Abstract
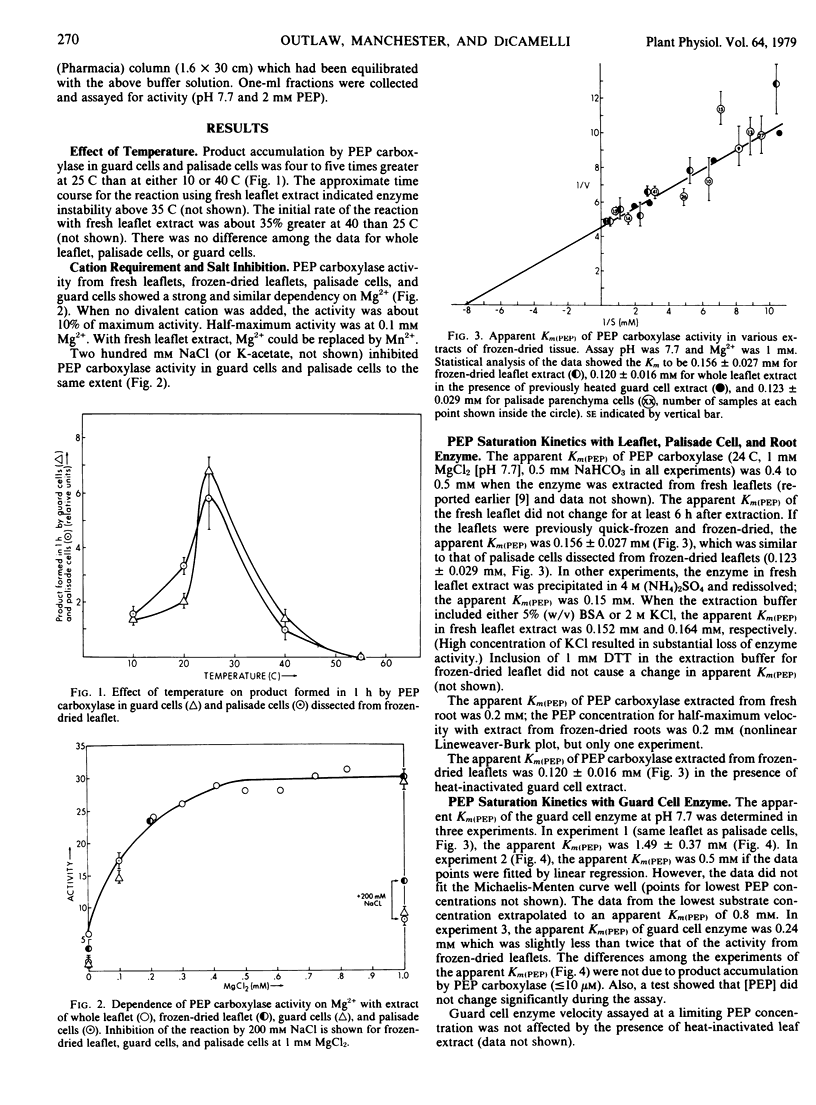
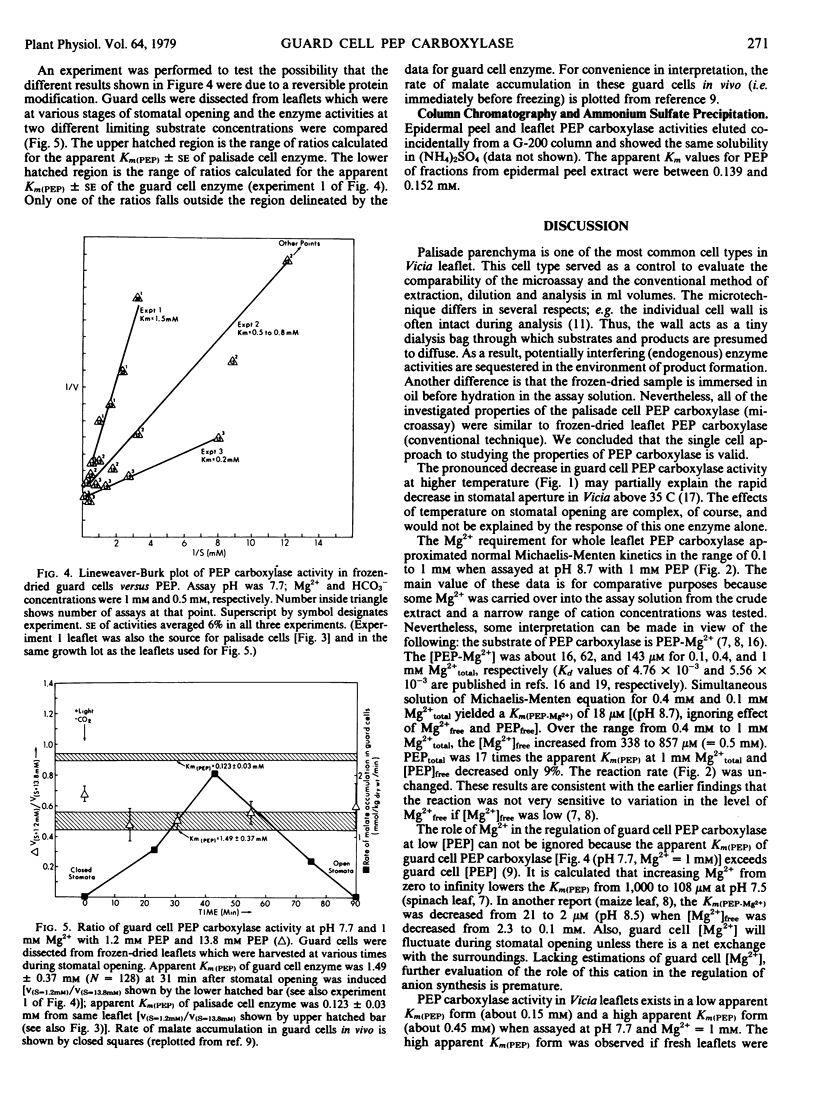
Properties of phosphoenolpyruvate carboxylase in guard cells dissected from frozen-dried Vicia faba L. leaflets were studied using quantitative histochemical techniques. Control experiments with palisade cells and whole leaflet extract proved that the single cell approach was valid. Most characteristics of enzyme activity in guard cells were identical to those in the leaflet extract. The activities were highly dependent on temperature, with maximum activity at 25 to 35 C. Half-maximum activity (with 1 millimolar phosphoenolpyruvate [PEP]) was observed at 0.1 millimolar Mg2+. Two-hundred millimolar NaCl inhibited the reaction by 50%. With frozen-dried leaflet extract, the apparent Km(PEP) was 0.15 millimolar at pH 7.7; with guard cells, the values were 1.49, 0.5 to 0.8, and 0.24 millimolar in three successive experiments. Additional experiments showed that apparent Km(PEP) of guard cell activity from plants within a single growth lot was reproducible and did not change during stomatal opening. Mixed extract experiments proved that soluble compounds were not responsible for the difference observed between leaflet and guard cell activities. The differences in apparent Km(PEP) of guard cell activity could not be unambiguously interpreted. The physiological implications of the properties of this enzyme in guard cells are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies D. D. Control of and by pH. Symp Soc Exp Biol. 1973;27:513–529. [PubMed] [Google Scholar]
