Abstract
Recombinations occur nonuniformly across the maize genome. To dissect the genetic mechanisms underlying the nonuniformity of recombination, we performed quantitative trait locus (QTL) mapping using recombinant inbred line populations. Genome-wide QTL scan identified hundreds of QTLs with both cis-prone and trans- effects for recombination number variation. To provide detailed insights into cis- factors associated with recombination variation, we examined the genomic features around recombination hot regions, including density of genes, DNA transposons, retrotransposons, and some specific motifs. Compared to recombination variation in whole genome, more QTLs were mapped for variations in recombination hot regions. The majority QTLs for recombination hot regions are trans-QTLs and co-localized with genes from the recombination pathway. We also found that recombination variation was positively associated with the presence of genes and DNA transposons, but negatively related to the presence of long terminal repeat retrotransposons. Additionally, 41 recombination hot regions were fine-mapped. The high-resolution genotyping of five randomly selected regions in two F2 populations verified that they indeed have ultra-high recombination frequency, which is even higher than that of the well-known recombination hot regions sh1-bz and a1-sh2. Taken together, our results further our understanding of recombination variation in plants.
Introduction
Recombination refers to the phenomenon of genomic exchange among chromatids, which leads to new alleles and new combinations of existing alleles1–3. Together with DNA mutation, recombination is a key driving force in genome evolution and can enhance the genetic diversity of species4–6. In crops, recombination contributes substantially to breeding by the rearrangement of genomic fragments, which results in the introduction of new and improved allele combinations as well as the elimination of deleterious mutations7, 8. Recombination events do not occur uniformly along the chromosomes, which may be affected by genetic factors and specific DNA sequences9–11. In animals, discovery of the genetic and genomic patterns furthered our understanding of recombination12–14.
Previous studies suggested variation in recombination frequency is under both cis- and trans- genetic control15–19. Several genes that regulate recombination have been identified. In humans, PR domain containing 9 (PRDM9) locus has been identified as a trans- regulator of recombination hot spots17. The phs1 and rad51 genes were reported to affect recombination variation at the whole genome scale in maize20–22. Besides trans-acting genes, cis- genomic features were also found to regulate recombination. Study of different PRDM9 alleles found that recombination break sites tend to cluster near transcript start sites (TSS) and simple repeat regions, and are associated with DNA motifs such as CCTCCCT and CCNCCNTNNCCNC17, 19. Gene density and CpG islands have also been reported to be positively correlated with recombination rate23, 24. In maize, a putative recombination hot region was reported to lie in an unusual gene-rich region including the Bz-a1 locus25. Although gene density is positively correlated with recombination frequency, certain transposons (TE) were found to be negatively correlated with recombination26. Using recombination crossover number as a quantitative trait, quantitative trait locus (QTL) mapping method was employed to dissect the genetic mechanism underlying recombination variation in maize segregating population, and several recombination QTLs were identified27. These studies suggest that the regulation of recombination is very complex in both animals and plants.
As a model for genetic studies, maize has a genome of 2.3 Gb and more than 85% of the genome is composed of transposons and other repetitive sequences28–31. The complex genomic composition of maize may affect recombination patterns32–34. Two putative recombination hot regions (a1-sh2 and sh1-bz) were identified 20 years ago, indicative of the nonuniform distribution of recombination events across maize genome35, 36. Further studies of these two recombination hot spots showed that genes and specific genomic contents were significantly enriched or depleted around them37–39. Recent studies have uncovered that recombination hot regions were related to phenotypic and gene expression variations, and different maize subgroups exhibited different genome-wide patterns in maize40, 41. However, the underlying genetic mechanisms of recombination variation in maize have not been completely explored.
Here, we conducted QTL mapping for recombination event number using 11 recombination inbred line (RIL) populations that have been genotyped with ~50000 SNPs41. We identified both cis- and trans- QTLs. We found candidate genes for some trans- QTLs. We also identified tens of genomic features as the cis- factors that are associated with the variation of recombination frequency. The cis- and trans- genetic and genomic factors were further validated in 23 DH populations and a panel of natural inbred lines40, 42. Furthermore, based on the identified features that are associated with recombination, we identified 41 putative recombination hot regions and 5 of them were verified in two separate F2 populations. Our results provide a comprehensive genome-wide scan of genetic and genomic factors conferring maize recombination variation, which will further our understanding of recombination and aid breeding in maize.
Results
Both cis- elements and trans- factors contribute to the nonuniformity of recombination events
Our previous study has provided the genome-wide landscape of recombination events across 11 RIL populations and one teosinte-maize introgression population, and identified 143 recombination hot regions where significantly more recombination events were identified than genome-wide average, indicative of nonuniformity of recombination in maize genome41. To decipher the genetic mechanisms conferring the nonuniformity of recombination events across maize genome, we considered the global recombination event number of all chromosomes (GRE) and the recombination number of each chromosome (GREchr) as phenotypic traits to perform QTL mapping across 11 RILs (Fig. 1A). GRE could indicate global recombination rate of each segregation population, while GREchr would be more accurate to quantify recombination variation for each chromosome. Totally, we mapped 26 QTLs for GRE in 11 populations. These QTLs were distributed on six chromosomes except chromosomes 6, 8, 9 and 10 (Table S1). For GREchr, a total of 245 QTLs were identified. The QTL number for GREchr ranged from 15 (for chromosome 1) to 30 (for chromosome 2) (Table 1). The highest number of recombination QTLs (39) were detected in population ZONG3/YU87-1, while the least (16) were identified in population KUI3/BY815 (Table S1). Meanwhile, an average of 16.1% of recombination number variation could be explained by recombination QTLs for GREchr, which is lower than an average of 21.2% for GRE. These may be due to that GRE QTLs confer larger recombination variation, while GREchr QTLs somehow partitioned the global recombination variation into different parts in each chromosome.
Figure 1.
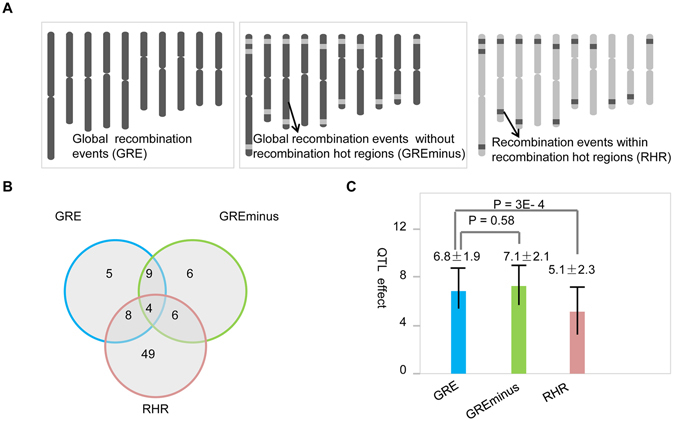
QTL mapping of recombination number variation in maize. (A) The schematic diagram of global recombination events (GRE, left panel), global recombination events substracting recombination number in recombination hot regions (GREminus, middle panel), and recombination event in recombination hot regions (RHR, right panel). Recombination number from dark genomic regions was counted for QTL mapping, while the ones from grey regions were excluded. (B) Venn Diagram of QTL mapping results among GRE, GREminus and RHR. (C) QTL effect variation among GRE, GREminus and RHR.
