Abstract
Accumulating evidence suggests an association between the SIRT1 gene and human psychiatric disorders. The aim of the study was to investigate the association between SIRT1 and predisposition to antisocial personality traits (ASP) in Chinese adolescents. Participants consisted of 327 controls and 261 juvenile offenders who were diagnosed with predisposition to ASP according to the Personality Diagnostic Questionnaire. Four tag single nucleotide polymorphisms (tagSNPs) of SIRT1, namely rs12778366, rs7896005, rs10823112, and rs4746720, were genotyped. Association analysis between individual SNPs and ASP risk revealed the CC genotype of rs4746720 to be significantly associated with reduced risk of ASP (OR = 0.51, 95% CI = 0.33–0.77, adjusted P = 0.007). Haplotype analysis showed the TAAC haplotype was associated with reduced susceptibility to ASP (OR = 0.72, 95% CI = 0.57–0.91, P = 0.005). Moreover, rs4746720 variants were found to not only have a direct impact on ASP susceptibility but also modulate the effect of alcohol consumption (Y = 0.022X + 0.431 vs. Y = −0.066X + 0.387). The present study is the first to report a significant association between SIRT1 polymorphisms and ASP in adolescents. This finding is expected to aid in the development of effective interventions for this socially and personally costly disorder.
Introduction
Antisocial personality traits (ASP) represent a serious psychiatric condition that poses a significant burden on the affected individuals and society1, and lacks an effective treatment1, 2. ASP is defined as a “pervasive pattern of disregard for, and violation of, the rights of others”. This pattern of behavior typically starts in childhood or adolescence and persists through adulthood3. ASP and major psychiatric disorders, such as mood swings, anxiety, and substance abuse, are common and present high comorbidity4, 5. The disorder has been well studied in community samples and is estimated to affect 4.4% of the general population6. It should be noted that ASP is particularly common in prison settings, with a worldwide prevalence of about 50% among male prisoners7, 8.
It is commonly accepted that genetics plays a substantial role in the etiology of psychopathic disorders, particularly in ASP9–11. Familial and twin registry studies indicate that ASP is highly heritable, with genetic factors accounting for 40–69% of the variation in diagnoses12–14. One neurochemical system that has been implicated, both theoretically and empirically, in ASP and psychopathy is the serotonin system (which consists of the 5-HTTLPR polymorphism in SLC6A4 and the genes HTR1B, MAOA, and MAOB)15–18. Recently, a genome-wide association study (GWAS) revealed that rs4714329 reached genome-wide significance (OR = 1.59, 95% CI = 1.37–1.85, P = 1.6 × 10−9)19. Genome-wide studies have confirmed only a minor contribution of single genetic polymorphisms to the incidence of ASP; thus, they are difficult to replicate. In summary, the etiology of ASP remains complex and poorly understood.
The SIRT family of nicotinamide adenine dinucleotide (NAD)-dependent deacetylases comprises seven members (SIRT1–7). These are emerging as having an important role in the regulation of metabolism, aging, oxidative stress, and circadian rhythms, and as modulators of synaptic plasticity and memory formation20–23. SIRT1, which localizes mainly to the nucleus, regulates gene transcription and DNA repair24. As such, SIRT1 plays an important functional role in numerous biological processes. One of these is the circadian clock system25, whereby SIRT1 associates and is recruited to the BMAL1 chromatin complex at circadian promoters26. Importantly, many studies evoke the involvement of circadian rhythm disruption in several psychiatric disorders such as, schizophrenia, anxiety disorders, bipolar disorders, and aggressive behaviors, which is the main characteristic of ASP27–29. Furthermore, a GWAS has identified various SIRT1 polymorphisms that confer susceptibility to major depressive disorders30. Notably, evidence has emerged showing that the SIRT1 gene is associated with anxiety disorders in animals31. Based on the above, we considered the SIRT1 gene to be a good candidate for ASP. At present, it is not known whether the SIRT1 gene is related to ASP.
Complex behavioral patterns such as those characteristic of ASP result from a combination of genetic and environmental factors, with a possible role for environmental experiences such as maltreatment during childhood32. Strikingly, previous studies have underscored the importance of the close interplay between genetic and environmental factors in the etiology of ASP. It has been shown that a functional polymorphism in the MAOA gene moderates the impact of childhood maltreatment on the development of ASP33. Biologically, childhood maltreatment has been shown to promote changes in brain structure, atypical development of the hypothalamic-pituitary-adrenal axis, elevated neurotransmitter levels, and gene methylation34–36. Based on the existing research on ASP, we hypothesize that: (1) The SIRT1 gene may be linked to ASP. (2) The SIRT1 gene may interact with environmental factors in determining the risk of ASP.
The aim of the present case-control study was to identify common genetic variants underlying variation and interplay between genetic and environmental factors in a population of Chinese adolescents with ASP. In particular, we attempted to obtain evidence for an association between ASP and SIRT1 in this population.
Results
Characteristics of the study subjects
Table 1 summarizes the characteristics of the cases and controls included in the study. The mean ages for the ASP and control groups were similar (19.79 ± 2.66 vs. 19.51 ± 2.44 years). Frequency of alcohol consumption, tobacco smoking (on the basis of which subjects were classified into the often, sometimes, and never groups, as shown in Table 1), and history of childhood maltreatment differed significantly between case and control groups (P < 0.001) (Table 1). When controlling for other environment variables (For example, alcohol consumption, network usage, emotional abuse, physical abuse, sex abuse emotional neglect and physical neglect were controlled, when calculate independent effect of tobacco smoking), only tobacco smoking (OR = 19.50, 95% CI = 10.87–34.99, P < 0.001) and sex abuse (OR = 21.35, 95% CI = 5.73–79.58, P < 0.001) were predictors of ASP, according to logistic regression.
