Abstract
Huperzine A (Hup-A) is a poorly water-soluble drug with low oral bioavailability. A self-microemulsifying drug delivery system (SMEDDS) was used to enhance the oral bioavailability and lymphatic uptake and transport of Hup-A. A single-pass intestinal perfusion (SPIP) technique and a chylomicron flow-blocking approach were used to study its intestinal absorption, mesenteric lymph node distribution and intestinal lymphatic uptake. The value of the area under the plasma concentration–time curve (AUC) of Hup-A SMEDDS was significantly higher than that of a Hup-A suspension (P<0.01). The absorption rate constant (Ka) and the apparent permeability coefficient (Papp) for Hup-A in different parts of the intestine suggested a passive transport mechanism, and the values of Ka and Papp of Hup-A SMEDDS in the ileum were much higher than those in other intestinal segments. The determination of Hup-A concentration in mesenteric lymph nodes can be used to explain the intestinal lymphatic absorption of Hup-A SMEDDS. For Hup-A SMEDDS, the values of AUC and maximum plasma concentration (Cmax) of the blocking model were significantly lower than those of the control model (P<0.05). The proportion of lymphatic transport of Hup-A SMEDDS and Hup-A suspension were about 40% and 5%, respectively, suggesting that SMEDDS can significantly improve the intestinal lymphatic uptake and transport of Hup-A.
Key words: Huperzine A, Self-microemulsion, Drug delivery systems, SMEDDS, Bioavailability, Single-pass intestinal perfusion, Lymphatic transport
Graphical abstract
Compared with the Hup-A suspension, SMEDDS formulation can enhance the oral bioavailability and intestinal absorption of Hup-A. According to the detection of Hup-A concentration in mesenteric lymph nodes and the results of the chylomicron flow blocking experiments, Hup-A SMEDDS was confirmed to be absorbed through the lymphatic route.
1. Introduction
Self-microemulsifying drug delivery systems (SMEDDS), as a type of lipid-based oral drug delivery system, can significantly enhance the oral bioavailability of poorly water-soluble drugs1. SMEDDS may affect drug absorption in many ways, including enhancing drug solubilization, increasing membrane permeability in the gastrointestinal tract, and increasing lymphatic drug uptake2, 3. Water-insoluble drugs can be transported into the systemic circulation through the intestinal lymphatic system without first-pass metabolism in the liver and so can increase the oral bioavailability4. Lymphatic uptake has been proven to be an important factor to increase the oral bioavailability of numerous highly lipophilic drugs, including halofantrine5, moxidectin6, dichlorodiphenyltrichloroethane (DDT)7, 8, probucol9, cyclosporine A10, lycopene11, saquinavir12 and puerarin13, 14.
The absorption of drugs in the intestine is a fundamental aspect of oral administration. The absorption rate constant (Ka) and the apparent permeability coefficient (Papp) reflect the extent of intestinal drug absorption15. The single-pass intestinal perfusion (SPIP) model is used to determine drug concentration in intestinal perfusion fluid from the perfused intestinal segment, and it can directly describe the intestinal drug absorption16.
In the study of lymphatic drug transport, the lymph duct-cannulated approach is the most direct method to investigate intestinal lymphatic drug uptake. However, this method requires a high level of surgical skill and the rate of success is low. In recent years, an indirect pharmacological method (named “chylomicron flow-blocking approach”) has been used to evaluate intestinal lymphatic drug transport. This method utilizes the intestinal chylomicron flow inhibitors Pluronic-L81 and cycloheximide to study intestinal lymphatic transport17. Numerous studies have proven that measurement of lymphatic drug absorption using the chylomicron flow blocking approach correlates well with the lymph duct-cannulated approach13, 18, 19, 20.
