (Agrostis stolonifera), negatively regulate plant responses to adverse environmental stresses in transgenic A. thaliana 22. Recently, OsHSP18.2, a sHSP from rice (Oryza sativa), was shown to play important roles in seed vigor, longevity and seedling establishment in transgenic A. thaliana 23.
Tea plants [Camellia sinensis (L.) O. Kuntze] are important leaf-type woody crops used for the production of non-alcoholic beverages worldwide. The plants must cope with various abiotic stress during their lifecycle24, including low and high temperatures25, drought26, and salinity27. High-temperatures are likely the most important abiotic stress for tea plants because they can considerably affect crop quality and yield. Thus, it is important to understand the molecular mechanisms regulating heat tolerance in C. sinensis. We previously isolated several HS-inducible genes from tea plants using a suppression subtractive hybridization method (data not shown). Pre-experimental results revealed that CsHSP17.2 (GenBank accession number: KU244518) was more highly expressed following exposure to HS than the other HS-inducible genes. These results suggested that CsHSP17.2 affected heat tolerance in C. sinensis. However, the exact relationship between CsHSP17.2 and thermotolerance has not been fully characterized. In the present study, we analyzed the CsHSP17.2 functions associated with thermotolerance by overexpressing CsHSP17.2 in E. coli (a prokaryote), P. pastoris and A. thaliana (eukaryotes). Furthermore, we report the cloning of its promoter by genome walking and its characterization using transgenic approaches. The results of this study may lead to a more thorough characterization of the molecular basis of thermotolerance in C. sinensis.
Results
Isolation and sequence analysis of CsHSP17.2 gene
The full-length cDNA of CsHSP17.2 gene consisted of 811 bp, with a 453-bp open reading frame (ORF) encoding a 150-amino-acid protein (predicted molecular mass of 17.2 kDa). Sequence alignments with the deduced CsHSP17.2 protein sequence and those of other plant sHSPs indicated that CsHSP17.2 contains a conserved 90-amino-acid C-terminal α-crystallin domain5, which can be further divided into two homologous regions, namely, consensus region I (positions +110 to +135) and consensus region II (positions +59 to +82) (Fig. S1a). A phylogenetic analysis revealed that CsHSP17.2 was highly similar to Class I sHSPs and was closely related to proteins from Gossypium hirsutum GhHSP17.9 (AEH30706) and Jatropha curcas JcHSP17.3 (XP_012066921) (Fig. S1b).
Subcellular localization of CsHSP17.2 protein
To clarify the CsHSP17.2 biological functions, subcellular localization investigations were completed using onion epidermal cells and tobacco leaves producing the CsHSP17.2:GFP fusion protein. The 35S:GFP signals were distributed throughout the cytosol and the nucleus in onion (Fig. 1A) and tobacco epidermal cells (Fig. 1B). As predicted by the protein subcellular localization prediction software WoLF PSORT28, diffuse 35S:CsHSP17.2:GFP signals were detected in the cytosol and nucleus of onion (Fig. 1A) and tobacco epidermal cells (Fig. 1B).
Figure 1.
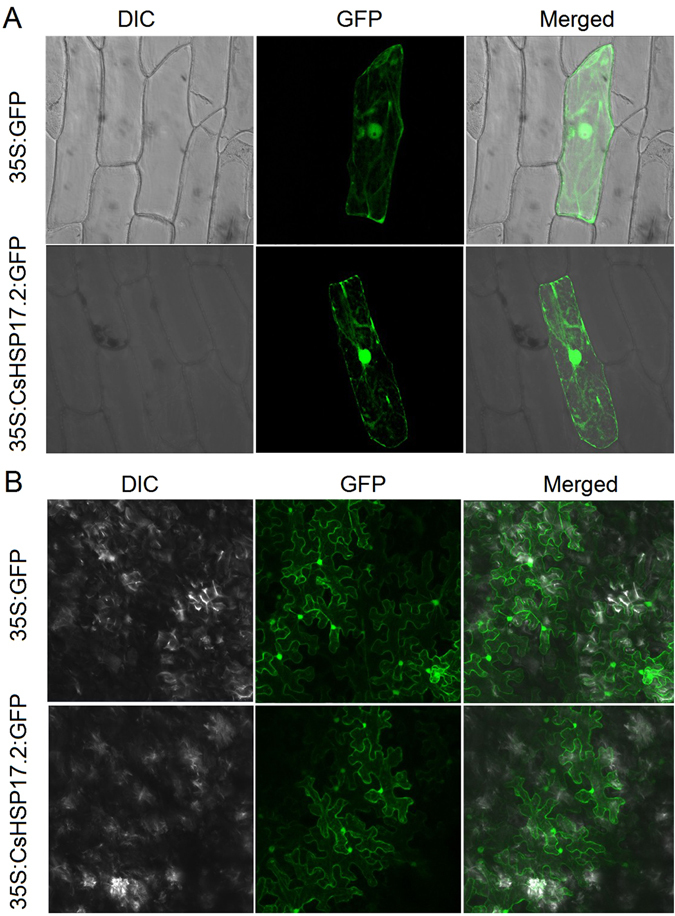
CsHSP17.2 protein accumulates in the nucleus and the cytosol. (A) Subcellular localization of CsHSP17.2 in onion (Allium cepa) epidermal cells. (B) Subcellular localization of CsHSP17.2 in tobacco (Nicotiana benthamiana) leaves. 35S:GFP indicates the vector control, and 35S:CsHSP17.2:GFP refers to the target protein.
CsHSP17.2 transcription profiles under heat shock (HS) and recovery conditions
To determine whether CsHSP17.2 expression under HS conditions is time-dependent, tea plants were incubated at high temperatures (40 °C) for different times (Fig. 2A). The abundance of CsHSP17.2 transcripts in plants exposed to HS initially increased very quickly, with the highest levels (approximately 1,450-fold higher than pre-treatment levels) occurring within 1 h. Transcript levels significantly decreased after 6 h (P < 0.01). Following the HS treatment for 1 h, the tea plants were returned to normal conditions to recover from the heat stress. The CsHSP17.2 transcript levels decreased significantly within 1 h of recovery and were almost undetectable after 3 h (P < 0.01) (Fig. 2B).
Figure 2.
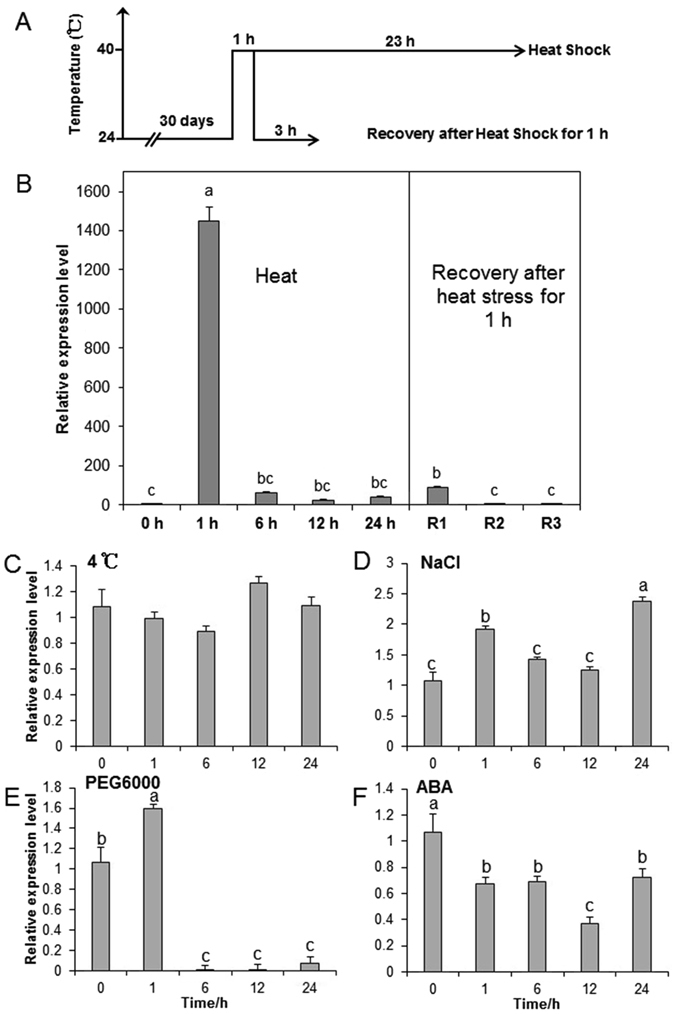
Transcription profiles of CsHSP17.2 gene under abiotic stress. (A) Schematic diagram of heat stress and recovery. (B) CsHSP17.2 expression levels under heat stress and recovery. R1, R2 and R3 indicate recovery from heat shock (HS) for 1, 2, and 3 h, respectively. Tea plants exposed to low temperature (4 °C) (C), 300 mM NaCl (D), 20% (w/v) PEG6000 (E) and 50 µM ABA (F). Different letters indicate significant differences (P < 0.05) between treatments and the control (0 h).