Table 1.
Characteristics of the study sample.
| Variables | Cases (n = 261) | Controls (n = 327) | χ2/t | P | OR (95% CI) | P |
|---|---|---|---|---|---|---|
| Age (in years) | 19.79 ± 2.66 | 19.51 ± 2.44 | 1.27 | 0.204 | 0.92 (0.79–1.06) | 0.24 |
| Tobacco smoking | 400.21 | <0.001 | 19.50 (10.87–34.99) | <0.001 | ||
| never | 15 | 277 | ||||
| sometimes | 51 | 41 | ||||
| Often | 192 | 9 | ||||
| Alcohol consumption | 107.73 | <0.001 | 1.17 (0.53–2.11) | 0.688 | ||
| never | 41 | 105 | ||||
| sometimes | 138 | 217 | ||||
| Often | 82 | 5 | ||||
| Network usage | 51.50 | <0.001 | 1.11 (0.59–2.11) | 0.743 | ||
| never | 22 | 15 | ||||
| sometimes | 46 | 151 | ||||
| Often | 188 | 161 | ||||
| Emotional abuse | 153.99 | <0.001 | 3.66 (0.99–13.63) | 0.053 | ||
| none to moderate | 121 | 300 | ||||
| moderate to extreme | 129 | 18 | ||||
| Physical abuse | 145.97 | <0.001 | 4.39 (0.98–21.21) | 0.066 | ||
| none to moderate | 136 | 302 | ||||
| moderate to extreme | 117 | 11 | ||||
| Sex abuse | 141.27 | <0.001 | 21.35 (5.73–79.58) | <0.001 | ||
| none to moderate | 141 | 296 | ||||
| moderate to extreme | 114 | 9 | ||||
| Emotional neglect | 1.80 (0.67–4.83) | 0.245 | ||||
| none to moderate | 113 | 265 | 93.12 | <0.001 | ||
| moderate to extreme | 136 | 51 | ||||
| Physical neglect | 103.13 | <0.001 | 1.49 (0.56–3.99) | 0.423 | ||
| none to moderate | 110 | 270 | ||||
| moderate to extreme | 146 | 54 |
Data calculated by two independent sample t-tests and logistic regression and other variables were controlled.
Positive results are in bold. Variable may not add up to the total number due to missing values.
Characteristics of tagSNPs
Four single nucleotide polymorphisms (SNPs) were successfully genotyped in a total of 588 subjects. SIRT1 was mapped to chromosome 10q21.3, and was approximately 33.7 kb in length. The distribution of genotypes did not deviate from the Hardy-Weinberg equilibrium in any of the groups or in the overall sample (P > 0.05). The general characteristics of tagSNPs are shown in Table 2. Minor allele frequencies (MAFs) of all four tagSNPs in our controls were similar to MAFs for CHBS_1000 g.
Table 2.
Characteristics of the four SNPs included in the current study.
| IDs | Location | Reference/Variant allele | W/H/V frequency (cases) | W/H/V frequency (controls) | MAF in cases | MAF in controls | MAF in CHBS_1000 g | P for HWE |
|---|---|---|---|---|---|---|---|---|
| rs12778366 | 5′FLANKING | T/C | 193/65/3 | 251/67/9 | 0.136 | 0.130 | 0.132 | 0.089 |
| rs7896005 | intron2 | A/G | 178/77/6 | 243/77/7 | 0.171 | 0.139 | 0.157 | 0.757 |
| rs10823112 | intron6 | A/G | 133/106/22 | 191/113/23 | 0.287 | 0.243 | 0.325 | 0.270 |
| rs4746720 | 3′UTR | T/C | 88/134/39 | 92/151/84 | 0.406 | 0.488 | 0.386 | 0.170 |
Abbreviations: W, wild type homozygote; H, heterozygote; V, variant homozygote.
MAF, minor allele frequency; CHB, Han Chinese in Beijing, China; HWE, Hardy-Weinberg equilibrium.
Association analysis between individual SNPs and ASP risk
Allele and genotype distributions of the four tagSNPs in cases and controls are shown in Table 3. Based on genetic model association analysis, only rs4746720 showed statistical significance. Compared to the CT/TT genotype, the CC genotype was associated with reduced AB risk (OR = 0.51, 95% CI = 0.33–0.77, adjusted P = 0.007) in a recessive model. C allele frequency of rs4746720 in patients with ASP was significantly lower than in the control group in an additive model (OR = 0.72, CI = 0.57–0.91, adjusted P = 0.024). Taken together, our data suggest that the SIRT1 tagSNP rs4746720 is associated with ASP risk, and that individuals carrying the C allele exhibit significantly reduced ASP susceptibility. This confirms hypothesis (1). It should be noted that we did not detect any association between rs12778366, rs7896005, or rs10823112 and the risk of ASP in allelic or genotypic analyses.
Table 3.