Table 1.
Summary of cis- and trans- QTL mapping results in 11 RIL and 23 DH populations.
| Trait | RIL | DH | ||
|---|---|---|---|---|
| Cis-prone | Trans | Cis-prone | Trans | |
| Chr1 | 2 | 13 | 12 | 24 |
| Chr2 | 8 | 22 | 9 | 18 |
| Chr3 | 3 | 21 | 26 | 25 |
| Chr4 | 13 | 16 | 13 | 34 |
| Chr5 | 13 | 9 | 15 | 25 |
| Chr6 | 11 | 14 | 7 | 27 |
| Chr7 | 7 | 19 | 17 | 25 |
| Chr8 | 0 | 21 | 9 | 29 |
| Chr9 | 0 | 27 | 10 | 20 |
| Chr10 | 7 | 19 | 4 | 25 |
| Suma | 64 | 181 | 122 | 252 |
ais the total number of QTL for each type (cis-prone, trans) across the ten chromosomes.
For GREchr, if QTL is located on the same chromosome, of which the recombination number variation was dissected, the QTL was defined as cis-prone QTL, otherwise it was defined as trans- QTL (see methods). Of the 245 QTLs underlying GREchr detectected in the 11 RIL populations, 64 were cis-prone QTLs, and 181 were trans-QTLs (Table 1). These 64 cis-prone QTLs were distributed on 8 maize chromosomes except chromosomes 8 and 9, while trans-QTLs were located on all maize chromosomes (Fig. S1). Recombination cis-prone QTLs had larger effects than trans-QTLs (P = 9.24E-13; Fig. S1). Of the cis-prone QTLs, the one located on chromosome 2 could explain up to 18.5% of the recombination variation of chromosome 2 (Table S1).
To validate our QTL mapping results for GREchr, we also mapped QTLs for recombination variation across 23 DH populations, which were constructed from 22 European maize inbred lines belonging to the Dent and Flint gene pools40. Comparable number and effects of cis-prone and trans- recombination QTLs were identified as in our 11 RIL populations (Table 1; Fig. S2; Table S2). Interestingly, of these 64 cis-prone recombination QTLs detected in RILs, 37 (58%) were also mapped in DH populations, and 161 out of 181 (89%) trans- QTL identified in our RIL populations could be detected in DHs as well, suggesting that similar genetic factors were employed to control recombination in these two different types of population.
To further dissect the recombination mechanisms that are related to the formation of recombination hot regions, we did QTL mapping for recombination events that occurred only outside recombination hot regions (GREminus), and recombination events that solely occurred within recombination hot regions (RHR) (Fig. 1A), and compared the QTL mapping results between GRE, GREminus, and RHR. In total, we mapped 26 and 25 recombination QTLs for GRE and GREminus, respectively. Comparative analyses between GRE and GREminus showed that 13 QTLs were shared between the two traits, while nearly half of the recombination QTLs were specific for each recombination trait (Fig. 1B). For the remaining 12 QTLs that are specific to GREminus, 7 out of the 12 specific recombination QTLs were located close to the recombination hot regions (Table S3). Futhermore, QTL mapping of RHR has identified 57 QTLs, of which only 18 were shared by GRE and GREminus (Fig. 1B). Comparative analysis between GRE, GREminus and RHR showed that only four recombination QTLs were detected simultaneously (Fig. 1B). A substantial number (49/67) of recombination QTLs were recombination hot region specific. These hot region specific recombination QTLs indicated that specific genetic mechanism might be involved in the regulation of recombination events in recombination hot regions. Expectedly, 11 QTLs of RHR were located in the same recombination hot regions (cis- recombination QTLs), while the others were not (trans- recombination QTLs) (Table S4). Interestingly, for RHR, cis- recombination QTLs had less effect (3.37%) than trans- QTLs (5.54%) (ANOVA; P = 1.22E-2). The QTL effects of RHR was significantly lower (ANOVA; P = 3E-4) than that of GRE and GREminus, while no significant difference was observed between GRE and GREminus (Fig. 1C).
A substantial number of recombination QTLs co-localized with well-known recombination pathway genes in maize
The molecular pathway related to recombination has been well studied, and several genes have already been cloned in Arabidopsis 43. Through comparative genomics, we identified 30 recombination pathway genes in maize. Co-localization of maize recombination pathway genes with recombination QTLs showed that 1, 3 and 5 recombination pathway genes were located in the QTLs for GRE, GREminus and RHR, respectively (Fig. 2A). Interestingly, ZmMER11, which encodes enzyme functioning in the upstream of recombination pathway, was located in the genomic region with detectable QTL for all three traits (GRE, GREminus and RHR). Moreover, two recombination pathway genes (ZmRAD51 and ZmDMC1) were within QTLs for both GREminus and RHR, and two genes (ZmTOP3 and ZmSHOH1) were located in QTLs that were only detected for RHR (Fig. 2B). These results showed that the more recombination QTLs could be mapped for recombination hot regions and the more downstream genes of recombination pathway could be identified through recombination QTL mapping for specific hot regions, suggesting that recombination pathway downstream genes are more likely to be involved in the process of recombination events in recombination hot regions
Figure 2.
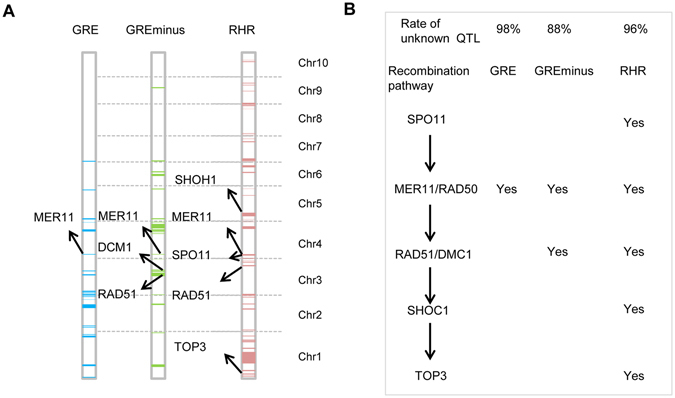
Relationships between recombination QTLs and recombination patyway genes. (A) Co-localization of recombination pathway genes with recombination QTLs for GRE, GREminus and RHR. (B) Scenario of recombination pathway genes that were detected by the QTL mapping for GRE, GREminus and RHR.