Analysis of CsHSP17.2 expression following exposure to cold, salinity and exogenous abscisic acid (ABA)
To investigate whether the CsHSP17.2 transcript levels were regulated by other abiotic stress, tea plants were exposed to various treatments. Under cold stress conditions, there were no significant changes in CsHSP17.2 expression levels within the first 24 h (Fig. 2C). The abundance of CsHSP17.2 transcripts in salt-stressed plants increased within 1 h of treatment, decreased significantly after 6 and 12 h (P < 0.05), and then peaked at 24 h (Fig. 2D). When exposed to drought stress, CsHSP17.2 transcript levels were high at 1 h and then decreased significantly after 6 h (P < 0.05) (Fig. 2E). Finally, exogenous ABA treatment significantly inhibited the CsHSP17.2 transcription levels in tea plants (P < 0.05) (Fig. 2F). These results indicated that CsHSP17.2 influenced drought and salinity stress responses and ABA signal transduction pathways.
CsHSP17.2 overexpression in E. coli cells
We completed reverse transcription polymerase chain reaction (RT-PCR) experiments to ensure that appropriate transgenic E. coli cells had been generated. The resulting amplicons were 202 and 655 bp long, which corresponded to the control and CsHSP17.2-overexpressing lines, respectively (Fig. S2a). The bands corresponding to the expected 22-kDa thioredoxin/histidine tag were larger in the control E. coli lines (i.e., those harboring the empty vector) treated with 0.2 mM isopropyl-β-D-thiogalac-topyranoside (IPTG) than in the non-induced control E. coli lines. Additionally, an approximately 36-kDa band was detected for CsHSP17.2-overexpressing lines induced with IPTG, which matched the expected size of the CsHSP17.2 protein fused to the thioredoxin/histidine tag (Fig. 3A).
Figure 3.
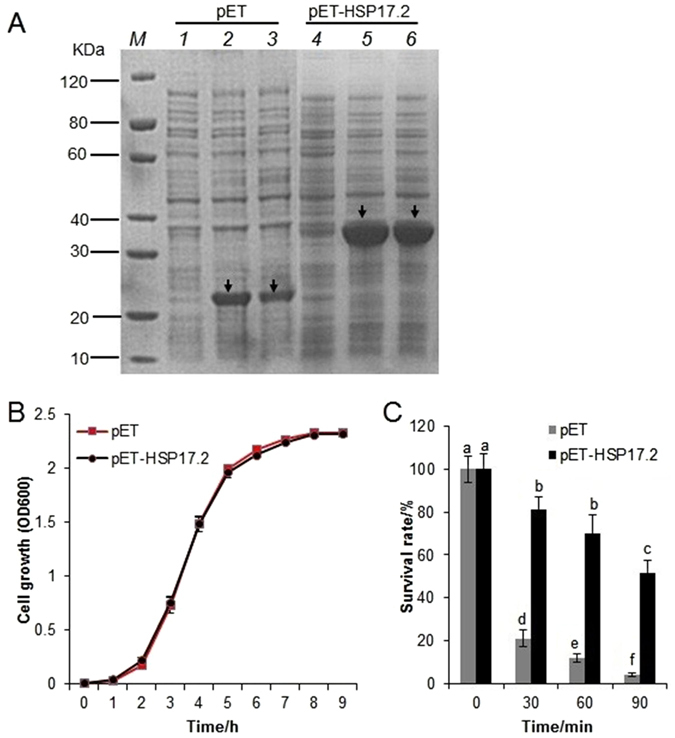
Heterologous expression of CsHSP17.2 in E. coli. (A) Expression of the CsHSP17.2 protein in E. coli assessed by SDS-PAGE analysis. M, protein molecular weight marker; lane 1: whole cell lysate of un-induced cells containing the empty vector pET; lane 2: whole cell lysate of cells cntaining the empty vector and induced by 0.2 mM IPTG at 15 °C for 16 h; lane 3: whole cell lysate of cells containing the empty vector and induced by 0.2 mM IPTG at 37 °C for 4 h; lane 4: whole cell lysate of un-induced cells containing pET-HSP17.2; lane 5: whole cell lysate of cells containing pET-HSP17.2 and induced by 0.2 mM IPTG at 15 °C for 16 h; lane 6: whole cell lysate of cells containing pET-HSP17.2 and induced by 0.2 mM IPTG at 37 °C for 4 h. The induced proteins are indicated by black arrows. (B) Growth analysis of transformed E. coli BL21 (DE3) cells under normal conditions. (C) Protective effect of recombinant CsHSP17.2 on cell viability during HS in vivo. The mean values of three independent experiments are plotted with error bars indicating standard deviations. The significant differences are indicated by different letters (P < 0.05).
Under the premise that the pET E. coli overexpression system works normally, we performed cell viability assays to investigate the possible functions of CsHSP17.2. There was no obvious difference in the growth rates of the pET and pET-HSP17.2 strains, indicating that CsHSP17.2 overexpression did not affect E. coli growth under normal conditions (Fig. 3B). Under heat stresses, the E. coli survival decreased rapidly, but the CsHSP17.2-overexpressing cells were more viable than the control cells (P < 0.01). After a 90-min heat treatment, more than 95% of the pET cells died, whereas approximately 51% of pET-HSP17.2 cells survived (Fig. 3C). This observation suggested that CsHSP17.2 increased the thermotolerance of the transgenic E. coli cells.
Constitutive expression of CsHSP17.2 confers thermotolerance in transgenic P. pastoris
To confirm that CsHSP17.2 influenced thermotolerance, we examined the expression of CsHSP17.2 in a eukaryotic organism. First, RT-PCR was used to verify that the recombinant plasmids were present in the transformed P. pastoris cells. The amplified fragments were 221 and 548 bp long, which were the expected sizes for the control and CsHSP17.2-overexpressing lines, respectively (Fig. S2b). The positive strains were used for subsequent thermotolerance assays. Similar growth rates were observed between the control and the CsHSP17.2-overexpressing lines at 30 °C. However, the growth rates were higher for the CsHSP17.2-overexpressing strains than the control after HS treatment for 30 or 60 min (Fig. 4). These results indicated that the constitutive expression of CsHSP17.2 confers thermotolerance in P. pastoris.
Figure 4.

Heterologous expression of CsHSP17.2 in P. pastoris under heat shock (HS). Control indicates the transformant harboring the empty vector pPIC3.5 K, and HSP17.2 indicates the P. pastoris strains with recombinant plasmid pPIC3.5K-HSP17.2.
CsHSP17.2 exhibits molecular chaperone activity in vitro
After the thioredoxin/hi-stidine-tagged CsHSP17.2 protein was bound to a nickel-charged affinity resin column, recombinant CsHSP17.2 (approximately 17 kDa) was separated from the fusion tag using the tobacco etch virus protease and then eluted from the column (Fig. 5A). To investigate whether CsHSP17.2 has molecular chaperone activity in vitro, citrate synthase (CS) were chemically denatured and renatured. When 300 nM CsHSP17.2 was added to the denatured CS under renaturing conditions, approximately 78% of the CS activity (threefold relative to the control) was recovered. In contrast, in the presence of H2O, only 26% of the CS activity was recovered after 75 min (Fig. 5B). These results suggested that CsHSP17.2 functioned as a molecular chaperone in vitro.
Figure 5.