Huperzine A (Hup-A), an alkaloid, is extracted from the traditional Chinese medicine Huperzia serrata (Thunb.) Trev21. Hup-A is a poorly water-soluble drug, easily soluble in methanol and ethanol, but insoluble in water. Thus, the present study was to prepare and characterize a SMEDDS formulation of Hup-A and to investigate the effect of Hup-A SMEDDS on intestinal absorption, mesenteric lymph nodes distribution, and intestinal lymphatic uptake with comparison to a Hup-A suspension, utilizing the SPIP approach and a chylomicron flow-blocking approach.
2. Materials and methods
2.1. Materials
Huperzine A (purity 99%) was purchased from Wanbangde Pharmaceutical Group Co., Ltd. (Zhejiang, China). Diphenhydramine hydrochloride (Lot No. 100066–200807) was purchased from the National Institutes for Food and Drug Control (Beijing, China). Propylene glycol was obtained from Tianjin Damao Chemical Reagent Factory (Tianjin, China). Polyoxyl 40 hydrogenated castor oil (Cremophor RH40®) was purchased from BASF, Germany. Castor oil was obtained from Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai, China). Cycloheximide was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Acetonitrile and methanol were HPLC grade and were supplied by the Oceanpak Alexative Chemical Co., Ltd. (Gothenburg, Sweden). Pure water was prepared by the Milli-Q Ultrapure water purification system (Millipore, Bedford, MA, USA). All other chemicals used in this study were analytical grade.
2.2. Preparation of Hup-A formulations
The composition of the SMEDDS was based on that used in our previous study with some modifications22, i.e., Hup-A SMEDDS was composed of castor oil (16%, w/w), Cremophor RH40 (50%, w/w) and propylene glycol (34%, w/w). Preparation of Hup-A SMEDDS was by simply mixing these components. Hup-A was initially dissolved in propylene glycol followed by dropping Cremophor RH40 and castor oil at room temperature until a homogeneous mixture formed. The mixture was stored overnight at room temperature. It subsequently was examined for signs of turbidity or phase separation before evaluation. The Hup-A suspension was prepared by dissolving Hup-A in 0.5% (w/v) sodium carboxymethyl cellulose (CMC-Na) solution by ultrasonication.
2.3. Characterization of the Hup-A-loaded self-microemulsion
The Hup-A SMEDDS was diluted 100-fold with distilled water and mixed by gentle shaking. Zetasizer Nano 3690 (Malvern Instruments Ltd., UK) was used to measure the particle size and zeta potential of the microemulsion at 25 °C23. Transmission electron microscopy (TEM; H-7650; Hitachi, Tokyo, Japan) was used to determine the morphology of microemulsion. After Hup-A SMEDDS was diluted 100-fold with distilled water, the sample was stained with 2% (w/v) phosphotungstic acid aqueous solution (PTA) for 5 min at 25 °C. Then one drop of stained sample was placed on a copper grid. After drying, it was examined under the TEM24.
2.4. Bioavailability study
Sprague-Dawley rats (male, 210–260 g; Center of Experimental Animals, Anhui, China; certificate No. SCXK (Wan) 2011-002) were utilized for all bioavailability and absorption studies. Animal experiments were performed according to the guidelines of our institution for the care and use of laboratory animals in Anhui University of Chinese Medicine (Hefei, China), and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals. All surgeries were performed under sodium pentobarbital anesthesia, and every effort was made to minimize suffering. The rats were fasted for 12 h with free access to water, and were divided into two groups at random before the experiments. The rats were administered a single oral dose of the Hup-A SMEDDS or Hup-A suspension and 3 mL of water was given to rats. Whole blood was collected from an eye socket vein into heparinized tubes at 0, 0.17, 0.33, 0.5, 1, 2, 4, 6, 8, 12 and 24 h after oral administration. After the whole blood was subjected to centrifugation (Multifuge X1R centrifuge, Thermo Fisher Scientific, MA, USA) at 10,000 rpm for 3 min, the supernatant was transferred into 5 mL tubes followed by addition of 50 μL of internal standard solution (diphenhydramine, 40 μg/L), a 100 μL solution of methanol:H2O (50:50, v/v), and 100 μL of phosphate buffer (pH 12). After the mixture was vortexed (SK-1 fast vortex mixer, Jintan Guowang Instruments Factory, Jiangsu, China) for 1 min, 3 mL of an extraction solvent of ethyl acetate:isopropanol (95:5, v/v) was added and vortexed for 10 min. The mixture was centrifuged at 4000 rpm for 5 min, and the organic layer was evaporated at 50 °C. The sample was reconstituted in 200 μL of mobile phase. The mixture was centrifuged at 5000 rpm for 5 min and the supernate was filtered and analyzed by UPLC/MS/MS.