Local recombination rate variation is strongly affected by local genomic contents
The identification of recombination hot regions and cis- QTLs suggests that local chromosome feature might affect the frequency of recombination. To identify these genomic features, we focused on the 143 recombination hot regions and assessed the gene density along the hot regions and their flanking regions41. We found that gene density peaks around the hot regions and decreases dramatically away from the hot regions. This pattern is significantly different from that of the randomly selected genomic regions (ANOVA; P < 2.2E-16; Fig. 3A). Gene density (32 genes/Mb) in the hot regions is much higher than the genomic average (16 genes/Mb) (ANOVA; P < 2.2E-16), in accordance with previous reports that crossovers are more likely to occur around gene islands44, 45. To complement the analysis of present populations that captured recent recombination events, we also estimated genome-wide recombination that occurred historically in the HapMap2 population46. We confirmed that recombination tends to occur near genes. We found that recombination showed a strong tendency to occur at the transcriptional start and 3′-UTR regions of the genes (Fig. 3B). We also observed a significant enrichment of CpG sites in the hot regions (ANOVA; P < 2.2E-16; Fig. S3). Moreover, we uncovered that GC frequency is higher at the start and terminal sites of genes than that of the other genic regions (Fig. S3 ). Our findings suggest that recombination rate is increased in the flanking regions of genes rather than within genes.
Figure 3.
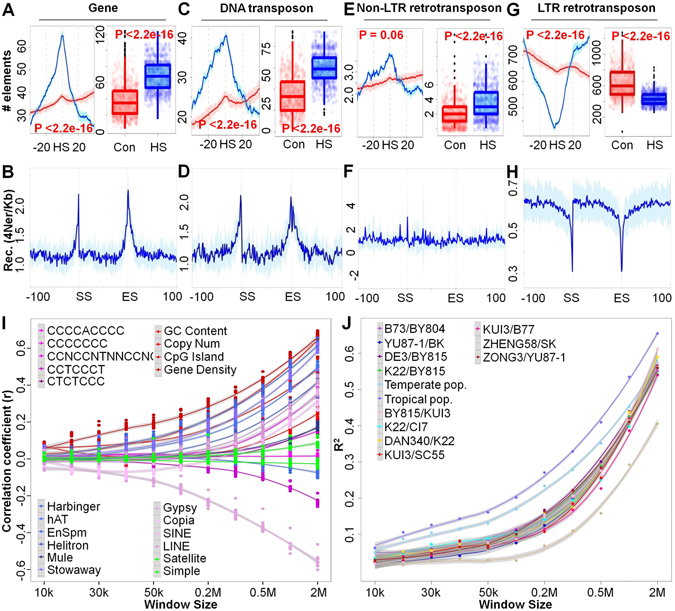
Relationships between recombination rate and local genomic features in maize. (A,C,E,G) Distribution of different genome components around recombination hot regions in segregating populations. “HS” is the short name of hot regions, while “Con” shows the random samples used as control. “−20” and “20” represent 20 Mb upstream and downstream genomic regions of hot regions. Blue and red represent recombination hot regions and control samples, respectively. (B,D,F,H) Recombination rate (4Ner/Kb) in 1 Kb bins spanning the 100 Kb upstream and downstream regions of different genome components in the natural temperate population from the Hapmap2 dataset. SS and ES indicate the start site and end site of genome content, respectively. (I) Correlation coefficients over 11 different sliding window sizes between 21 different genome components and recombination rate in 11 segregation populations. (J) The linear regression determination coefficient of genomic components in different window size relative to recombination variation in 11 segregation populations, and in temperate and tropical sub-populations of natural population.
A substantial proportion of recombination events occurred in intergenic regions41, which largely consist of transposable elements28, 30. To clarify the relationship between transposons and recombination rate, we first examined DNA transposons and their flanking genomic regions. More than 162,990 putative DNA transposons were identified and classified into six types according to their DNA sequence features (Table S5). These DNA transposons were distributed unevenly across the whole genome (Fig. S4). By comparing the average number of DNA transposons in the flanking genomic regions of the hot regions with that of a set of randomly selected control regions, we found a significant enrichment for five out of the six DNA transposon families, including Helitron, Harbinger, hAT, MULE and Stowaway, in the vicinity of recombination hot regions (ANOVA; P < 2.2E-16; Fig. 3C). However, EnSpm was not enriched, consistent with results that EnSpm is retrotransposon-like in the Triticeae 45 (ANOVA; P = 0.58, Fig. S5).
To further validate the relationship between recombination and DNA transposons, we analyzed recombination variation in DNA transposons and their flanking regions in natural maize populations based on the high density Hapmap2 SNP42. We found that all classes of DNA transposons and their flanking regions tend to have higher recombination rates than randomly selected non-genic regions (ANOVA; P < 2.2E-16; Fig. 3D). Intriguingly, recombination rate had a distinct probability peak around both ends of DNA transposons, similar to the distribution of recombination near genes (Fig. 3B). Among all the related DNA transposons, Harbinger exhibits the strongest correlation with recombination not only in the segregating populations but also in the natural populations, while Mule transposon showed the weakest correlation, perhaps due to the high genetic diversity in their terminal inverted repeats (TIRs) compared to all other DNA transposons47. Moreover, we also noted that EnSpm and their adjacent regions showed a significant positive relationship with recombination in natural populations (Fig. S5), while such a relationship was not observed in artificial segregating populations.
Genome-wide recombination events are more likely to occur in telomere and decrease toward pericentromeric regions, which mainly consist of retrotransposons, indicative of potential negative relationships between retrotransposons and recombination in maize. To test this hypothesis, we analyzed the density distribution of retrotransposons around recombination hot regions detected in the 11 RIL populations as well as the recombination sites in the natural populations. Plant retrotransposons were classified into two distinct groups: LTR retrotransposons (Copia and Gypsy) and non LTR retrotransposons (LINE and SINE)47. For non LTR retrotransposons, we identified enrichment peaks around the hot regions in 11 RIL populations and compared these with the randomly selected genomic regions (ANOVA; P < 2.2E-16; Fig. 3E). We did not see a relationship between non-LTR retrotransposons and recombination rates in natural populations (Fig. 3F). This might be due to the hitchhiking effect of other recombination-related elements in the artificial segregating populations, since we observed that non LTR transposons are more likely to occur in the autosomal regions and are tightly linked with DNA transposons.
For Gypsy LTR retrotransposons, we found a distinct negative correlation (Pearson’s Correlation Test; P < 2.2E-16) with recombination in both artificial segregating populations and natural populations (Fig. S6), indicative of potential repressive regulatory roles of LTR retrotransposons in the process of recombination. Although the adjacent regions of Copia and Gypsy showed significantly lower levels of recombination than other genomic regions (ANOVA; P < 2.2E-16; Fig. 3G,H), this may be related to their closeness to the pericentromere. However, the LTRs of both Copia and Gypsy had low recombination rates (less than 50% of the recombination rate of flanking regions) in natural populations (Fig. 3G,H). The difference of association between non-LTR retrotransposons and LTR retrotransposons with recombination suggested that the recombination repression effects may be related to the presence of LTRs.