- Humble G. D., Raschke K. Stomatal opening quantitatively related to potassium transport: evidence from electron probe analysis. Plant Physiol. 1971 Oct;48(4):447–453. doi: 10.1104/pp.48.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Berger S. J., Carter J. A., Lowry O. H. An enzymatic cycling method for nicotinamide-adenine dinucleotide with malic and alcohol dehydrogenases. Anal Biochem. 1973 May;53(1):86–97. doi: 10.1016/0003-2697(73)90409-0. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M., Nowak T., Mildvan A. S. Spinach leaf phosphoenolpyruvate carboxylase: purification, properties, and kinetic studies. Arch Biochem Biophys. 1974 Jul;163(1):378–389. doi: 10.1016/0003-9861(74)90489-5. [DOI] [PubMed] [Google Scholar]
- Mukerji S. K. Corn leaf phosphoenolpyruvate carboxylases. The effect of divalent cations on activity. Arch Biochem Biophys. 1977 Jul;182(1):352–359. doi: 10.1016/0003-9861(77)90316-2. [DOI] [PubMed] [Google Scholar]
- Outlaw W. H., Kennedy J. Enzymic and substrate basis for the anaplerotic step in guard cells. Plant Physiol. 1978 Oct;62(4):648–652. doi: 10.1104/pp.62.4.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Lowry O. H. Organic acid and potassium accumulation in guard cells during stomatal opening. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4434–4438. doi: 10.1073/pnas.74.10.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Manchester J. Guard cell starch concentration quantitatively related to stomatal aperture. Plant Physiol. 1979 Jul;64(1):79–82. doi: 10.1104/pp.64.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. E. Escherichia coli phosphoenolpyruvate carboxylase: studies on the mechanism of multiple allosteric interactions. Arch Biochem Biophys. 1977 Oct;183(2):538–552. doi: 10.1016/0003-9861(77)90389-7. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLD F., BALLOU C. E. Studies on the enzyme enolase. I. Equilibrium studies. J Biol Chem. 1957 Jul;227(1):301–312. [PubMed] [Google Scholar]