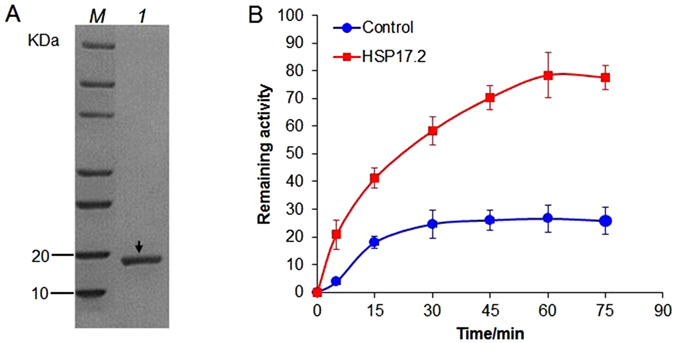
Purified recombinant CsHSP17.2 protein was separated on a 4–22% native gel (A) and the effect of CsHSP17.2 protein on the renaturation of chemically denatured citrate synthase (CS) (B). M, protein marker; lane 1: recombinant CsHSP17.2 protein. CS (15 μM) was denatured in 6 M guanidine hydrochloride for 2 h and then placed under refolding conditions containing 150 nM CsHSP17.2 proteins or H2O (Control). The remaining activity of CS is presented as the percentage of non-denatured CS activity. Error bars in figures represent standard errors generated from at least three replicate trials.
Variations in thermotolerance, maximum photochemical efficiency (Fv/Fm), soluble protein and free proline content
An RT-PCR analysis indicated that CsHSP17.2 was substantially expressed in six independent transgenic A. thaliana lines (i.e., OE-8, OE-18, OE-21, OE-28, OE-29, and OE-30) but not in the wild-type (WT) plants (Fig. 6A). The three transgenic lines with the highest transgene expression levels (i.e., OE-8, OE-21, and OE-30) were used for subsequent experiments. The survival rates were higher in transgenic lines than in the WT plants after HS for 45 min, suggesting that CsHSP17.2 overexpression enhanced thermotolerance in A. thaliana plants (Fig. 6B). To estimate the level of HS-induced damage to A. thaliana plants, related physiological indices were recorded after a 4-h HS treatment. Exposure to HS resulted in a significant decrease in the Fv/Fm value in the CsHSP17.2-overexpressing and WT plants (P < 0.05) (Fig. 6C), but photosystem II was more active in the transgenic lines than in the WT plants, implying that CsHSP17.2 overexpression stabilized Fv/Fm under HS conditions. Similarly, the soluble protein content decreased more in WT plants than in the CsHSP17.2-overexpressing lines following HS treatment (Fig. 6D). This change indicated that CsHSP17.2 overexpression resulted in increased amounts of soluble protein in transgenic plants. In addition, after exposure to HS, more free proline accumulated in the transgenic lines than in WT plants (Fig. 6E), indicating that CsHSP17.2 overexpression promoted proline synthesis.
Figure 6.
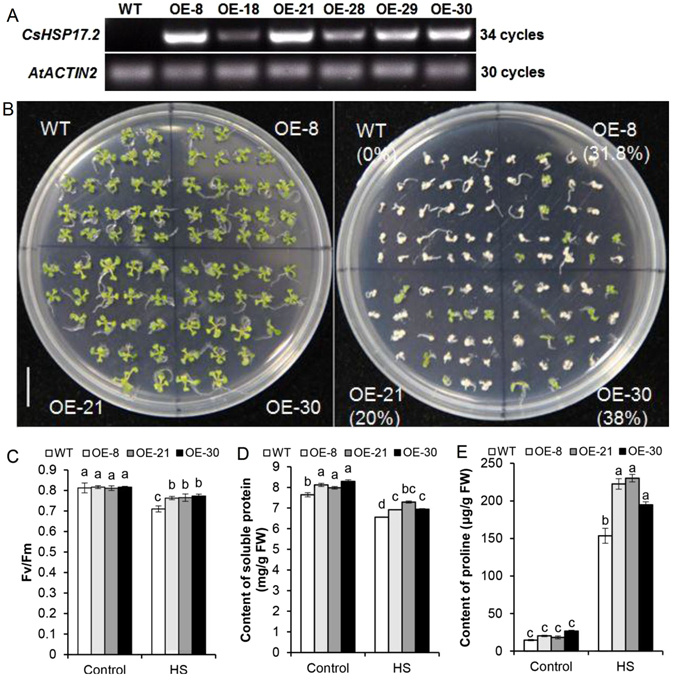
Thermotolerance of WT and transgenic A. thaliana. (A) RT-PCR identification of CsHSP17.2 transgenic A. thaliana lines. Total RNA was extracted from rosette leaves of 4-week-old plants, and the AtACTIN2 (AT3G18780) gene was used as a control for equal loading. (B) The survival rates of WT and transgenic A. thaliana plants under HS. The detection of chlorophyll fluorescence (C), soluble protein content (D) and proline content (E) in transgenic and WT plants. The A. thaliana seedlings were cultivated for four weeks under normal temperature (22 °C; Control) before HS (45 °C for 4 h), and the rosette leaves were harvested for the experiments. Data represent the means ± standard deviations of three replicates. Significant differences between WT and transgenic plants are indicated by different letters (P < 0.05). WT: wild-type; OE-8, OE-18, OE-21, OE-28, OE-29, and OE-30: six transgenic A. thaliana lines.
CsHSP17.2 overexpression in A. thaliana promotes seed germination and hypocotyl development under HS conditions
To determine whether CsHSP17.2 affected seed vigor, we examined the germination rates of WT and transgenic A. thaliana seeds under HS conditions. Without the HS treatment, nearly 100% of WT and transgenic seeds incubated at 22 °C germinated within 3 days (Figs 7A and S3a), indicating the high quality of the seeds used for the germination assays. However, the germination rates of transgenic and WT seeds gradually decreased with increasing duration of HS treatment. Additionally, the transgenic seeds exhibited enhanced vigor compared with the WT seeds after HS treatment (Figs 7B–D and S3b–d). Only 95.9% and 91.6% of WT seeds germinated after 1 and 2 h of heat treatment, respectively, whereas 98.7% of the transgenic seeds germinated even after 2 h. The germination rates of transgenic and WT seeds were 95.9–100.0% and 72.5% after a 3-h heat treatment, respectively. These results implied that CsHSP17.2 overexpression in plants enhanced the basal thermotolerance of seeds.
Figure 7.
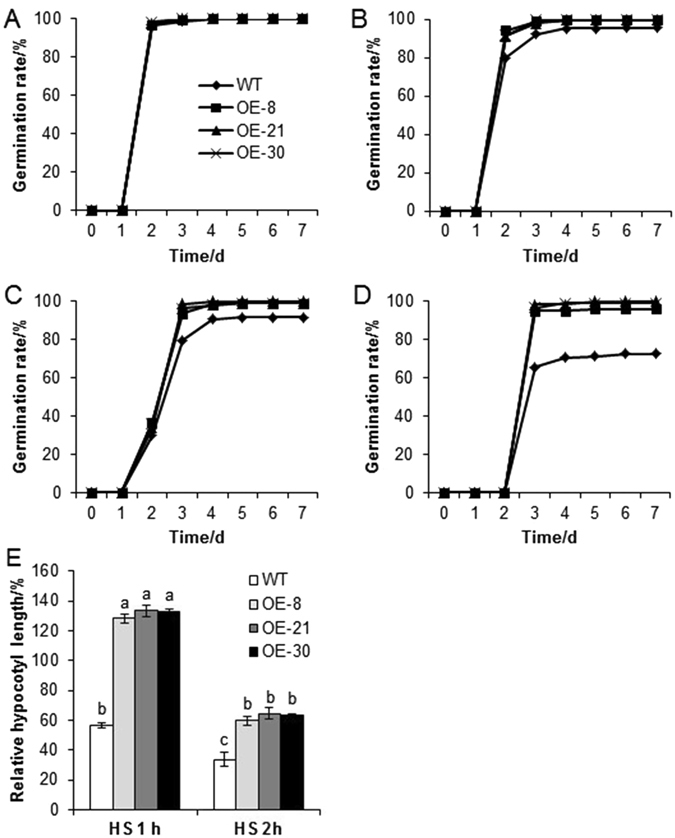
Germination rates (A–D) and relative hypocotyl lengths (E) of WT and transgenic A. thaliana under HS. (A) Control group, (B) HS for 1 h, (C) HS for 2 h, and (D) HS for 3 h. Error bars represent standard deviations and are based on data for at least 10 seedlings from each line. Significant differences between WT and transgenic plants are indicated by different letters (P < 0.05).