To uncover other genetic factors underlying the variation of recombination, we examined the relationship between recombination rate and 21 genomic features (Table S6) using linear regression in a range of sliding window sizes (10 kb, 20 kb, 30 kb, 40 kb, 50 kb, 100 kb, 200 kb, 300 kb, 500 kb, 1 Mb, 2 Mb). As described above, we confirmed that some among the 21 surveyed genomic features, including gene density, gene copy number, CpG island, GC content and DNA transposons, had a high positive correlation with recombination rate variation, while two among the 21 surveyed genomic features, including the Gypsy retrotransposons and microsatellite sequence, were negatively associated with recombination rate in 11 segregating populations. Notably, Copia exhibited a negative correlation with recombination at a fine scale of less than 100 kb, but a positive correlation at a large scale (greater than100 kb) (Pearson’s Correlation Test; P < 0.001; (Fig. 3I; Table S6). This is inconsistent with the observation that Copia showed a negative correlation in natural populations that have a high recombination resolution and a positive correlation in segregating populations that have low recombination resolution (Fig. S7; Tables S6 and S7). We also tried to relate recombination rate to DNA sequence motifs that were found to be associated with recombination in humans17, 19. In maize, we found positive correlations of CCCCCCC, CCNCCNTNNCCNC and CCCCACCCC with recombination rate (Tables S6 and S7) which are consistent as in humans17, 19. Interestingly, CCTCCCT was negatively associated with recombination rate in our study, while this motif was positively correlated with recombination hot regions in humans17 (Tables S6 and S7). These results suggested a possible difference in the mechanism underlying selection of recombination sites between plant and animals. Furthermore, to sum up the potential local effects of genomic contents contributing to the recombination variation in maize, linear regression analysis with an empirical window size identified that the above described associated genomic factors explain up to 60% of the recombination variation over all populations at the 2 Mb genomic window size (Fig. 3J), suggestive of strong cis-regulation of recombination variation in maize.
Tens of novel recombination hot regions were verified in maize
Previous studies have identified 143 recombination hot regions with 2 Mb window-size, indicative of dramatic recombination frequency variation in maize41. Here, we elected to accurately measure the recombination event variation in order to fine-map recombination hot regions by our 11 RIL populations within sliding 500 kb genomic bins. The recombination coefficient analysis (see the Materials and Methods section), which takes local recombination events, genomic contents and the relative distance to centromere of sliding 500 Kb window-size regions into account simultaneously, was employed. Based on the recombination coefficient estimation, we identified 41 genomic regions that showed the highest 1% recombination coefficient values and might be the putative recombination hot regions (Fig. 4A; Table S8). These 41 recombination hot regions are scattered on chromosomes 2, 5, 6, 7, 8, 9 and 10, of which chromosome 9 has most (15), while chromosome 2 and 6 have least (one) (Table S8). These 41 recombination hot regions spanned 0.8% of the maize genome; however, they captured 17.4% of recombination events across different populations41. Interestingly, not all the recombination hot regions are located in the ends of chromosomes, 39.1% are also distributed in pericentromeic genomic regions.
Figure 4.
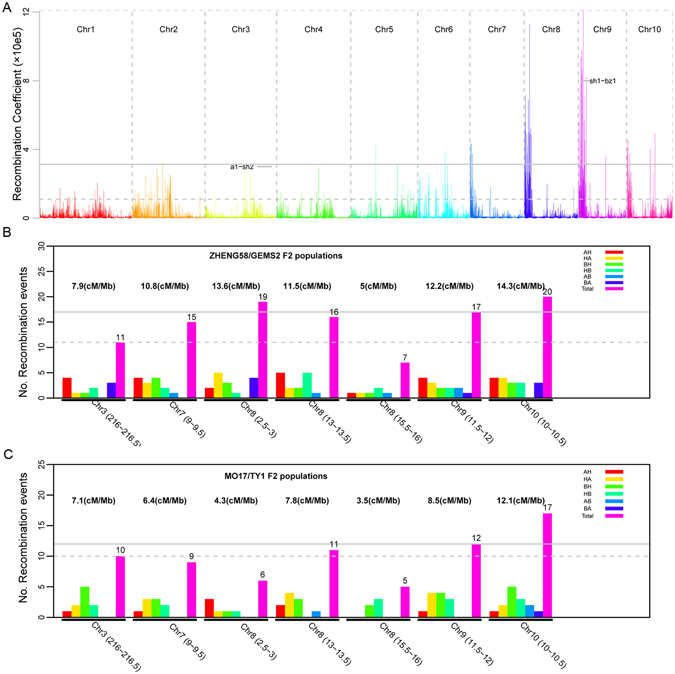
Distribution and validation of novel recombination hot spots in maize. (A) Distribution of recombination coefficient indicated a large number of recombination hot spots across the maize genome. Two putative recombination hot spots of a1-sh2 and sh1-bz1 were labelled. The gray solid line is the threshold for top 1% recombination coefficients. The Gray dashed line is the threshold line for top 5% recombination coefficients. (B) and (C) High-density genotypeing validation of five randomly selected recombination hot spots in two F2 populations - ZHENG/GEMS2 and Mo17/TY1. A, B, and H in (B) and (C) graphs represent the genotypes of parent 1, parent 2, and heterozygosity, respectively. The number of crossover was obtained by counting the number of individuals with genotype switching in the target recombination region. For example, AH means there was a genotype switch from parent 1 to heterozygosity. The gray solid line shows the recombination rate at the sh1-bz1 locus, while Grey dashed line shows the recombination rate of the a1-sh2 locus.
There were two well known recombination hot regions (a1-sh2, sh1-bz) in maize35, 36. As expected, one well-known recombination hot regions (sh1-bz) was also identified to have top 1% recombination coefficient values across the whole maize genome. Another well-known recombination hot region (a1-sh2) in maize was not detected in the hot regions of top 5% coefficient values but captured within top 37%. Using these two well-known recombination hotspots as negative and positive controls, we randomly selected five recombination hot spots with top 1% recombination coefficient values to be verified. These five new recombination hot spots are located in chromosome 7:9–9.5 Mb, 8:2.5–3, 8:13–13.5 Mb, 8:15.5–16 and 10:10–10.5 Mb with an average tract length of 500 Kb. We tested these five novel and two well known (a1-sh2, sh1-bz) recombination hot spots in two different F2 segregation populations with a population size of 279 and 282, respectively. All the five recombination hot spots harbored exceptional high recombination events in both F2 populations (Fig. 4B,C). Of these five recombination hotspots, 8:13–13.5 and 10:10–10.5 Mb had even higher recombination rate than a1-bz2 in both two populations, and one located in chromosome 10:10–10.5 Mb had higher recombination rate (14.3 and 12.1 cM/Mb) than both sh1-bz and a1-sh2 loci in both F2 populations (Fig. 4B,C). Taken together, 41 novel recombination hot spots were identified and five were verified, showed comparable recombination frequency as these two putative well-known recombination hot spots, and could be the new targets of future recombination research.