Association analysis between individual SNPs and ASP risk.
| SNP | Variables | Cases (%) | Controls (%) | OR (95% CI) | P | FDR_BH adjusted |
|---|---|---|---|---|---|---|
| rs12778366 | TT | 193 (73.9) | 251 (76.8) | 1 | ||
| TC | 65 (24.9) | 67 (20.5) | 1.26 (0.86–1.86) | 0.242 | ||
| CC | 3 (1.1) | 9 (2.8) | 0.43 (0.12–1.62) | 0.215 | 0.426 | |
| Dom. | 1.16 (0.80–1.70) | 0.431 | 0.431 | |||
| Rec. | 0.41 (0.11–1.53) | 0.186 | 0.371 | |||
| Add. | 1.05 (0.75–1.47) | 0.764 | 0.764 | |||
| rs7896005 | AA | 178 (68.2) | 243 (74.3) | 1 | ||
| AG | 77 (29.5) | 77 (23.5) | 1.37 (0.94–1.98) | 0.099 | ||
| GG | 6 (2.3) | 7 (2.1) | 1.17 (0.39–3.54) | 0.781 | 0.781 | |
| Dom. | 1.35 (0.94–1.93) | 0.103 | 0.193 | |||
| Rec. | 1.08 (0.36–3.24) | 0.897 | 0.897 | |||
| Add. | 1.28 (0.93–1.76) | 0.137 | 0.182 | |||
| rs10823112 | AA | 133 (51.0) | 191 (58.4) | 1 | ||
| AG | 106 (40.6) | 113 (34.6) | 1.35 (0.95–1.90) | 0.091 | ||
| GG | 22 (8.4) | 23 (7.0) | 1.37 (0.74–2.57) | 0.953 | 0.426 | |
| Dom. | 1.35 (0.97–1.88) | 0.071 | 0.193 | |||
| Rec. | 1.22 (0.66–2.24) | 0.528 | 0.704 | |||
| Add. | 1.25 (0.96–1.61) | 0.094 | 0.182 | |||
| rs4746720 | TT | 88 (33.7) | 92 (28.1) | 1 | ||
| CT | 134 (51.3) | 151 (46.2) | 0.93 (0.64–1.35) | 0.694 | ||
| CC | 39 (14.9) | 84 (25.7) | 0.49 (0.30–0.78) | 0.003 | 0.012 | |
| Dom. | 0.77 (0.54–1.09) | 0.145 | 0.193 | |||
| Rec. | 0.51 (0.33–0.77) | 0.002 | 0.007 | |||
| Add. | 0.72 (0.57–0.91) | 0.006 | 0.024 |
Abbreviations: Dom, dominant model; Rec, recessive model; Add, additive model; OR, odds ratio; 95% CI, 95% confidence interval; FDR_BH, false discovery rate-Benjamini and Hochberg.
Positive results are in bold.
Association between SIRT1 tagSNPs haplotypes and ASP risk
As shown in Fig. 1, all four tagSNPs were located in one haplotypic block. Therefore, we further compared the haplotype frequencies of the four tagSNPs between the ASP group and controls. Four common haplotypes (frequency >3%) derived from the four tagSNPs accounted for almost 100% of haplotype variations. Among the four common haplotypes, only rs12778366T-rs7896005A-rs10823112A-rs4746720C was associated with a reduced ASP risk (OR = 0.72, 95% CI = 0.57–0.91 P = 0.005, Table 4).
Figure 1.
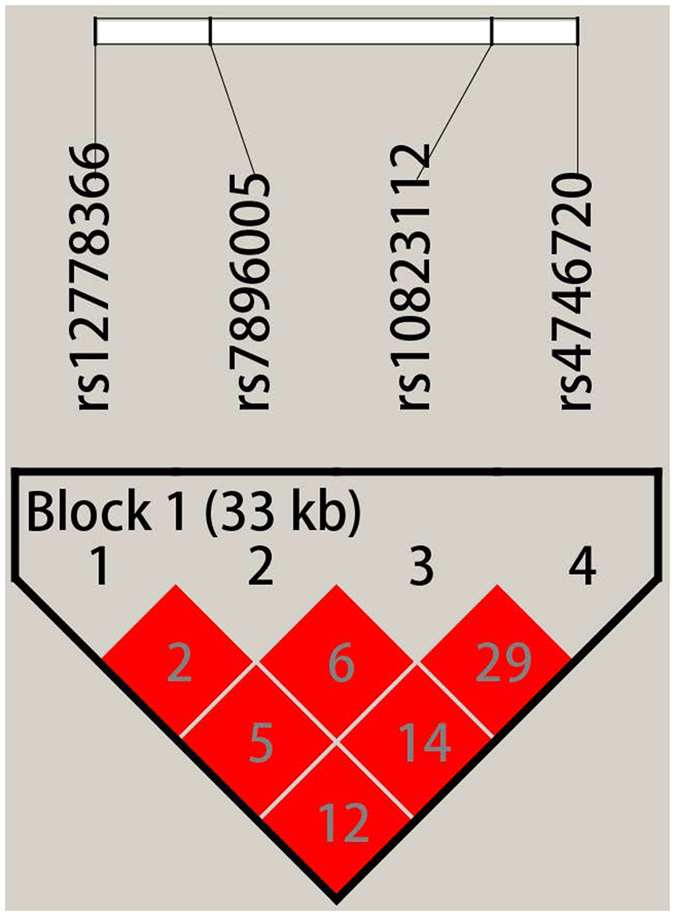
Four tagSNPs haplotypic block analysis.
Table 4.