2.5. Single-pass intestinal perfusion studies
Sprague-Dawley rats (male, 200–250 g) were fasted overnight with free access to water. The rats were divided into different groups at random before the experiments. The surgical procedure for the single-pass intestinal perfusion experiments was performed as previously described15, 25. The process was as follows: the rats were anesthetized with an intraperitoneal injection of sodium pentobarbital. The surgery was performed under a surgical lamp to keep the body temperature at 37 °C. After the abdomen was opened by a median incision of about 3 cm, the duodenum, jejunum, ileum, and colon was exposed and cannulated with flexible tube (approximately 10 cm) and then ligated at both ends. The surgery was performed gently to minimize the damage and keep blood circulation intact. A wet gauze was placed on the exposed intestinal segment to maintain moisture.
In this study, we explored the absorption of Hup-A in four different intestinal segments. The visible Peyer׳s patches (PPs) in ileum were ligatured with silk thread before the perfusion experiment in order to study the effect of ligature of PPs on the ileal absorption of Hup-A SMEDDS. In order to investigate whether the absorption of Hup-A was dose-dependent, the Hup-A SMEDDS and Hup-A suspension were dispersed in Krebs–Ringer׳s buffer at a low, middle and high drug concentration (5, 10 and 20 μg/mL) as the perfusion solution, and the perfusion solution was placed in a 37 °C water bath to keep the temperature.
At the beginning, in order to clean out any residual debris in the intestine, the isolated intestinal segment was rinsed with normal saline solution (37 °C) at a flow rate of 0.5 mL/min. The experimental intestinal segment was perfused with the perfusion solution at a flow rate of 0.25 mL/min for 30 min in order to achieve absorption equilibrium utilizing a peristaltic pump (HL-2; Shanghai Qingpu-Huxi Instruments Factory, Shanghai, China). The intestinal perfusion samples were collected at 10-min intervals for 90 min. All samples including perfusion samples were collected from inlet and outlet drug perfusion solution at different time points. All samples were filtered and analyzed by HPLC. All glass vials were weighted, respectively, before and after the perfusion experiment. At the end of the experiment, the length and radius of the perfused intestinal segments were carefully measured.
The gravimetric method was used to calculate the correction perfusion fluid volume change caused by intestinal moisture absorption. The 0.5 mL perfusion solution was put in the tube that had been weighed and the solution weight was used to calculate the perfusion solution density (ρin). Similarly, the 0.5 mL solution from all intestinal perfusion samples was put into tubes that had been weighed, and the intestinal perfusion samples density (ρout) was calculated. The Ka and Papp of Hup-A were calculated according the following Eqs. (1)–(5)26, 27:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
where min and mout are the weight (g) of the inlet perfusion solution and outlet perfusion solution, respectively; ρin and ρout are the density (g/mL) of the inlet perfusion solution and outlet perfusion solution, respectively; Vin and Vout are the volume (mL) of the inlet perfusion solution and outlet perfusion solution, respectively; Cin and Cout are the concentration (μg/mL) of the drug in the inlet perfusion solution and outlet perfusion solution, respectively. Q is the perfusion flow rate (0.25 mL/min); V is the volume (mL) of the perfused intestinal segment; l is the length (cm) of the perfused intestinal segment; r is the radius (cm) of the perfused intestinal segment.