Discussion
The genetic mechanisms of recombination events are largely unknown in maize. Only a few genes such as phs1, rad50, and rad51 have been identified to affect recombination events through homologous or mutant cloning20–22. However, due to the severe defect of these mutations in plants, utilization of these genes was largely limited. In this study, we identified 41 novel recombination hot spots, which confirmed the nonuniformity of recombination frequency across the maize genome. Further, we mapped the variation of recombination events across 11 RIL populations, and found loci that underpin recombination variation in both cis- and trans- manners. Comparative genomic analysis of recombination-related genes showed a lot of recombination QTLs are coincident with recombination genes, suggestive of the robustness of QTL mapping for the genetic dissection of recombination variation in plants, as suggested by27.
Genomic features are associated with recombination rate variation in maize
In maize, recombination break point shows strong chromosome- and locus-specific patterns40. Cis- regulation of recombination break point was demonstrated in a series of studies. The interplay of retrotransposon polymorphisms and intragenic recombination was also observed in maize48. The maize genome, which is comprised of more than 85% repeat sequences, more than 39,000 protein-coding genes, and ~20,000 long non-coding elements, exhibits extreme complexity30, 49. Thus, the lack of high-resolution genetic maps from diverse genetic backgrounds makes the identification of cis-elements underlying recombination variation and their effects elusive. In this study, we provided a comprehensive genome-wide scan of 21 genomic features associated with the recombination variation in maize at a high-resolution.
We confirmed the positive correlation between gene content and recombination break point and further identified that recombination break point is more likely to occur in the 5′ and 3′ UTRs of genes, in agreement with reports in mice and Arabidopsis 33, 34. The recombination pattern along maize genes and their flanking regions appears to be related to chromatin status, such as the DNA methylation levels and histone methylation levels in the 5′ and 3′ UTRs32, 50, 51. Open chromatin status may allow easy access to recombination/DSB complexes, and be related to the GC bias at the 5′ and 3′ ends of genes (Fig. S3). It has been reported that GC bias at the 5′ and 3′ UTRs of human genes could form R-loops52. R-Loop and its associated DNA nicks may lead to the initiation of DNA breakage and lead to genome instability53, which could be associated with recombination.
Interestingly, we found that recombination is positively associated with nearly all DNA transposons and negatively associated with LTR-retrotransposons, in both current and historical populations. The formation of stem-loop structures by TIRs seems to facilitate formation of recombination intermediates54–56. LTR-retrotransposons are mainly clustered around the centromeric regions30. It has been suggested that LTRs could recruit the DNA binding factor switch-activating protein 1 and block the formation of the replication fork in centromeric regions, enhancing the stability of the genome57. It is likely that LTR could use a similar mechanism to block the formation of Holliday structures in the centromere and repress the recombination.
Noteworthily, there is a dramatic structural variation driven by transposons among maize inbreds31, 58, possibly reducing the observed correlation between transposons and recombination variation because some transposons may not exist in the non-B73 parental founders, which was used for all analyses. However, structural variation is not likely to change our overall conclusion, because 1) the possibility of structural variation existing in both parents of the segregating populations should be low; 2) similar association trends between transposons and recombination were found in populations developed from non-B73 lines. Although cis-effects of genomic features on recombination frequency were found, the overall cis-effects account for only ~60% of the variation, indicative of the relevance of other (trans-) effects.
Recombination mechanism in maize may be complex and different from that in other species
It is important to understand mechanisms regulating recombination since it contributes to species evolution7, 59. In this study, we used QTL mapping for four different types of recombination events (GRE, GREchr, GREminus and RHR) to capture loci which were associated with recombination variation and identified hundreds of recombination QTLs. Some recombination QTLs were coincident with the well-known recombination related genes43, but others were not. This might be caused by either the fact that only a few recombination related genes have been cloned or that maize has different genetic mechanisms underlying recombination variation. Furthermore, through association analysis between genomic features and recombination variations across different genome regions, we found that some specific cis- motifs that show different associations with recombination in animals and maize. For example, the CCTCCCT motif17, 19 were positively related with recombination rate in animals, but showed negative relationship with recombination variation in maize. All these results indicate that the genetic mechanisms (both cis- and trans- genetic factors) underlying recombination variation may vary across different species.
Utilization of recombination patterns in modern maize breeding
Recombination is important in animal and plant breeding because it can create different combinations of alleles60. The more the allele combinations, the higher probability of elite hybrids that could be obtained60. Although all crop and animal breeding utilized recombination, how to efficiently improve the rate of recombination is still a big challenge. Here, we combined conventional genetic mapping method and association analysis between genomic features and recombination hot regions, not only were cis- genetic factors identified, but also hundreds of trans- factors were uncovered to explain most of recombination variation in maize. These trans- QTLs provide us the selection targets for high recombination inbreds with all favorable recombination alleles. Specifically, our recombination QTL mapping indicated that recombination pathway genes and other unknown genomic loci are both associated with recombination number variation in recombination hotspots. This QTL mapping information could potentially allow us to increase and decrease recombination events in specific genomic regions. Additionally, these cis-QTLs mapped in our study could inform us of how many plants or generations will be needed for the improvement of specific genomic regions. All these cis- and trans- recombination QTL information and the newly-idenfied recombination hot spots will accelerate our progress of maize breeding.
Methods
QTL mapping of global recombination variation in maize
In previous study, we’ve constructed 11 recombination inbred line populations -B73/BY804, SK/ZHENG58, BK/YU87-1, K22/CI7, K22/DAN340, KUI3/B77, ZONG3/YU87-1, DE3/BY815, K22/BY815, KUI3/BY815 and KUI3/SC55, and obtained high density linkage maps of these segregating populations41. Based on these 11 RIL high-density linkage maps, we could estimate the recombination events by counting the recombination break points. Took the number of recombination evets of all chromosomes (GRE) and each chromosome (GREchr) of each family individuals as phenotype, together with high-density linkage map, we mapped quantitative traits locus (QTL) associated with recombination variation by composite interval mapping. The software Winqtlcart 2.561 was employed. We conducted the 1000 permutations for all recombination traits both in 11 RIL and 23 DH populations using R/qtl permutation test with “scanone” function and “hk” method. The significance of LOD values with 0.05 were obtained. The average LOD cut off value was 2.58 for all recombination traits. To make it more comparable, the genomic region with Likelyhood (LOD) beyond the average LOD score 2.58 was defined as a QTL, and the QTL confidence interval was identified according to genomic range with 1 LOD drop from the LOD peak of the QTL (p < 0.01). To verify our mapping results, 23 DH populations40 genotyped by the same SNP platform were also collected to dissect recombination number variation through QTL mapping. For the QTL mapping of recombination variation in each chromosome, if QTL was located in the chromosome, of which the recombination number was analyzed, such QTL was defined as cis-prone recombination QTL. If QTL is not mapped to the chromosome, of which the recombination number was analyzed, the QTL was defined as trans- recombination QTL.