Association between SIRT1 tagSNP haplotypes and the risk of ASP.
| Haplotype | Cases (%) | Controls (%) | OR | 95% CI | P |
|---|---|---|---|---|---|
| TAGT | 150 (0.29) | 79 (0.24) | 1.26 | 0.97–1.63 | 0.087 |
| CAAT | 71 (0.14) | 39 (0.12) | 1.05 | 0.75–1.48 | 0.761 |
| TAAC | 212 (0.41) | 151 (0.46) | 0.72 | 0.57–0.91 | 0.005 |
| TGAT | 89 (0.17) | 57 (0.18) | 1.27 | 0.93–1.75 | 0.139 |
Positive results are in bold.
Association of pairwise interactions with ASP risk by logistic and moderate analysis
Compared to the CT/TT genotype in the often-tobacco smoking group, the CC genotype was associated with reduced ASP risk (OR = 0.21, 95% CI = 0.05–0.84) by univariate logistic regression; however, the interaction was not significant according to multivariate logistic regression. Furthermore, the CC genotype exhibited reduced ASP risk compared to the CT/TT genotype in the sometimes- (OR = 0.44, 95% CI = 0.25–0.77) and often-alcohol consumption groups (OR = 0.09, 95% CI = 0.01–0.62) (Table 5). The rs4746720 CC genotype exhibited reduced ASP risk compared to the CT/TT genotype in the subgroup defined by maltreatment history in childhood. Childhood experiences of emotional, physical, and sexual abuse, as well as physical and emotional neglect were all strong predictors of ASP (P < 0.001). The interaction between rs4746720 genotype and alcohol consumption was statistically significant (OR = 0.43, 95% CI = 0.20–0.94, P = 0.034). However, no other statistically significant gene-environmental interactions were detected (Table 5). Furthermore, rs4746720 variants were found to not only have a direct impact on ASP susceptibility but to also modulate the effects of alcohol consumption (Y = 0.022X + 0.431 vs. Y = −0.066X + 0.387) (Table 6, Fig. 2), thus confirming also hypothesis (2).
Table 5.
Pairwise interactions between genetic variants of rs4746720.
| Variables | OR (95%CI)a | OR (95%CI)b | OR (95%CI)b | OR (95%CI)b | |
|---|---|---|---|---|---|
| CT + TT | CC | Variables | rs4746720 | Interaction | |
| Tobacco smoking | 23.57 (14.22–39.07) *** | 1.64 (0.24–10.95) | 0.54 (0.22–1.37) | ||
| never | Ref. | 0.71 (0.20–2.65) | |||
| sometimes | Ref. | 0.66 (0.23–1.90) | |||
| Often | Ref. | 0.21 (0.05–0.84) | |||
| Alcohol consumption | 4.69 (3.21–6.85)*** | 2.64 (0.56–12.53) | 0.43 (0.20–0.94)* | ||
| never | Ref. | 1.15 (0.49–2.69) | |||
| sometimes | Ref. | 0.44 (0.25–0.77) | |||
| Often | Ref. | 0.09 (0.01–0.62) | |||
| Network usage | 1.81 (1.33–2.46)*** | 0.39 (0.04–3.76) | 1.07 (0.46–2.47) | ||
| never | Ref. | 0.40 (0.24–0.68) | |||
| sometimes | Ref. | 0.78 (0.33–1.84) | |||
| Often | Ref. | 0.40 (0.24–0.68) | |||
| Emotional abuse | 15.38 (8.54–27.70)*** | 0.48(0.27–0.84)* | 2.02 (0.48–8.53) | ||
| none to moderate | Ref. | 0.48 (0.27–0.84) | |||
| moderate to extreme | Ref. | 0.97 (0.26–3.65) | |||
| Physical abuse | 21.15 (10.31–43.37)*** | 0.49 (0.28–0.83)** | 1.68 (0.31–9.21) | ||
| none to moderate | Ref. | 0.49 (0.28–0.84) | |||
| moderate to extreme | Ref. | 0.81 (0.16–4.10) | |||
| Sex abuse | 24.48 (11.01–54.43)*** | 0.45 (0.26–0.76)** | 1.47 (0.26–8.34) | ||
| none to moderate | Ref. | 0.32 (0.14–0.73) | |||
| moderate to extreme | Ref. | 0.52 (0.21–1.28) | |||
| Emotional neglect | 7.02 (4.48–10.98)*** | 0.66 (0.38–1.14) | 0.55 (0.21–1.44) | ||
| none to moderate | Ref. | 0.66 (0.38–1.14) | |||
| moderate to extreme | Ref. | 0.37 (0.17–0.80) | |||
| Physical neglect | 7.93 (5.05–12.45)*** | 0.55 (0.30–1.00) | 0.58 (0.23–1.47) | ||
| none to moderate | Ref. | 0.55 (0.30–0.99) | |||
| moderate to extreme | Ref. | 0.32 (0.16–0.65) | |||
aData calculated by univariate logistic regression; bData calculated by multivariate logistic regression (Y = a0 + a1 X1 + a2 X2 + a1a2 X1 * X2); Positive results are in bold. *P < 0.05 (two-tailed), **P < 0.01 (two-tailed), ***P < 0.001 (two-tailed).
Table 6.