Hypocotyls were much longer in the CsHSP17.2-overexpressing lines than in the WT plants after exposure to high temperatures for 1 or 2 h (Fig. 7E), indicating that CsHSP17.2 plays an important role in hypocotyl development under HS conditions.
Cloning of the putative promoter of CsHSP17.2
The 863-bp upstream sequence of the CsHSP17.2 translation initiation site was obtained via thermal asymmetric interlaced PCR (TAIL-PCR) (Fig. S4), and the presence of core promoter elements was assessed using the PlantCARE database29. The presence of hormone-responsive motifs together with abiotic stress-related motifs and developmental stage specific elements was observed in the putative promoter sequence (Fig. 8 and Table S1). The presence of these regulatory elements suggested that the promoter region of CsHSP17.2 might be responsive to a wide variety of abiotic stress and hormones and induced in some developmental stages.
Figure 8.
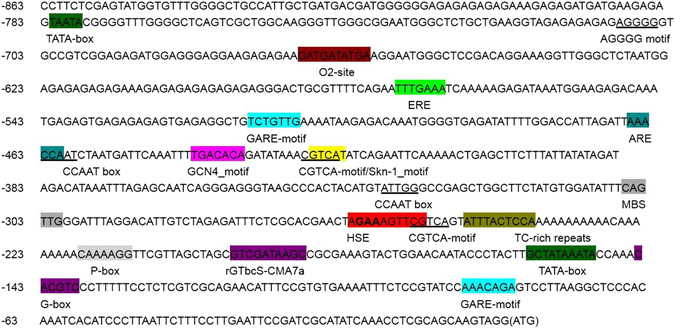
Analysis of the promoter sequences of CsHSP17.2 using the PlantCARE promoter motif analysis tool. The start codon is shown in parentheses. The underlines and different colors signify different functional elements.
Responsiveness of the CsHSP17.2 promoter to abiotic stress and hormones
The induction of the GUS under abiotic stress and hormones was monitored in 3-week-old transgenic A. thaliana plants via quantitative real-time PCR (qRT-PCR) and GUS histochemical staining. The GUS transcript gradually increased and peaked at approximately 12 h of heat stress treatment; thereafter, the transcription levels decreased significantly (Fig. 9A). The GUS histochemical assay revealed protein accumulation until 24 h, suggesting that although the transcript might not be required in high amounts, the corresponding protein accumulated (Fig. S5).
Figure 9.
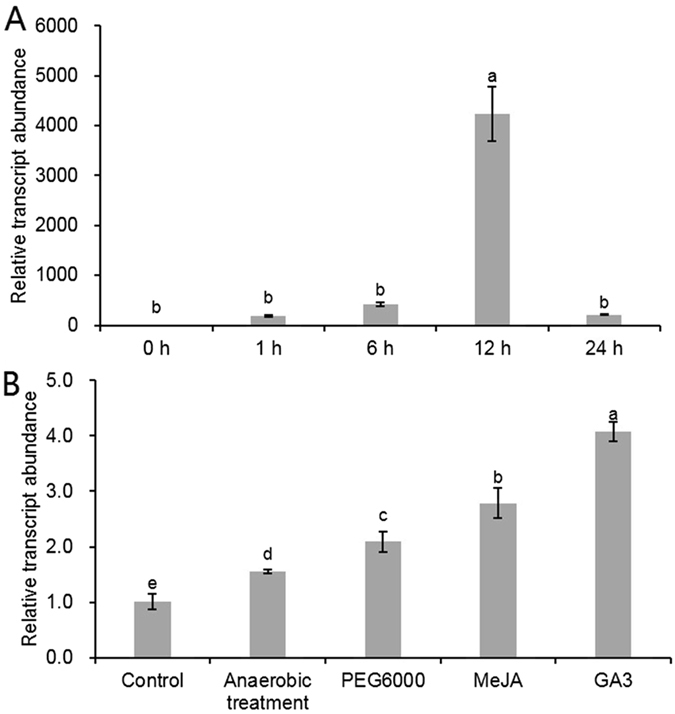
Induction of GUS gene governed by the CsHSP17.2 promoter. (A) qRT-PCR for the GUS transcript under heat stress. (B) qRT-PCR for the GUS transcript under other abiotic stress and hormone treatments. Data represent the means ± standard deviations of four replicates, and the significant differences are indicated by different letters (P < 0.05).
In addition to heat stress, the CsHSP17.2 promoter activity was also induced by simulated drought (10% PEG6000) and plant hormones (40 μM MeJA or 20 μM GA3) (Figs 9B and S5). These findings were consistent with the presence of multiple abiotic stress- and hormone-responsive elements in the CsHSP17.2 promoter sequence.
Discussion
Since the first sHSP was discovered in Drosophila melanogaster, numerous sHSPs have been identified in various plant species5, 30. Plant sHSPs are classified into different subfamilies based on amino acid sequence similarities and localization to distinct subcellular compartments. In this study, the green fluorescence of the CsHSP17.2:GFP fusion protein was detected in the cytosol and nucleus, in agreement with our results from analyses of homology and phylogenetic relationships. These findings suggested that CsHSP17.2 is a Class I sHSP.
Numerous studies have previously demonstrated that sHSPs are regulated by various abiotic stress. A Primula sHSP gene, PfHSP17.1, is induced by heat, cold, salt, PEG-induced drought stress, and oxidative stress14. Similarly, a Tamarix hispida sHSP gene, ThHSP18.3, is induced by cold, heat, salt, and drought stress12. In addition, a David Lily sHSP gene, LimHSP16.45 is triggered by cold and heat stress16. Our observations revealed that the expression of CsHSP17.2 was differentially regulated by heat, cold, drought, salinity, and ABA treatments, suggesting that it has different roles in various abiotic stress responses. However, CsHSP17.2 expression was not obviously affected by cold stress, which suggested that CsHSP17.2 might not participate in the cold stress response. Interestingly, CsHSP17.2 transcription was down-regulated by ABA treatment, which was consistent with the results of Sun et al.31, suggesting that CsHSP17.2 may function in an ABA-dependent manner.
Several recent studies have used the expression of sHSP genes in E. coli cells to investigate their possible functions in vivo. The heterologous expression of a Medicago sativa mitochondrial HSP23 protected E. coli from salinity and arsenic stresses32, and the overexpression of a Rosa chinensis RcHSP17.8 enhanced E. coli viability during exposure to heat and cold stresses33. Additionally, as a major eukaryotic model organism, P. pastoris produces sHSPs and is often used to investigate sHSP functions33, 34. Similarly, the E. coli and P. pastoris cells constitutively expressing CsHSP17.2 were more viable when exposed to heat stress than the control cells, implying that CsHSP17.2 overexpression results in greater thermotolerance.
The Fv/Fm value is a good indicator of the photosynthetic functions of plants under adverse environmental conditions. A previous study concluded that overexpression of a tomato (Lycopersicon esculentum) HSP21 protected photosystem (PS) II from the effects of temperature-dependent oxidative stress35. In our study, the Fv/Fm values of CsHSP17.2-overexpressing plants were significantly higher than those of WT plants under HS conditions, suggesting that CsHSP17.2 has a role in protecting of PSII against oxidative stress created by high temperature. Previous studies have demonstrated that protein synthesis positively correlates with stress tolerance, and heat-tolerant plants maintain a higher protein synthesis rate and a lower protein degradation rate than heat-intolerant plants36, 37. The overall protein levels in A. thaliana expressing exogenous CsHSP17.2 significantly increased compared with WT plants, indicating that CsHSP17.2 overexpression enhances thermotolerance by facilitating protein synthesis. It has been well established that excess H2O2 in plant cells leads to the occurrence of oxidative stress38, 39. In our study, the histochemical detection of H2O2 indicated that CsHSP17.2 overexpression resulted in increased scavenging of H2O2 under HS conditions (Fig. S6), suggesting that CsHSP17.2 overexpression confers thermo-tolerance in A. thaliana. Ascorbate peroxidases (APXs) and peroxidases (PODs) are considered to have essential roles in scavenging reactive oxygen species (ROS) and protecting cells from oxidative damage in higher plants40, 41. During exposure to HS, the transcription levels of AtAPX1 and AtPOD were higher in the CsHSP17.2-overexpressing lines than in the WT plants (P < 0.05) (Fig. S7). Consequently, we hypothesize that CsHSP17.2 increases the capacity for ROS-scavenging by elevating AtAPX1 and AtPOD transcript levels. Proline confers osmotic tolerance during exposure to stress conditions, and AtP5CS2 and AtProT1 are two key genes involved in proline biosynthesis and transport42, 43. The transcription levels of these two genes were higher in the transgenic lines than in WT plants under normal and HS conditions (Fig. S7), which was consistent with the content of proline, implying that CsHSP17.2 overexpression may enhance proline biosynthesis and transport and ultimately increase the thermotolerance in A. thaliana.