QTL mapping of local recombination (recombination hot region depletion and recombination hot region specific) variation
Our previous study has identified 143 recombination hot regions, which harbored a substantial number of recombination events. The total number of recombination and the number of recombination in each chromosome quantify the overall recombination variation across the whole genome or whole chromosome, showing the global recombination variation. The recombination hot spots largely represent the local recombination variation, which might be different from global recombination variation. To test the difference of genetic mechanisms underlying global and local recombination variation, we’ve also conducted QTL mapping on the variation of whole-genome recombination number subtracting the recombination number from recombination hot regions (GREminus), and the QTL mapping for the variation of recombination number from recombination hot regions (RHR). Then, we compared the mapping results between GRE, GREminus and RHR. The QTL, which was mapped to the neighboring region (downstream and upstream 10 Mb) of recombination hot spots, was defined as cis-QTL, otherwise it’s trans- QTL for RHR.
Estimation of historical recombination rate in maize natural population
To estimate the historical recombination of maize, we downloaded the HapMap2 SNP dataset42, and employed the previously described Markov-Chain Monte Carlo method-Ldhat62 to estimate recombination rate in the natural population. The natural population from the HapMap2 dataset42 was divided into two subgroups: temperate and tropical sub-groups. The recombination rate value was estimated as 4N e r/kb separately for each chromosome in each population62. SNP data in each chromosome was divided into segments of 2,000 SNPs, with an overlap of 500 SNPs according to the physical coordinate in the B73 reference genome. The 4N e r/kb was calculated with a block penalty of 5 and 10 million iterations and a removal burn-in of the first quarter of the samples.
Estimation of the sequence patterns and genome parameter
We downloaded the B73 V2 reference genome sequence data from the Maizesequence web site http://ftp.maizesequence.org/. In the reference genome of B73, 11 sliding window sizes (10 kb, 20 kb, 30 kb, 40 kb, 50 kb, 100 kb, 200 kb, 300 kb, 500 kb, 1 Mb, and 2 Mb) were used to find distinct sequence motifs. Maize gene number and gene copy number variance (CNV) information was downloaded from the maize genome sequence project (http://ftp.maizesequence.org/ and http://plants.ensembl.org/). CpG islands were predicted as described in previous studies23 and the motifs CCTCCCT, CCCCCCC, CTCTCCC, CCCCACCCC, CCNCCNTNNCCNC and GC contents were obtained from an in-house Perl script by parsing the B73 V2 genome sequence. Repetitive sequence features were estimated by the RepeatMasker software downloaded from http://www.repeatmasker.org/ with default parameters. In this study, 12 types of TE-like elements, including Harbinger, MULE, hAT, LINE, Copia, Helitron, EnSpm, Sine, Gypsy, Satellite, Simple, and Stowaway, were annotated.
Correlation and regression analysis
To study the correlation between recombination rate and genomic patterns, we used 11 different sliding window sizes in an empirical analysis to estimate the correlation value. These windows were defined as 10 kb, 20 kb, 30 kb, 40 kb, 50 kb, 100 kb, 200 kb, 300 kb, 500 kb, 1 Mb, and 2 Mb to verify the stabilitity of the results. For the regression analysis, we used ‘lm’ function in R by treating the genomic patterns as the independent variable, explaining how these patterns contributed to the recombination rate to uncover the relationships between total genomic patterns and recombination rate. For relationships between genomic patterns and recombination rate, R function cor.test was used to do the analysis. The analysis was also conducted in 11 different sliding window sizes. All these analyses were conducted by R 2.15.3 (http://www.r-project.org/).
Prediction of recombination hotspot
From the 11 RIL populations, we firstly counted the recombination events in a 500k sliding window across the whole genome. Simultaneously taking the number of recombination events, gene density, transposon density and the distance from centromere into account, we used the formula to calculate recombination coefficient (Y) as follows:
| 1 |
where, X is total recombination events; Z is gene desntiy; M is TE density; N is length from centromere (Mb) in (1). Recombination coefficient is a normalized statistic, which is more than 0. The higher the recombination coefficient, the higher the probability of recombination, which make it a robust statistic to identify the recombination hotspot. Accordingly, the genomic regions with recombination coefficient in the top 5 were considered as recombination hotspots. Two F2 populations reported in previous study were also used to verify recombination hotspots indefied in this study63. Two putatitve recombination hot spots (a1-sh2, sh1-bz1)35, 36 and five new randomly selected recombination hotspots were tested with high-density PCR markers using these two segregating populations63. The primer information of these seven recombination hotspots could be found in Table S9.
Electronic supplementary material
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31525017), the National Hi-Tech Research and Development Program of China (2012AA10A307) to Jianbing Yan, and Huazhong Agricultural University Scientific & Technological Self-Innovation Foundation (Program No. 2015RC016) to Lin Li. We are grateful to the editor and the anonymous reviewers for their helpful comments and suggestions.