Hierarchical regression analysis of the effect of rs4746720 polymorphisms on the relationship between alcohol consumption and ASP.
| Variables | Step1 (β) | Step2 (β) | Step3 (β) | Step4 (β) |
|---|---|---|---|---|
| Constant | 0.439*** | 0.439*** | 0.451*** | 0.451*** |
| Age (in years) | −0.003 | −0.003 | −0.002 | −0.002 |
| Tobacco smoking | 0.414*** | 0.416*** | 0.413*** | 0.412*** |
| Network usage | −0.008 | −0.007 | −0.005 | −0.006 |
| Alcohol consumption | −0.005 | −0.005 | 0.009 | |
| rs4746720 (Recessive) | −0.058* | −0.064* | ||
| rs4746720 (Recessive) × Alcohol consumption | −0.075** | |||
| ΔR2 | 0.000 | 0.002* | 0.003** |
*P < 0.05 (two-tailed), **P < 0.01 (two-tailed), ***P < 0.001 (two-tailed).
Positive results are in bold.
Figure 2.
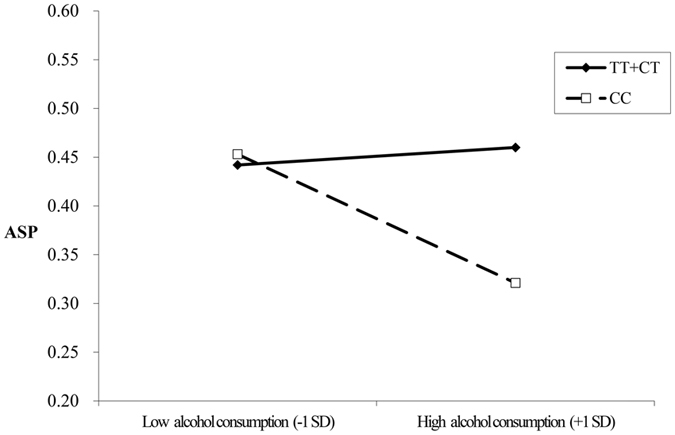
Relationship between rs4746720 and alcohol consumption, and ASP risk.
Discussion
In the present case-control study, we investigated whether four tagSNPs in SIRT1 were associated with ASP risk in Chinese adolescents. Our results suggest that a genetic variant of rs4746720, namely the CC genotype, is significantly associated with a reduced risk of ASP. Furthermore, a significantly lower frequency of the rs12778366T-rs7896005A-rs10823112A-rs4746720C haplotype was observed in ASP cases compared to controls. Genetic background plays an important role in the development of ASP, a phenomenon that has been extensively investigated in recent years. SIRT1 is unlikely to be the only gene that modulates ASP risk. The role of other genes, most notably MAOA, in determining susceptibility to ASP remains to be elucidated18, 37. Compelling evidence suggests that numerous genes are involved in determining an individual’s predisposition to psychiatric disorders38, 39. Therefore, the combined role of multiple genes should be considered. In recent years, GWASs have identified multiple genetic loci associated with ASP40, 41. However, genetic information is merely a proxy for the underlying structure and function of biological systems; these can only be understood by determining the specific function of a gene.
Rs4746720 is a functional polymorphism located in the 3′ UTR region of SIRT1. We used haploreg software to predict the function of rs4746720, however there is no specific evidence supporting any functional or structural alterations to SIRT1 caused by this variant (http://archive.broadinstitute.org/mammals/haploreg/haploreg.php; http://www.regulomedb.org/). Nevertheless, the strong linkage between rs4746720 and rs75818614 (R2 = 0.86), and a likely effect of rs75818614 on the binding of transcription factors as deduced from ChIP-Seq experiments, suggests a possible function of rs47467204242. Moreover, it has been reported that, among healthy Han Chinese, carriers of the C allele variant of rs4746720 have a higher risk of natural aging than non-carriers43. Recently, preclinical studies have suggested that SIRT1 signaling influences the dopaminergic system44, 45 and is associated with defects in synaptic plasticity in the hippocampus22, 46. SIRT1 variations associated with ASP may exert biological effects by disrupting circadian rhythms and the dopaminergic system, thus leading to the development of ASP.
In line with the results of numerous previous studies, our findings highlight the significant role of childhood maltreatment in ASP risk. We suggest that the environment plays a major role in determining susceptibility to ASP; as a result, the effect of rs4746720 is relatively negligible. Additionally, we studied the effect of gene-environment interactions; however, the genetic variants examined in this study did not show strong effects when linked to certain environmental factors. It should be noted that epigenetic regulation may be at least partially mediated by the environment, resulting in lasting phenotypic effects47. Therefore, further investigation of the role of epigenetic regulation is necessary.
ASP often co-exists with alcohol-related disorders48–50. The highest estimates of ASP (over 70%) have been reported in clinically ascertained populations of males with substance use disorders51. Based on the findings herein, genotype and environment have a “fan-shaped” interaction. Its key characteristic is a modest difference in the level of the outcome variable as a function of genetic liability when individuals exhibit a “virtuous” conduct. In other words, the role of specific genes is minimal under such circumstances. More specifically, the genotype may act as a barrier when ASP co-exists with alcohol-related disorders, rendering the relationship between these two weak or non-existent, and raising questions of ASP comorbidity52. High scores of impulsivity/disinhibition traits are associated with alcohol-related problems, because individuals with high impulsivity scores are more likely to be diagnosed as alcoholics53. Therefore, the SIRT1 variant described in this study seems associated with the impulsivity antisocial trait rather than lack of empathy. Further research should be performed to allow for better grouping of antisocial personality traits. In addition, the researchers speculate that the genotype of SIRT1 may be associated with certain metabolic enzymes, which can affect individual alcohol consumption. But there is no literature reports. Further research should to explore the SIRT1 gene function when ASP co-exists with alcohol-related disorders. At the same time, mechanisms underlying the relationship between genotype and ASP remain relatively unexplored. As research on the behavioral genetics of ASP expands beyond studies of heritability, it becomes increasingly clear that understanding the role of genes and behavior requires the characterization of probabilistic and interdependent influences of genetic predispositions and comorbidity risk factors.