Heat shock transcription factors (HSFs) are transcription factors that activate the expression of genes in response to stress, thereby playing a central role in cellular homeostatic control mechanisms44. An HSF binds to a DNA sequence motif, the heat shock element (HSE), which is characterized by an array of inverted repeats of the motif nGAAn. Besides an HSE motif, AGGGG motif (designated as STRE) and CCAAT box were also found in the promoter region of CsHSP17.2, which have been demonstrated to act cooperatively with the HSEs to enhance HSP promoter activity under high temperature45, 46. Therefore, these combined results strongly suggest that CsHSP17.2 is regulated by HSFs and induced by heat stress conditions. The transcript levels of AtHSF genes (especially AtHSFA4a and AtHSFC1) were higher in CsHSP17.2 transgenic lines than in WT plants. Thus, we speculated that CsHSP17.2 overexpression might enhance the thermotolerance of transgenic plants by co-regulating HSF gene expression.
Molecular chaperones, including sHSPs, are important for maintaining cellular homeostasis under optimal and adverse growth conditions. They can bind to partially denatured proteins to prevent further denaturation or aggregation and promote the correct refolding of proteins4, 47. Our results indicated that CsHSP17.2 functions as a molecular chaperone in vitro by inducing the renaturation and reactivation of chemically denatured CS, suggesting that CsHSP17.2 may help to maintain proteins in their functional conformations and to prevent the aggregation of non-native proteins in host cells. AtHSP17.4 and AtHSP70, which are associated with molecular chaperone activities11, 48, 49, were more actively transcribed in transgenic A. thaliana plants under HS conditions (Fig. S7). These findings suggested that CsHSP17.2 overexpression induces HSP synthesis and confers thermotolerance in plants.
The sHSPs have protective roles in seed germination. Transgenic A. thaliana seeds expressing NnHSP17.5 displayed enhanced seed germination vigor to heat stress50. Similarly, ectopic expression of LimHSP16.45 enhanced the seed viability of A. thaliana exposed to high temperature, salinity, and oxidative stress51. In this study, seeds from CsHSP17.2-overexpressing lines exhibited enhanced germination rates under HS conditions, suggesting that CsHSP17.2 may contribute to basal thermotolerance during seed germination. Induced thermotolerance is defined as the capacity of an organism to survive a normally lethal temperature if it is first conditioned by pretreatment at a milder temperature and can be measured in hypocotyl elongation assays52. CsHSP17.2-overexpressing lines exhibited higher hypocotyl elongation lengths, suggesting that CsHSP17.2 overexpression conferred induced thermotolerance in transgenic plants. To the best of our knowledge, this report is the first demonstrating that a Class I sHSP is correlated with induced thermotolerance. Thus, we speculate that CsHSP17.2 is essential for basal and induced thermotolerance in transgenic A. thaliana.
Previous studies have demonstrated that overexpression of sHSPs have different roles in plant growth and development. Overexpression of TaHSP26 in A. thaliana produced higher biomass and seed yield than WT plants3. Besides, transgenic tomato (Lycopersicon esculentum) plants constitutively expressing LeHSP21 promoted fruit ripening under normal growth temperature35. However, overexpression of NnHSP17.5 had no positive or detrimental effects on plant development in A. thaliana. Similarly, overexpressing CsHSP17.2 did not cause any obvious phenotypic changes. The germination times and rates, growth rates, time to flowering, and seed yields of the transgenic plants were similar to those of WT plants (Fig. S8). For example, under normal conditions, there were no significant differences between WT and CsHSP17.2-overexpressing plants in terms of the fresh weights of rosette leaves from 4- or 5-week-old seedlings (Fig. S9). These results suggested that heterologous expression of CsHSP17.2 may be used for enhancing thermotolerance in economically important crops.
In summary, our results demonstrate that CsHSP17.2 is a heat-inducible gene, and CsHSP17.2 has molecular chaperone activities in vitro. CsHSP17.2 has the capacity to confer thermotolerance not only on E. coli and P. pastoris, but also on A. thaliana under heat stress. We propose that CsHSP17.2 increases plant thermotolerance through several pathways, including the maintenance of photosynthetic rates and protein synthesis, enhancement of ROS-scavenging, and expression of HS-responsive genes. These findings should help to clarify the complex mechanisms and roles of HSPs in regulating plant responses to environmental stresses.
Methods
Plant materials and stress treatments
Two-year-old C. sinensis cv. ‘Yingshuang’ plants were grown in a light incubator with a 12-h light (200 μmol·m−2·s−1; 24 °C)/12-h dark (20 °C) photoperiod for 30 days before treatments. For HS treatments, plants were placed in a light incubator (40 °C) for 24 h. For recovery treatments, the plants (HS for 1 h) were allowed to recover at 24 °C for 3 h, and samples were collected every hour. For the low-temperature treatment, tea plants were transferred to another chamber maintained at 4 °C. To simulate high salinity and drought stresses, tea plants were treated with 300 mM NaCl and 20% (w/v) PEG6000, respectively. For the exogenous ABA treatment, tea leaves were sprayed with 50 µM ABA solution. All treatments were completed under normal conditions (as described above), unless otherwise indicated. Additionally, the third fully expanded leaves from the top buds were harvested at 0, 1, 6, 12, and 24 h after various treatments, immediately frozen with liquid nitrogen, and stored at −70 °C for subsequent RNA extraction.
Molecular cloning of CsHSP17.2 gene and its promoter
Total RNA was isolated from tea leaves using a Quick RNA Isolation Kit (Huayueyang, Beijing, China), and 1 μg of total RNA was reverse transcribed to generate first-strand cDNA using Reverse Transcriptase M-MLV (RNase H−) (TaKaRa, Dalian, China) according to the manufacturer’s instructions. To obtain the ORF of CsHSP17.2, a primer pair (ORF-F/-R, Table S2) was designed and employed for PCR amplification. The resulting amplicons were purified and cloned into the pEASY-T1 Simple Cloning Vector (Transgen, Beijing, China) for sequencing (Genscript, Nanjing, China).
Genomic DNA was extracted from tea buds using Plant Genomic DNA Kit (TIANGEN, Beijing, China) according to the manufacturer’s instructions. The promoter of CsHSP17.2 gene was amplified using Genome Walking Kit (TaKaRa, Dalian, China) with three gene-specific primers (SP1, SP2 and SP3, Table S2). The target products were cloned into the pEASY-T1 simple vector and sequenced. A search for regulatory elements in the promoter was performed using the PlantCARE (Plant Cis-Acting Regulatory Element) database29.
Analyses of sequences and phylogenetic relationships
The ClustalX program was used for multiple sequence alignments53, and a phylogenetic tree was constructed using MEGA5 according to the neighbor-joining method54.
Subcellular localization of the CsHSP17.2 protein in onion (Allium cepa) epidermal cells and tobacco (Nicotiana benthamiana) leaves
To construct a transient expression vector, the ORF of the CsHSP17.2 gene (without the stop codon) was amplified using the sub-F/-R primer pair (Table S2). The amplified CsHSP17.2 coding region was inserted into the pCAMBIA2300-GFP vector at the Bam HI and Xba І sites to generate the CsHSP17.2::GFP construct. Onion epidermal cells were transformed with the recombinant plasmid or the empty vector using a PDS-1000/He particle delivery system (Bio-Rad, Hercules, CA, USA). The transformed onion epidermal cells were then incubated on Murashige and Skoog (MS) agar medium for 16 h at 25 °C in darkness. Finally, the GFP signal was detected using a Zeiss LSM700 confocal laser-scanning microscope (Carl Zeiss Inc., USA).
For transient expression in tobacco cells, Agrobacterium tumefaciens strain GV3101 cells were independently transformed with the recombinant plasmid or empty vector. The transformed A. tumefaciens cells were infiltrated into tobacco leaves55, and then plants were incubated for 3 days in darkness. The GFP signal was detected as described above.