Author Contributions
L.L. designed and supervised this study. Q.P. and L.L. performed the data analysis. M.D. performed the experiment. Q.P. and L.L. prepared the manuscript. J.Y. reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01240-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Villeneuve AM, Hillers KJ. Whence meiosis? Cell. 2001;106:647–50. doi: 10.1016/S0092-8674(01)00500-1. [DOI] [PubMed] [Google Scholar]
- 2.Hamant O, Ma H, Cande WZ. Genetics of meiotic prophase I in plants. Annu Rev Plant Biol. 2006;57:267–302. doi: 10.1146/annurev.arplant.57.032905.105255. [DOI] [PubMed] [Google Scholar]
- 3.Mezard C, Vignard J, Drouaud J, Mercier R. The road to crossovers: plants have their say. Trends Genet. 2007;23:91–9. doi: 10.1016/j.tig.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Gaut BS. Does recombination shape the distribution and evolution of tandemly arrayed genes (TAGs) in the Arabidopsis thaliana genome? Genome Res. 2003;13:2533–40. doi: 10.1101/gr.1318503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulathinal RJ, Bennett SM, Fitzpatrick CL, Noor MA. Fine-scale mapping of recombination rate in Drosophila refines its correlation to diversity and divergence. Proc Natl Acad Sci USA. 2008;105:10051–6. doi: 10.1073/pnas.0801848105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kent CF, Minaei S, Harpur BA, Zayed A. Recombination is associated with the evolution of genome structure and worker behavior in honey bees. Proc Natl Acad Sci USA. 2012;109:18012–7. doi: 10.1073/pnas.1208094109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijnker E, de Jong H. Managing meiotic recombination in plant breeding. Trends Plant Sci. 2008;13:640–6. doi: 10.1016/j.tplants.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Martin OC, Wagner A. Effects of recombination on complex regulatory circuits. Genetics. 2009;183:673–84. doi: 10.1534/genetics.109.104174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Hsia AP, Zhang L, Nikolau BJ, Schnable PS. Meiotic recombination break points resolve at high rates at the 5′ end of a maize coding sequence. Plant Cell. 1995;7:2151–61. doi: 10.1105/tpc.7.12.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu H, Zheng Z, Dooner HK. Recombination rates between adjacent genic and retrotransposon regions in maize vary by 2 orders of magnitude. Proc Natl Acad Sci USA. 2002;99:1082–7. doi: 10.1073/pnas.022635499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steiner WW, Steiner EM, Girvin AR, Plewik LE. Novel nucleotide sequence motifs that produce hot regions of meiotic recombination in Schizosaccharomyces pombe. Genetics. 2009;182:459–69. doi: 10.1534/genetics.109.101253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillon H, de Massy B. An initiation site for meiotic crossing-over and gene conversion in the mouse. Nat Genet. 2002;32:296–9. doi: 10.1038/ng990. [DOI] [PubMed] [Google Scholar]
- 13.Buhler C, Borde V, Lichten M. Mapping meiotic single-strand DNA reveals a new landscape of DNA double-strand breaks in Saccharomyces cerevisiae. PLoS Biol. 2007;5:e324. doi: 10.1371/journal.pbio.0050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Backström N, et al. The recombination landscape of the zebra finch Taeniopygia guttata genome. Genome Res. 2010;20:485–95. doi: 10.1101/gr.101410.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldman AS, Tran H, Goldsmith EC, Resnick MA. Long inverted repeats are an at-risk motif for recombination in mammalian cells. Genetics. 1999;153:1873–83. doi: 10.1093/genetics/153.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright SI, Agrawal N, Bureau TE. Effects of recombination rate and gene density on transposable element distributions in Arabidopsis thaliana. Genome Res. 2003;13:1897–903. doi: 10.1101/gr.1281503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers S, Bottolo L, Freeman C, McVean G, Donnelly P. A fine-scale map of recombination rates and hotspot across the human genome. Science. 2005;310:321–4. doi: 10.1126/science.1117196. [DOI] [PubMed] [Google Scholar]
- 18.Serre D, Nadon R, Hudson TJ. Large-scale recombination rate patterns are conserved among human populations. Genome Res. 2005;15:1547–52. doi: 10.1101/gr.4211905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers S, Freeman C, Auton A, Donnelly P, McVean G. A common sequence motif associated with recombination hot spots and genome instability in humans. Nat Genet. 2008;40:1124–9. doi: 10.1038/ng.213. [DOI] [PubMed] [Google Scholar]
- 20.Pawlowski WP, Golubovskaya IN, Cande WZ. Altered nuclear distribution of recombination protein RAD51 in maize mutants suggests the involvement of RAD51 in meiotic homology recognition. Plant Cell. 2003;15:1807–16. doi: 10.1105/tpc.012898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawlowski WP, Golubovskaya IN, Timofejeva L, Meeley RB, Sheridan WF, Cande WZ. Coordination of meiotic recombination, pairing, and synapsis by PHS1. Science. 2004;303:89–92. doi: 10.1126/science.1091110. [DOI] [PubMed] [Google Scholar]
- 22.Li J, et al. Functional analysis of maize RAD51 in meiosis and double-strand break repair. Genetics. 2007;176:1469–82. doi: 10.1534/genetics.106.062604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fullerton SM, Carvalho AB, Clark AG. Local rates of recombination are positively correlated with GC content in the human genome. Mol Biol Evol. 2001;18:1139–42. doi: 10.1093/oxfordjournals.molbev.a003886. [DOI] [PubMed] [Google Scholar]
- 24.Nachman MW. Single nucleotide polymorphisms and recombination rate in humans. Trends Genet. 2001;17:481–5. doi: 10.1016/S0168-9525(01)02409-X. [DOI] [PubMed] [Google Scholar]
- 25.Yao H, et al. Molecular characterization of meiotic recombination across the 140-kb multigenic a1-sh2 interval of maize. Proc Natl Acad Sci USA. 2002;99:6157–62. doi: 10.1073/pnas.082562199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzon, C., Marais, G., Gouy, M. & Biemont, C. Recombination rate and the distribution of transposable elements in the Drosophila melanogaster genome. Genome Res. 12, 400–7, doi:10.1101/gr.210802. Article published online before print in February 2002 (2002). [DOI] [PMC free article] [PubMed]
- 27.Esch E, Szymaniak JM, Yates H, Pawlowski WP, Buckler ES. Using crossover breakpoints in recombinant inbred lines to identify quantitative trait loci controlling the global recombination frequency. Genetics. 2007;177:1851–8. doi: 10.1534/genetics.107.080622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baucom RS, et al. Exceptional diversity, non-random distribution, and rapid evolution of retroelements in the B73 maize genome. PLoS Genet. 2009;5:e1000732. doi: 10.1371/journal.pgen.1000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gore MA, et al. A first-generation haplotype map of maize. Science. 2009;326:1115–7. doi: 10.1126/science.1177837. [DOI] [PubMed] [Google Scholar]
- 30.Schnable PS, et al. The B73 maize genome: complexity, diversity, and dynamics. Science. 2009;326:1112–5. doi: 10.1126/science.1178534. [DOI] [PubMed] [Google Scholar]