The main strengths of this paper are represented by the target group and the significant results obtained after correcting for multiple comparisons. The present tagSNPs contain almost all the information required for a genetic model. There are, nevertheless, also several limitations to this study, some of which are related to its methodology. First, the study was based on the use of self-report questionnaires; as such, the participants were not evaluated by a mental health professional to diagnose ASP. All participants in our sample were men; therefore, our findings may not be extrapolated to women. Third, experimental data from cell line or animal studies did not reveal any SNP function. Finally, sample size was relatively small; consequently, the results of this study should be confirmed by analyzing a larger sample. Despite these limitations, our study contributes significantly to the understanding of the role of SIRT1 tagSNPs on ASP risk in adolescence by demonstrating the importance of individuals’ genetic make-up; to our knowledge, this has not been identified in previous studies.
In summary, to our knowledge, no previous study has investigated whether SIRT1 genetic polymorphisms determined the onset of ASP in adolescents, or identified the underlying mechanisms. The present results provide preliminary evidence that SIRT1 polymorphisms may contribute to the risk of ASP. These findings may enable the elucidation of the genetic basis determining predisposition to ASP.
Materials and Methods
Participants
A total of 261 male juvenile offenders (under the age of 25 years) from youth correctional facilities of Hubei province, who were diagnosed with ASP according to Personality Diagnostic Questionnaire-4+ (PDQ-4+) criteria, were selected for our study. Juvenile offenders were excluded if they had severe mental retardation or a major physical illness. Finally, 327 volunteers recruited from universities and high schools, matched to the cases in terms of age, gender, place of birth, and ethnicity, were included in the control group. All participants were male and native of Hubei province. This study was reviewed and approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology. The study was performed according to the approved ethical guidelines. Informed written consent was obtained from each participant prior to enrollment.
Instruments
Sociodemographic variables
Data regarding sociodemographic variables, such as age, place of birth, alcohol consumption, tobacco smoking, and social networks were obtained.
Diagnostic assessment
The PDQ-4+ was used to evaluate ASP of juvenile delinquents and healthy individuals. PDQ-4+ is a self-report questionnaire composed of 107 true/false items. To date, the PDQ-4+ has been shown to have good reliability and validity, and has been widely used in clinical practice54, 55. A total score of 30 or more indicates that the subject has a personality disturbance. Cut-off scores for each subscale are 3–556. Corresponding alpha coefficients were 0.85 in the current study. Scores higher than 5 indicated a disposition to ASP. This subscale included 8 items, the last of which contained 15 sub-items. The participants received a score of 1 if more than 3 sub-items were chosen for the last item.
History of childhood maltreatment
Maltreatment history was evaluated using the Childhood Trauma Questionnaire57, a 28-item self-report instrument, developed by Bernstein, that evaluates emotional, physical, and sexual abuse, as well as physical and emotional neglect during childhood. Responses range from 1 (never true) to 5 (very true). Internal consistency in our study was high for the sample (Cronbach’s α = 0.75).
Materials
Gene selection
Tag SNPs were identified from the Han Chinese data set of the International HapMap Consortium (http://hapmap.ncbi.nlm.nih.gov/). First, the selected gene region was extended by 2000 base pairs both upstream and downstream in the genome. Haploview 4.2 software was then used to run the tagger program with the parameters set to a minor allele frequency of at least 0.1 in the Chinese Han population and a pairwise r2 of at least 0.8. Four tagSNPs of SIRT1 were selected: rs12778366, rs7896005, rs10823112, and rs4746720.
Genotyping techniques
Genomic DNA was isolated from buccal epithelial cells using standard methods.
All genotypes of interest were tested for Hardy-Weinberg equilibrium using a goodness-of-fit χ2-test. SNP genotyping was performed using a custom-design 2 × 48-Plex SNPscanTM kit (Genesky Biotechnologies Inc., Shanghai, China) based on double ligation and multiplex fluorescence PCR. To validate the genotyping accuracy of the kit, a 5% random sample of cases and controls was genotyped twice for all SNPs by different analysts. Specifically, we included 22 pairs of blind duplicates, and concordance rate was more than 98%.
Statistical and bioinformatics analyses
All statistical analyses were performed using SPSS 18 software (SPSS Inc., Chicago, IL, USA). Means and standard deviations were computed for continuous variables. Counting data was analyzed using a χ2- test. Univariate or multivariate logistic regression analysis was performed to calculate the OR and 95% CI for the factors related to ASP. Multiple linear regression models were generated by entering the covariates first, followed by the predictors, and then the interaction term. Changes in R2 value between each step and the P values associated with the R2 change were noted. SHEsis software (http://shesisplus.bio-x.cn/SHEsis.html) was used to test a possible association of statistically inferred haplotypes with ASP. All presented P values are two-sided, and P < 0.05 was considered to represent statistical significance. The false discovery rate adjustment was applied for multiple comparisons.