Gene expression analysis by qRT-PCR
Total RNAs were extracted from tea leaves exposed to different treatments using the Quick RNA Isolation Kit (Huayueyang, Beijing, China). After assessing the quality and determining the concentration of total RNA using the ONE Drop OD-1000+ spectrophotometer (ONE Drop, USA), 1 μg of total RNA was reverse transcribed to single-stranded cDNA using the PrimeScript RT Reagent Kit with gDNA Eraser (TaKaRa, Dalian, China) following the manufacturer’s instructions. The CsHSP17.2-qF/-qR primer pair (Table S2) was used to analyze CsHSP17.2 expression levels. The C. sinensis β-actin gene (GenBank accession number: HQ420251, Table S2) was used as the reference gene.
A. thaliana transgenic plants (3-week-old) were exposed to various abiotic stress and hormone treatments. For heat stress treatment, plants were cultured in a growth cabinet at 40 °C (dark) for different time periods. Drought stress was provided by treating with 10% PEG6000 for 4 h, and anaerobic treatment by immersing seedlings in distilled water for 3 h. For hormone treatments, leaves of plants were sprayed with 40 μM methyl jasmonate (MeJA) or 20 μM gibberellin A3 (GA3) for 24 h. After these treatments, the A. thaliana rosette leaves were sampled and flash-frozen in liquid nitrogen and stored at −80 °C for RNA extraction. All of these treatments were performed under a growth regime of 16/8 h light/darkness at 22 °C unless otherwise mentioned. qRT-PCR was performed with GUS-specific primers (GUS-qF/-qR, Table S2), and AtACTIN2 (AT3G18780) was selected as a housekeeping gene.
To analyze the expression profiles of HS-responsive genes, total RNA was extracted from the leaves of 4-week-old A. thaliana plants that had been incubated at 45 °C for 0, 0.5, 1, 2, and 4 h. The expression profiles of twelve HS-responsive genes were determined by qRT-PCR, with the A. thaliana ACTIN2 (AT3G18780) gene used as the housekeeping gene. All of the relevant primers are listed in Table S3.
The qRT-PCR was completed using SYBR Premix Ex Taq II (Tli RnaseH Plus) (TaKaRa, Dalian, China) and an iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad). Each 20 μl qRT-PCR sample consisted of 10 μl of SYBR Premix Ex Taq II (2x), 0.2 μM of each primer, and 10 ng of cDNA template. The PCR program was as follows: 95 °C for 30 s, 40 cycles at 95 °C for 5 s, and 58 °C for 30 s. All experiments were repeated three times with independent RNA samples, and the relative expression levels were calculated using the 2−ΔΔCT method56.
CsHSP17.2 overexpression in E. coli cells
The full-length CsHSP17.2 coding region was amplified using the pro-F/-R primer pair (Table S2) and KOD FX Neo polymerase (TOYOBO, Shanghai, China). The amplicons were subcloned into the pEASY-Blunt Simple Cloning Vector (Transgen, Beijing, China). The intermediate vector was digested with Bam HІ and Eco RІ (Thermo Scientific, Waltham, USA), and the CsHSP17.2 fragment was ligated into a pET-32a(+) vector digested with the same enzymes. The pET-CsHSP17.2 recombinant plasmid and the empty vector were used to transform E. coli BL21 (DE3) cells. The fusion proteins were analyzed via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to the previously described methods and techniques57.
Thermotolerance of transgenic E. coli exposed to HS
Thermotolerance assays were conducted according to the method described by Soto et al.58 with some modifications. Briefly, when the cultures (grown at 37 °C) of E. coli harboring the pET-CsHSP17.2 plasmid or the empty vector reached an optical density (at 600 nm, OD600) of 1.0, they were diluted 100-fold with fresh Luria-Bertani liquid medium supplemented with ampicillin (100 μg·ml−1 final concentration). After the E. coli cells were treated with 1 mM IPTG for 4 h, 1-ml samples were transferred to a temperature-controlled water bath (50 °C), and 30-μl aliquots were added to Luria-Bertani agar plates after 0, 30, 60, and 90 min. Cell viability was estimated by counting the number of colony-forming units after an overnight incubation at 37 °C.
CsHSP17.2 purification and chemical denaturation and renaturation experiments
Recombinant proteins were purified using a nickel-charged affinity resin column (Qiagen GmbH, Germany) according to the method described by Liu et al.8. The fusion proteins were cleaved with tobacco etch virus protease for 4 h at 30 °C and then analyzed by SDS-PAGE. Gels were stained with Coomassie brilliant blue R-250 and photographed. The concentrations of the purified proteins were determined using the Bradford method59 with bovine serum albumin (BSA) as the standard.
Refolding assays involving chemically denatured citrate synthase (CS) were conducted as described by Collada et al.60 to determine whether CsHSP17.2 exhibited molecular chaperone activities. First, 15 μM CS (Sigma, St. Louis, MO, USA) was denatured with 6 M guanidine hydrochloride for 2 h, and then diluted 100-fold in refolding buffer (100 mM Tris-HCl, pH 8.0) supplemented with 300 nM CsHSP17.2 protein or distilled water. We then analyzed 20-μl aliquots for CS activity by monitoring the breakage of the thioester bond of acetyl CoA, which absorbs at 233 nm.
Constitutive expression of CsHSP17.2 in P. pastoris
The coding region of CsHSP17.2 was inserted into the pPIC3.5 K vector (Invitrogen, Carlsbad, CA, USA) to construct a P. pastoris overexpression plasmid. After being linearized with Sal I (Thermo Scientific, Waltham, USA), the recombinant vector (pPIC3.5K-HSP17.2) and the empty vector pPIC3.5 K were independently inserted into P. pastoris strain SMD1168 cells using the MicroPulser electroporator (Bio-Rad). Positive clones were identified by PCR with the ORF-F/3′AOX1 primer pair and the universal primers 5′AOX1/3′AOX1 (Table S2).
For thermotolerance assays, cultures of P. pastoris carrying pPIC3.5K-HSP17.2 (OD600 = 1.5) were incubated at 50 °C for 0, 30, and 60 min, and 10-μl aliquots of 10-fold serial dilutions were spotted onto yeast extract/peptone/dextrose (YEPD) agar medium. After incubation at 30 °C for 3 days, samples were observed and photographed using a digital camera (Canon, Japan). The P. pastoris strains harboring the pPIC3.5 K were used as controls.
Plasmid construction and transformation of A. thaliana
To investigate the functions of CsHSP17.2, a plant expression vector containing 35S:CsHSP17.2:GFP (Fig. S10a) was constructed and inserted into A. tumefaciens strain EHA105 cells. A floral dip method61 was used to transform A. thaliana. Transgenic A. thaliana plants were screened on 1/2 MS agar medium supplemented with 50 μg·ml−1 kanamycin. The T3 generations were analyzed by RT-PCR with gene-specific primers (ORF-F/-R, Table S2) before being used in subsequent experiments. All plants were grown at 22 °C in a light incubator with a 16-h light (200 μmol·m−2·s−1)/8-h dark cycle unless otherwise specified.
For characterization, the putative CsHSP17.2 promoter (PHSP17.2) was cloned into the pEASY-T1 Simple Cloning Vector with the primer pair pHSP17.2-F/-R (Table S2). Then, the amplified product was digested with Hind III and Bam HI and inserted into the pBI121 vector (Invitrogen) to generate the PHSP17.2:GUS fusion vector (Fig. S10b). Agrobacterium strain EHA105 harboring the binary vector PHSP17.2:GUS was used for A. thaliana transformation. The independent transformants were selected on 1/2 MS agar medium containing 50 μg·ml−1 kanamycin and the T3 homozygous plants were raised for subsequent histochemical staining and GUS transcription assays. The PCR primers used to confirm the transgenic A. thaliana were T-pHSP17.2-F and T-pHSP17.2-R (Table S2).
Thermotolerance test
Plates containing 1-week-old A. thaliana plants were submerged in a water bath at 45 °C for 45 min according to the method described by Charng et al.62.