2.6. Determination of Hup-A in perfusion samples by HPLC
All samples were analyzed by HPLC (Agilent 1100; Agilent technologies Inc., USA). The HPLC system consisted of a G1311A Quatpump, a G1322A Online solvent degasser and a G1315A DAD detector. Hup-A was fractionated by a C18 column (250 mm×4.6 mm, 5 μm; Agilent, USA) at 25 °C. The mobile phase was composed of acetonitrile and potassium dihydrogen orthophosphate buffer (0.02 mol/L; pH 2.5) (16:84, v/v). The flow rate was 1.0 mL/min. The detection wavelength was 308 nm. The method was linear over the range 0.5–25.0 μg/mL. The mean recovery of Hup-A after intestinal perfusion was 96.37±0.75%.
2.7. Assessment of drug concentration in mesenteric lymph nodes
For determination of Hup-A concentration in mesenteric lymph nodes, the rats were divided into different groups at random before the experiments. These rats were administered a single oral dose of Hup-A SMEDDS or Hup-A suspension and samples collected at different times (viz. 0, 0.5, 2, 4, 6, 8 and 10 h). The rats were euthanized via cervical dislocation at designated time points and the mesenteric lymph nodes were collected, washed, and carefully weighed. One mL of normal saline was added to each (15±3 mg, 4–5 nodes each). All samples were homogenized (Ultra-turrax homogenizer, IKA T18, IKA Werke GmbH & Co., Germany) for 5 min. After homogenization, these samples were treated as described above in Section 2.4.
2.8. Lymphatic uptake study
Sprague–Dawley rats (male, 220–250 g) were fasted for 12 h with free drinking water, and were divided into four groups at random before the experiments. One hour before the experiment, the rats were treated with either an intraperitoneal injection of 3 mg/kg cycloheximide solution in normal saline (0.6 mg/mL) or an equal volume of normal saline17, 18. After 1 h, the rats were further administered a single oral dose of the Hup-A SMEDDS or Hup-A suspension. Then, 3 mL of water was given and whole blood was collected from an eye socket vein in a heparinized tube at 0, 0.17, 0.33, 0.5, 1, 2, 4, 6, 8, 12 and 24 h after oral administration. After homogenization the samples were treated as described above in Section 2.4.
2.9. Determination of Hup-A in blood samples and mesenteric lymph nodes by UPLC/MS/MS
The UPLC equipment was an Agilent 1290 UPLC system. The UPLC system consisted of a binary pump, vacuum degasser, automatic injector and thermostatic column compartment (Agilent technologies Inc., USA). Hup-A was separated by a Waters Xbridge C18 column (50 mm×2.1 mm, 1.7 μm, Waters, USA) at 30 °C. The mobile phase was methanol and 0.1% formic acid solution in water (30:70, v/v). The flow rate was 0.2 mL/min. Mass spectrometric analysis was performed on a 4500 QTRAP triple quadrupole mass spectrometer. The triple quadrupole mass spectrometer was equipped with an electrospray ionization (ESI) source. The detection mode was the positive ionization mode (AB SCIEX, USA) and the scanning mode utilized multiple reaction monitoring (MRM). Quantitative analyses of ionic reactions were m/z 243.3→m/z 210.0 for Hup-A and m/z 256→m/z 167 for the internal standard (diphenhydramine). The main parameters for mass spectrometric analysis were set as follows: ionspray voltage was 5500 V; collision gas (He), nebulizer gas (N2) and curtain gas were 50, 50 and 45 psi, respectively; the declustering potential and collision energy were 90 and 39 V, respectively; the temperature of nebulizer gas was 500 °C. The method was linear over the range 0.1–10.0 μg/L. The mean absolute recovery of Hup-A and the internal standard in blood samples was 84.8% and 88.4%, respectively. The mean absolute recovery of Hup-A and the internal standard in mesenteric lymph nodes was 86.9% and 90.7%, respectively.