Acknowledgements
The authors thank all the participants and their families. The study was supported by the National Natural Science Foundation of China (grant number: 81373022 and 81573172). The funding agencies had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Author Contributions
Hongjuan Chang was responsible for the data analysis, interpretation of the data, and drafting of the manuscript. Yizhen Yu was involved in the coordination of the project, conception of the study, and critical revision of the manuscript. Qiuge Yan was involved in the conception and design of the study and revision of the manuscript. Jie Tang, Juan Huang, Yanmei Zhang, Yuqiao Ma, Xiaozhou Ye, Lina Tang, Linguo Wu and Chunxia Wu conducted the data collection.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thylstrup B, Schroder S, Hesse M. Psycho-education for substance use and antisocial personality disorder: a randomized trial. BMC psychiatry. 2015;15:283. doi: 10.1186/s12888-015-0661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warren, F. et al. Review of treatments for severe personality disorder. Home Office Online Report30 (2003).
- 3.Association, A. P. Diagnostic and statistical manual of mental disorders (DSM-5®). (American Psychiatric Pub, 2013). [DOI] [PubMed]
- 4.Goodwin RD, Hamilton SP. Lifetime comorbidity of antisocial personality disorder and anxiety disorders among adults in the community. Psychiatry Res. 2003;117:159–166. doi: 10.1016/S0165-1781(02)00320-7. [DOI] [PubMed] [Google Scholar]
- 5.Merikangas KR, Kalaydjian A. Magnitude and impact of comorbidity of mental disorders from epidemiologic surveys. Curr Opin Psychiatry. 2007;20:353–358. doi: 10.1097/YCO.0b013e3281c61dc5. [DOI] [PubMed] [Google Scholar]
- 6.Coid J, Yang M, Tyrer P, Roberts A, Ullrich S. Prevalence and correlates of personality disorder in Great Britain. Br J Psychiatry. 2006;188:423–431. doi: 10.1192/bjp.188.5.423. [DOI] [PubMed] [Google Scholar]
- 7.Fazel S, Danesh J. Serious mental disorder in 23 000 prisoners: a systematic review of 62 surveys. The Lancet. 2002;359:545–550. doi: 10.1016/S0140-6736(02)07740-1. [DOI] [PubMed] [Google Scholar]
- 8.Justice JV, Young MH, Erdberg P. Assault in prison and assault in prison psychiatric treatment. Forensic Sci Int. 2003;49:1–9. [PubMed] [Google Scholar]
- 9.Garcia LF, Aluja A, Fibla J, Cuevas L, Garcia O. Incremental effect for antisocial personality disorder genetic risk combining 5-HTTLPR and 5-HTTVNTR polymorphisms. Psychiatry Res. 2010;177:161–166. doi: 10.1016/j.psychres.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Li JJ, Lee SS. Latent class analysis of antisocial behavior: interaction of serotonin transporter genotype and maltreatment. J Abnorm Child Psychol. 2010;38:789–801. doi: 10.1007/s10802-010-9409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latendresse SJ, et al. Differential susceptibility to adolescent externalizing trajectories: examining the interplay between CHRM2 and peer group antisocial behavior. Child Dev. 2011;82:1797–1814. doi: 10.1111/j.1467-8624.2011.01640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Q, et al. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Arch Gen Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- 13.Torgersen S, et al. Dimensional representations of DSM-IV cluster B personality disorders in a population-based sample of Norwegian twins: a multivariate study. Psychol Med. 2008;38:1617–1625. doi: 10.1017/S0033291708002924. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson KC, Prescott CA, Kendler KS. Sex differences in the genetic and environmental influences on the development of antisocial behavior. Dev Psychopathol. 2002;14:395–416. doi: 10.1017/S0954579402002110. [DOI] [PubMed] [Google Scholar]
- 15.Shah SS, Mohyuddin A, Colonna V, Mehdi SQ, Ayub Q. Monoamine Oxidase A gene polymorphisms and self reported aggressive behaviour in a Pakistani ethnic group. J Pak Med Assoc. 2015;65:818–824. [PubMed] [Google Scholar]
- 16.Reti IM, et al. Monoamine oxidase A regulates antisocial personality in whites with no history of physical abuse. Compr Psychiatry. 2011;52:188–194. doi: 10.1016/j.comppsych.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas K, et al. 5-HTTLPR as a potential moderator of the effects of adverse childhood experiences on risk of antisocial personality disorder. Psychiatr Genet. 2011;21:240–248. doi: 10.1097/YPG.0b013e3283457c15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrd AL, Manuck SB. MAOA, childhood maltreatment, and antisocial behavior: meta-analysis of a gene-environment interaction. Biol Psychiatry. 2014;75:9–17. doi: 10.1016/j.biopsych.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rautiainen MR, et al. Genome-wide association study of antisocial personality disorder. Transl Psychiatry. 2016;6:e883. doi: 10.1038/tp.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakahata Y, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao J, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunet, A. et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science303 (2004). [DOI] [PubMed]
- 24.Park S, Mori R, Shimokawa I. Do sirtuins promote mammalian longevity? A critical review on its relevance to the longevity effect induced by calorie restriction. Mol Cells. 2013;35:474–480. doi: 10.1007/s10059-013-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishi T, et al. SIRT1 gene, schizophrenia and bipolar disorder in the Japanese population: an association study. Genes Brain Behav. 2011;10:257–263. doi: 10.1111/j.1601-183X.2010.00661.x. [DOI] [PubMed] [Google Scholar]