Chlorophyll fluorescence, soluble protein and proline content measurements and H2O2 detection
The chlorophyll fluorescence of rosette leaves was measured using an Imaging-PAM Chlorophyll Fluorometer (M-series; Heinz Walz GmbH, Germany). All A. thaliana plants were incubated in darkness for 10 min immediately before measurements, and the Fv/Fm was calculated automatically. The soluble protein contents were analyzed using Coomassie brilliant blue G250 according to a published procedure37. The free proline content was determined using the acid-ninhydrin method63. We detected H2O2 in A. thaliana rosette leaves according to the method described by Orozco-Cardenas and Ryan64. For these experiments, 4-week-old seedlings were incubated at 45 °C for 4 h, and each experiment was performed three times.
Germination assays
Seeds of WT and CsHSP17.2-overexpressing plants (i.e., OE-8, OE-21, and OE-30) were plated on 1/2 MS medium. After a 3-day cold treatment (4 °C) in darkness, the plates were transferred to a water bath at 45 °C and incubated for 0, 1, 2, and 3 h. The plates were then placed in a light incubator under normal conditions for 7 days, and the germination rates were calculated every day. After the 7-day incubation, representative plates were photographed.
Hypocotyl elongation assays
Hypocotyl elongation assays were conducted according to a published method52 with minor modifications. For all assays, seeds were plated on 1/2 MS medium, and the plates were covered with foil. After a 2.5-day cold treatment, the plates were incubated at 22 °C for an additional 2.5 days. The seeds were then incubated at 38 °C for 90 min, followed by a 2-h recovery period at 22 °C and then a 1- or 2-h treatment at 45 °C (i.e., HS treatment). After another 2.5 days, the hypocotyl lengths of all seedlings were measured. The relative hypocotyl length was calculated using the following formula: (hypocotyl lengths of 5-day-old seedlings under HS conditions–hypocotyl lengths of 2.5-day-old seedlings)/hypocotyl lengths of 2.5-day-old seedlings. Experiments included at least 10 seedlings from each line and were repeated at least three times.
Phenotypic analysis
The phenotypes of each A. thaliana line were observed and photographed after 7 days (grown in 1/2 MS), 14 days (grown in 1/2 MS), 5 weeks (grown in soil), and 7 weeks (grown in soil), respectively. Additionally, the fresh weights of rosette leaves were measured after 4 and 5 weeks of cultivation under normal conditions.
Histochemical GUS staining
The GUS histochemical staining of transgenic A. thaliana plants containing the PHSP17.2:GUS fusion construct followed the method described previously65. The explants were then observed with a bright field microscope and photographed (Leica Q500MC, Cambridge, England).
Statistical analysis
All data were statistically analyzed with SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) using Duncan’s multiple range test at a 0.05 level of significance.
Electronic supplementary material
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (31570689) and the China Earmarked Fund for Modern Agro-industry Technology Research System (CARS-23).
Author Contributions
X.L. designed the study and guided the research. M.W. conducted the experiments and wrote the main manuscript text. M.W., Z.Z., Q.L., K.S., X.C. and X.L. edited the manuscript text. X.L. and X.C. supervised the project. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01407-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- 2.Hong SW, Vierling E. Hsp101 is necessary for heat tolerance but dispensable for development and germination in the absence of stress. Plant J. 2001;27:25–35. doi: 10.1046/j.1365-313x.2001.01066.x. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan H, Khurana N, Nijhavan A, Khurana JP, Khurana P. The wheat chloroplastic small heat shock protein (sHSP26) is involved in seed maturation and germination and imparts tolerance to heat stress. Plant Cell Environ. 2012;35:1912–1931. doi: 10.1111/j.1365-3040.2012.02525.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang WX, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9:244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Scharf KD, Siddique M, Vierling E. The expanding family of Arabidopsis thaliana small heat stress proteins and a new family of proteins containing α-crystallin domains (Acd proteins) Cell Stress Chaperon. 2001;6:225–237. doi: 10.1379/1466-1268(2001)006<0225:TEFOAT>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun WN, Van Montagu M, Verbruggen N. Small heat shock proteins and stress tolerance in plants. BBA-Gene Struct. Expr. 2002;1577:1–9. doi: 10.1016/S0167-4781(02)00417-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Gao YK, Pan HT, Zhang QX. The small heat shock proteins and their roles in abiotic stress tolerance in horticultural plants. Acta Hortic. 2014;1035:247–251. doi: 10.17660/ActaHortic.2014.1035.28. [DOI] [Google Scholar]
- 8.Liu JA, Shono M. Characterization of mitochondria-located small heat shock protein from tomato (Lycopersicon esculentum) Plant Cell Physiol. 1999;40:1297–1304. doi: 10.1093/oxfordjournals.pcp.a029518. [DOI] [PubMed] [Google Scholar]
- 9.Yonehara M, Minami Y, Kawata Y, Nagai J, Yahara I. Heat-induced chaperone activity of HSP90. J. Biol. Chem. 1996;271:2641–2645. doi: 10.1074/jbc.271.5.2641. [DOI] [PubMed] [Google Scholar]
- 10.Joshi CP, Nguyen HT. Understanding the roles of heat-shock proteins in acquired thermotolerance of wheat plants through molecular-genetic analysis. J. Cell Biochem. 1994;18A:96. [Google Scholar]
- 11.Vierling E. The Roles of Heat-Shock Proteins in Plants. Annu. Rev. Plant Phys. 1991;42:579–620. doi: 10.1146/annurev.pp.42.060191.003051. [DOI] [Google Scholar]
- 12.Gao CQ, Jiang B, Wang YC, Liu GF, Yang CP. Overexpression of a heat shock protein (ThHSP18.3) from Tamarix hispida confers stress tolerance to yeast. Mol. Biol. Rep. 2012;39:4889–4897. doi: 10.1007/s11033-011-1284-2. [DOI] [PubMed] [Google Scholar]
- 13.Sabehat A, Lurie S, Weiss D. Expression of small heat-shock proteins at low temperatures–A possible role in protecting against chilling injuries. Plant Physiol. 1998;117:651–658. doi: 10.1104/pp.117.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Gao YK, Pan HT, Hu WJ, Zhang QX. Cloning and characterisation of a Primula heat shock protein gene, PfHSP17.1, which confers heat, salt and drought tolerance in transgenic Arabidopsis thaliana. Acta Physiol. Plant. 2013;35:3191–3200. doi: 10.1007/s11738-013-1354-2. [DOI] [Google Scholar]
- 15.Song LL, Jiang YL, Zhao HQ, Hou MF. Acquired thermotolerance in plants. Plant Cell Tiss. Org. 2012;111:265–276. doi: 10.1007/s11240-012-0198-6. [DOI] [Google Scholar]
- 16.Mu CJ, et al. Small heat shock protein LimHSP16.45 protects pollen mother cells and tapetal cells against extreme temperatures during late zygotene to pachytene stages of meiotic prophase I in David Lily. Plant Cell Rep. 2011;30:1981–1989. doi: 10.1007/s00299-011-1106-y. [DOI] [PubMed] [Google Scholar]
- 17.Zou J, Liu CF, Liu AL, Zou D, Chen XB. Overexpression of OsHsp17.0 and OsHsp23.7 enhances drought and salt tolerance in rice. J. Plant Physiol. 2012;169:628–635. doi: 10.1016/j.jplph.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Ramakrishna W, Deng ZP, Ding CK, Handa AK, Ozminkowski RH. A novel small heat shock protein gene, vis1, contributes to pectin depolymerization and juice viscosity in tomato fruit. Plant Physiol. 2003;131:725–735. doi: 10.1104/pp.012401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn YJ, Zimmerman JL. Introduction of the carrot HSP17.7 into potato (Solanum tuberosum L.) enhances cellular membrane stability and tuberization in vitro. Plant Cell Environ. 2006;29:95–104. doi: 10.1111/j.1365-3040.2005.01403.x. [DOI] [PubMed] [Google Scholar]
- 20.Sanmiya K, Suzuki K, Egawa Y, Shono M. Mitochondrial small heat-shock protein enhances thermotolerance in tobacco plants. FEBS Lett. 2004;557:265–268. doi: 10.1016/S0014-5793(03)01494-7. [DOI] [PubMed] [Google Scholar]
- 21.Ruibal C, Castro A, Carballo V, Szabados L, Vidal S. Recovery from heat, salt and osmotic stress in Physcomitrella patens requires a functional small heat shock protein PpHsp16.4. BMC Plant Biol. 2013;13:174. doi: 10.1186/1471-2229-13-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun X, Sun C, Li Z, Luo H. Over-expression of AsHSP17, a creeping bentgrass (Agrostis stolonifera) small heat shock protein, increases abiotic stress sensitivity in transgenic Arabidopsis thaliana. In Vitro Cell Dev-An. 2014;50:S60–S61. [Google Scholar]