- 31.Springer NM, et al. Maize inbreds exhibit high levels of copy number variation (CNV) and presence/absence variation (PAV) in genome content. PLoS Genet. 2009;5:e1000734. doi: 10.1371/journal.pgen.1000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eichten SR, et al. Heritable epigenetic variation among maize inbreds. PLoS Genet. 2011;7:e1002372. doi: 10.1371/journal.pgen.1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brick K, Smagulova F, Khil P, Camerini-Otero RD, Petukhova GV. Genetic recombination is directed away from functional genomic elements in mice. Nature. 2012;485:642–5. doi: 10.1038/nature11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi K, et al. Arabidopsis meiotic crossover hot spots overlap with H2AZ nucleosomes at gene promoters. Nat Genet. 2013;45:1327–36. doi: 10.1038/ng.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Civardi L, Xia Y, Edwards KJ, Schnable PS, Nikolau BJ. The relationship between genetic and physical distances in the cloned a1-sh2 interval of the Zea mays L genome. Proc Natl Acad Sci USA. 1994;91:8268–72. doi: 10.1073/pnas.91.17.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dooner HK, Martínez-Férez IM. Recombination occurs uniformly within the bronze gene, a meiotic recombination hotspot in the maize genome. Plant Cell. 1997;9:1633–46. doi: 10.1105/tpc.9.9.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu H, Park W, Yan X, Zheng Z, Shen B, Dooner HK. The highly recombinogenic bz locus lies in an unusually gene-rich region of the maize genome. Proc Natl Acad Sci USA. 2001;98:8903–8. doi: 10.1073/pnas.141221898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dooner HK, He L. Polarized gene conversion at the bz locus of maize. Proc Natl Acad Sci USA. 2014;111:13918–23. doi: 10.1073/pnas.1415482111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yandeau-Nelson MD, Nikolau BJ, Schnable PS. Effects of trans-acting genetic modifiers on meiotic recombination across the a1-sh2 interval of maize. Genetics. 2006;174:101–12. doi: 10.1534/genetics.105.049270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauer E, et al. Intraspecific variation of recombination rate in maize. Genome Biol. 2013;14:R103. doi: 10.1186/gb-2013-14-9-r103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan Q, et al. Genome-wide recombination dynamics are associated with phenotypic variation in maize. New Phytol. 2016;210:1083–94. doi: 10.1111/nph.13810. [DOI] [PubMed] [Google Scholar]
- 42.Chia JM, et al. Maize HapMap2 identifies extant variation from a genome in flux. Nat Genet. 2012;44:803–7. doi: 10.1038/ng.2313. [DOI] [PubMed] [Google Scholar]
- 43.Osman K, Higgins GD, Sanchez-Moran E, Armstrong SJ, Franklin FC. Pathways to meiotic recombination in Arabidopsis thaliana. New Phytol. 2011;190:523–44. doi: 10.1111/j.1469-8137.2011.03665.x. [DOI] [PubMed] [Google Scholar]
- 44.Paape T, Zhou P, Branca A, Briskine R, Young N, Tiffin P. Fine-scale population recombination rates, hot regions, and correlates of recombination in the Medicago truncatula genome. Genome Biol Evol. 2012;4:726–37. doi: 10.1093/gbe/evs046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auton A, et al. Genetic recombination is targeted towards gene promoter regions in dogs. PLoS Genet. 2013;9:e1003984. doi: 10.1371/journal.pgen.1003984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wicker T, Guyot R, Yahiaoui N, Keller B. CACTA transposons in Triticeae A diverse family of high-copy repetitive elements. Plant Physiol. 2003;132:52–63. doi: 10.1104/pp.102.015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casacuberta JM, Santiago NP. LTR-retrotransposons and MITEs: control of transposition and impact on the evolution of plant genes and genomes. Gene. 2003;311:1–11. doi: 10.1016/S0378-1119(03)00557-2. [DOI] [PubMed] [Google Scholar]
- 48.Dooner HK, He L. Maize genome structure variation: interplay between retrotransposon polymorphisms and genic recombination. Plant Cell. 2008;20:249–58. doi: 10.1105/tpc.107.057596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L, et al. Genome-wide discovery and characterization of maize long non-coding RNAs. Genome Biol. 2014;15:R40. doi: 10.1186/gb-2014-15-2-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eichten SR, Vaughn MW, Hermanson PJ, Springer NM. Variation in DNA methylation patterns is more common among maize inbreds than among tissues. Plant. Genome. 2013;6:1–10. [Google Scholar]
- 51.Regulski M, et al. The maize methylome influences mRNA splice sites and reveals widespread paramutation-like switches guided by small RNA. Genome Res. 2013;23(10):1651–62. doi: 10.1101/gr.153510.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ginno PA, Lim YW, Lott PL, Korf I, Chedin F. GC skew at the 5′ and 3′ ends of human genes links R-loop formation to epigenetic regulation and transcription termination. Genome Res. 2013;23:1590–600. doi: 10.1101/gr.158436.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wimberly H, Shee C, Thornton PC, Sivaramakrishnan P, Rosenberg SM, Hastings PJ. R-loops and nicks initiate DNA breakage and genome instability in non-growing Escherichia coli. Nat Commun. 2013;4:2115. doi: 10.1038/ncomms3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forsdyke DR. Reciprocal relationship between stem-loop potential and substitution density in retroviral quasispecies under positive Darwinian selection. J Mol Evol. 1995;41:1022–37. doi: 10.1007/BF00173184. [DOI] [PubMed] [Google Scholar]
- 55.Olsthoorn RC, Bruyere A, Dzianott A, Bujarski JJ. RNA Recombination in brome mosaic virus: effects of strand-specific stem-loop inserts. J Virol. 2002;76:12654–62. doi: 10.1128/JVI.76.24.12654-12662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Draghici HK, Varrelmann M. Evidence for similarity-assisted recombination and predicted stem-loop structure determinant in potato virus X RNA recombination. J Gen Virol. 2010;91:552–62. doi: 10.1099/vir.0.014712-0. [DOI] [PubMed] [Google Scholar]
- 57.Zaratiegui M, et al. CENP-B preserves genome integrity at replication forks paused by retrotransposon LTR. Nature. 2011;469:112–5. doi: 10.1038/nature09608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lai J, et al. Genome-wide patterns of genetic variation among elite maize inbred lines. Nat Genet. 2010;42:1027–30. doi: 10.1038/ng.684. [DOI] [PubMed] [Google Scholar]
- 59.Trickett AJ, Butlin RK. Recombination suppressors and the evolution of new species. Heredity. 1994;73:339–45. doi: 10.1038/hdy.1994.180. [DOI] [PubMed] [Google Scholar]
- 60.McClosky B, Tanksley SD. The impact of recombination on short-term selection gain in plant breeding experiments. Theor Appl Genet. 2013;126:2299–312. doi: 10.1007/s00122-013-2136-3. [DOI] [PubMed] [Google Scholar]
- 61.Wang, S., Basten, C. J. & Zeng, Z. Windows QTL Cartographer 25 (North Carolina State University) (2005).
- 62.McVean GA, Myers SR, Hunt S, Deloukas P, Bentley DR, Donnelly P. The fine-scale structure of recombination rate variation in the human genome. Science. 2004;304:581–4. doi: 10.1126/science.1092500. [DOI] [PubMed] [Google Scholar]
- 63.Yang Q, et al. CACTA-like transposable element in ZmCCT attenuated photoperiod sensitivity and accelerated the postdomestication spread of maize. Proc Natl Acad Sci USA. 2013;110:16969–74. doi: 10.1073/pnas.1310949110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