- 26.Nakahata Y, et al. The NAD+-Dependent Deacetylase SIRT1 Modulates CLOCK-Mediated Chromatin Remodeling and Circadian Control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang RH, et al. Negative reciprocal regulation between Sirt1 and Per2 modulates the circadian clock and aging. Sci Rep. 2016;6:28633. doi: 10.1038/srep28633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takaesu Y, et al. Prevalence of Circadian Rhythm Sleep-Wake Disorders and Associated Factors in Euthymic Patients with Bipolar Disorder. PLoS One. 2016;11:e0159578. doi: 10.1371/journal.pone.0159578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bronsard G, Bartolomei F. Rhythms, rhythmicity and aggression. J Physiol Paris. 2013;107:327–334. doi: 10.1016/j.jphysparis.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature523, 588–591 (2015). [DOI] [PMC free article] [PubMed]
- 31.Libert S, et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147:1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 33.Caspi A, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 34.McCrory E, De Brito SA, Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry. 2010;51:1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- 35.Williams LM, et al. A polymorphism of the MAOA gene is associated with emotional brain markers and personality traits on an antisocial index. Neuropsychopharmacology. 2009;34:1797–1809. doi: 10.1038/npp.2009.1. [DOI] [PubMed] [Google Scholar]
- 36.Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA. Methylation at 5HTT mediates the impact of child sex abuse on women’s antisocial behavior: an examination of the Iowa adoptee sample. Psychosom Med. 2011;73:83–87. doi: 10.1097/PSY.0b013e3181fdd074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ficks CA, Waldman ID. Candidate genes for aggression and antisocial behavior: a meta-analysis of association studies of the 5HTTLPR and MAOA-uVNTR. Behav Genet. 2014;44:427–444. doi: 10.1007/s10519-014-9661-y. [DOI] [PubMed] [Google Scholar]
- 38.Salvatore, J. E. & Dick, D. M. Genetic Influences on Conduct Disorder. Neurosci Biobehav Rev (2016). [DOI] [PMC free article] [PubMed]
- 39.Pluess M, Belsky J. Conceptual issues in psychiatric gene-environment interaction research. Am J Psychiatry. 2012;169:222–223. doi: 10.1176/appi.ajp.2011.11111614. [DOI] [PubMed] [Google Scholar]
- 40.Salvatore JE, et al. Genome-wide association data suggest ABCB1 and immune-related gene sets may be involved in adult antisocial behavior. Transl Psychiatry. 2015;5:e558. doi: 10.1038/tp.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tielbeek JJ, et al. Unraveling the genetic etiology of adult antisocial behavior: a genome-wide association study. PLoS One. 2012;7:e45086. doi: 10.1371/journal.pone.0045086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunham I, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang WG, Bai XJ, Chen XM. SIRT1 variants are associated with aging in a healthy Han Chinese population. Clin Chim Acta. 2010;411:1679–1683. doi: 10.1016/j.cca.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 44.Lee J-Y, et al. Nicotinamide reduces dopamine in postnatal hypothalamus and causes dopamine-deficient phenotype. Neurosci Lett. 2009;461:163–166. doi: 10.1016/j.neulet.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Kishi T, et al. SIRT1 gene is associated with major depressive disorder in the Japanese population. J Affect Disord. 2010;126:167–173. doi: 10.1016/j.jad.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Michán S, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA. Methylation at 5HTT mediates the impact of child sex abuse on women’s antisocial behavior: an examination of the Iowa adoptee sample. Psychosom Med. 2011;73:83–87. doi: 10.1097/PSY.0b013e3181fdd074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasin D, et al. Personality disorders and the 3-year course of alcohol, drug, and nicotine use disorders. Arch Gen Psychiatry. 2011;68:1158–1167. doi: 10.1001/archgenpsychiatry.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chávez JX, Dinsmore JA, Hof DD. Assessing the incidence rates of substance use disorders among those with antisocial and borderline personality disorders in rural settings. Int J Psychol. 2010;6:57–66. [Google Scholar]
- 50.Compton WM, Conway KP, Stinson FS, Colliver JD, Grant BF. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J clin Psychiatry. 2005;66:677–685. doi: 10.4088/JCP.v66n0602. [DOI] [PubMed] [Google Scholar]
- 51.Moran P. The epidemiology of antisocial personality disorder. Soc Psychiatry Psychiatr Epidemiol. 1999;34:231–242. doi: 10.1007/s001270050138. [DOI] [PubMed] [Google Scholar]
- 52.Dick DM, Kendler KS. The impact of gene–environment interaction on alcohol use disorders. Alcohol Res. 2012;34:318–324. doi: 10.35946/arcr.v34.3.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zernicke KA, Cantrell H, Finn PR, Lucas J. The association between earlier age of first drink, disinhibited personality, and externalizing psychopathology in young adults. Addictive Behaviors. 2010;35:414–418. doi: 10.1016/j.addbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hyler SE, Skodol AE, Oldham JM, Kellman HD, Doidge N. Validity of the Personality Diagnostic Questionnaire-Revised: A replication in an outpatient sample. Compr Psychiatry. 1992;33:73–77. doi: 10.1016/0010-440X(92)90001-7. [DOI] [PubMed] [Google Scholar]
- 55.Kounou KB, et al. Childhood maltreatment and personality disorders in patients with a major depressive disorder: A comparative study between France and Togo. Transcult Psychiatry. 2015;52:681–699. doi: 10.1177/1363461515572001. [DOI] [PubMed] [Google Scholar]
- 56.Hyler, S. PDQ-4 and PDQ-4+: Instructions for use. Unpublished manuscript, Columbia University (1994).
- 57.Bernstein, D. P. & Fink, L. Childhood trauma questionnaire: A retrospective self-report: Manual. (Harcourt Brace & Company, 1998).