- 23.Kaur H, et al. Differentially expressed seed aging responsive heat shock protein OsHSP18.2 implicates in seed vigor, longevity and improves germination and seedling establishment under abiotic stress. Front. Plant Sci. 2015;6:713. doi: 10.3389/fpls.2015.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao HL, et al. Isolation and expression analysis of 18 CsbZIP genes implicated in abiotic stress responses in the tea plant (Camellia sinensis) Plant Physiol. Bioch. 2015;97:432–442. doi: 10.1016/j.plaphy.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Liu ZW, et al. Identification, classification, and expression profiles of heat shock transcription factors in tea plant (Camellia sinensis) under temperature stress. Gene. 2016;576:52–59. doi: 10.1016/j.gene.2015.09.076. [DOI] [PubMed] [Google Scholar]
- 26.Liu SC, et al. Transcriptomic analysis of tea plant responding to drought Stress and recovery. PLoS ONE. 2016;11:e0147306. doi: 10.1371/journal.pone.0147306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng WW, Wang S, Chen Q, Zhang ZZ, Hu XY. Effect of salt treatment on theanine biosynthesis in Camellia sinensis seedlings. Plant Physiol. Bioch. 2012;56:35–40. doi: 10.1016/j.plaphy.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Horton P, et al. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35:W585–W587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lescot M, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddique M, Gernhard S, von Koskull-Doring P, Vierling E, Scharf KD. The plant sHSP superfamily: five new members in Arabidopsis thaliana with unexpected properties. Cell Stress Chaperon. 2008;13:183–197. doi: 10.1007/s12192-008-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun XB, et al. AsHSP17, a creeping bentgrass small heat shock protein modulates plant photosynthesis and ABA-dependent and independent signalling to attenuate plant response to abiotic stress. Plant Cell Environ. 2016;39:1320–1337. doi: 10.1111/pce.12683. [DOI] [PubMed] [Google Scholar]
- 32.Lee KW, et al. Overexpression of alfalfa mitochondrial HSP23 in prokaryotic and eukaryotic model systems confers enhanced tolerance to salinity and arsenic stress. Biotechnol. Lett. 2012;34:167–174. doi: 10.1007/s10529-011-0750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang CH, et al. A cytosolic class I small heat shock protein, RcHSP17.8, of Rosa chinensis confers resistance to a variety of stresses to Escherichia coli, yeast and Arabidopsis thaliana. Plant Cell Environ. 2009;32:1046–1059. doi: 10.1111/j.1365-3040.2009.01987.x. [DOI] [PubMed] [Google Scholar]
- 34.Praekelt UM, Meacock PA. Hsp12, a new small heat-shock gene of Saccharomyces cerevisiae–Analysis of structure, regulation and function. Mol. Gen. Genet. 1990;223:97–106. doi: 10.1007/BF00315801. [DOI] [PubMed] [Google Scholar]
- 35.Neta-Sharir I, Isaacson T, Lurie S, Weiss D. Dual role for tomato heat shock protein 21: Protecting photosystem II from oxidative stress and promoting color changes during fruit maturation. Plant Cell. 2005;17:1829–1838. doi: 10.1105/tpc.105.031914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastenes C, Horton P. Effect of high temperature on photosynthesis in beans (II. CO2 assimilation and metabolite contents) Plant Physiol. 1996;112:1253–1260. doi: 10.1104/pp.112.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang LQ, Xu CX, Wang C, Wang YC. Characterization of a eukaryotic translation initiation factor 5A homolog from Tamarix androssowii involved in plant abiotic stress tolerance. BMC Plant Biol. 2012;12:118. doi: 10.1186/1471-2229-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 39.Wilson RA, Sangha MK, Banga SS, Atwal AK, Gupta S. Heat stress tolerance in relation to oxidative stress and antioxidants in Brassica juncea. J. Environ. Biol. 2014;35:383–387. [PubMed] [Google Scholar]
- 40.Apel K, Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 41.Pnueli L, Liang H, Rozenberg M, Mittler R. Growth suppression, altered stomatal responses, and augmented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plants. Plant J. 2003;34:187–203. doi: 10.1046/j.1365-313X.2003.01715.x. [DOI] [PubMed] [Google Scholar]
- 42.Hong ZL, Lakkineni K, Zhang ZM, Verma DPS. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000;122:1129–1136. doi: 10.1104/pp.122.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grallath S, et al. The AtProT family. Compatible solute transporters with similar substrate specificity but differential expression patterns. Plant Physiol. 2005;137:117–126. doi: 10.1104/pp.104.055079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hahn JS, Hu ZZ, Thiele DJ, Iyer VR. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol. Cell Biol. 2004;24:5249–5256. doi: 10.1128/MCB.24.12.5249-5256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MartinezPastor MT, et al. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress-response element (STRE) EMBO J. 1996;15:2227–2235. [PMC free article] [PubMed] [Google Scholar]
- 46.Rieping M, Schoffl F. Synergistic effect of upstream sequences, CCAAT box elements, and HSE sequences for enhanced expression of chimeric heat-shock genes in transgenic tobacco. Mol. Gen. Genet. 1992;231:226–232. doi: 10.1007/BF00279795. [DOI] [PubMed] [Google Scholar]
- 47.Volkov RA, Panchuk II, Schoffl F. Small heat shock proteins are differentially regulated during pollen development and following heat stress in tobacco. Plant Mol. Biol. 2005;57:487–502. doi: 10.1007/s11103-005-0339-y. [DOI] [PubMed] [Google Scholar]
- 48.Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007;61:199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- 49.Waters ER, Lee GJ, Vierling E. Evolution, structure and function of the small heat shock proteins in plants. J. Exp. Bot. 1996;47:325–338. doi: 10.1093/jxb/47.3.325. [DOI] [Google Scholar]
- 50.Zhou YL, et al. NnHSP17.5, a cytosolic class II small heat shock protein gene from Nelumbo nucifera, contributes to seed germination vigor and seedling thermotolerance in transgenic Arabidopsis. Plant Cell Rep. 2012;31:379–389. doi: 10.1007/s00299-011-1173-0. [DOI] [PubMed] [Google Scholar]
- 51.Mu CJ, et al. Overexpression of small heat shock protein LimHSP16.45 in Arabidopsis enhances tolerance to abiotic stresses. PLoS ONE. 2013;8:e82264. doi: 10.1371/journal.pone.0082264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Queitsch C, Hong SW, Vierling E, Lindquist S. Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell. 2000;12:479–492. doi: 10.1105/tpc.12.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larkin MA, et al. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 54.Tamura K, et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sparkes IA, Runions J, Kearns A, Hawes C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006;1:2019–2025. doi: 10.1038/nprot.2006.286. [DOI] [PubMed] [Google Scholar]
- 56.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 57.He S, et al. Molecular characterization and functional analysis by heterologous expression in E. coli under diverse abiotic stresses for OsLEA5, the atypical hydrophobic LEA protein from Oryza sativa L. Mol. Genet. Genomics. 2012;287:39–54. doi: 10.1007/s00438-011-0660-x. [DOI] [PubMed] [Google Scholar]
- 58.Soto A, et al. Heterologous expression of a plant small heat-shock protein enhances Escherichia coli viability under heat and cold stress. Plant Physiol. 1999;120:521–528. doi: 10.1104/pp.120.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 60.Collada C, Gomez L, Casado R, Aragoncillo C. Purification and in vitro chaperone activity of a class I small heat-shock protein abundant in recalcitrant chestnut seeds. Plant Physiol. 1997;115:71–77. doi: 10.1104/pp.115.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang XR, Henriques R, Lin SS, Niu QW, Chua NH. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006;1:641–646. doi: 10.1038/nprot.2006.97. [DOI] [PubMed] [Google Scholar]
- 62.Charng YY, Liu HC, Liu NY, Hsu FC, Ko SS. Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. Plant Physiol. 2006;140:1297–1305. doi: 10.1104/pp.105.074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- 64.Orozco-Cardenas M, Ryan CA. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. P. Natl. Acad. Sci. USA. 1999;96:6553–6557. doi: 10.1073/pnas.96.11.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