2.10. Data analysis and statistics
The pharmacokinetic parameters, including the area under the plasma concentration–time curve (AUC), the maximum plasma concentration (Cmax), and the time to reach maximum plasma concentration (Tmax) were calculated by the DAS 2.0 software (issued by the State Food and Drug Administration of China for Pharmokinetic Study). All data were analyzed by the SPSS statistical software (version 17.0; SPSS Inc., Chicago, USA), and expressed as mean±standard deviation (SD). Data with P<0.05 were considered to have statistical significance.
3. Results and discussion
3.1. Characterization of Hup-A–loaded self-microemulsion
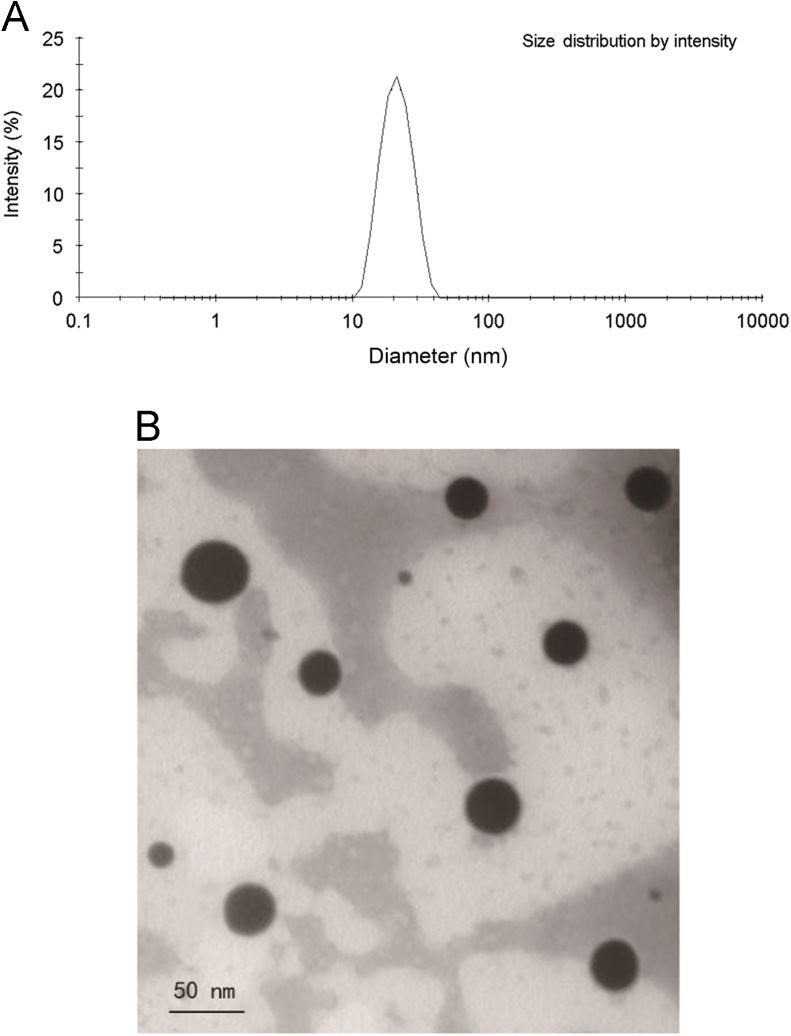
The mean droplet size of the Hup-A microemulsion was 20.33±0.68 nm with a polydispersity index (PDI) 0.050±0.004. The Zeta potential of the Hup-A microemulsion was −15.7±0.39 mV. The droplet size distribution is shown in Fig. 1A, suggesting that nanosized emulsion droplets were obtained in this experiment. TEM was used to observe the morphology of the Hup-A microemulsion. The morphological image is shown in Fig. 1B, in which the emulsion droplets with the size of 0–50 nm were spherical and uniform.
Figure 1.
(A) Size distribution of huperzine A SMEDDS in water; (B) TEM photograph of huperzine A microemulsion after negative staining.
3.2. Bioavailability studies
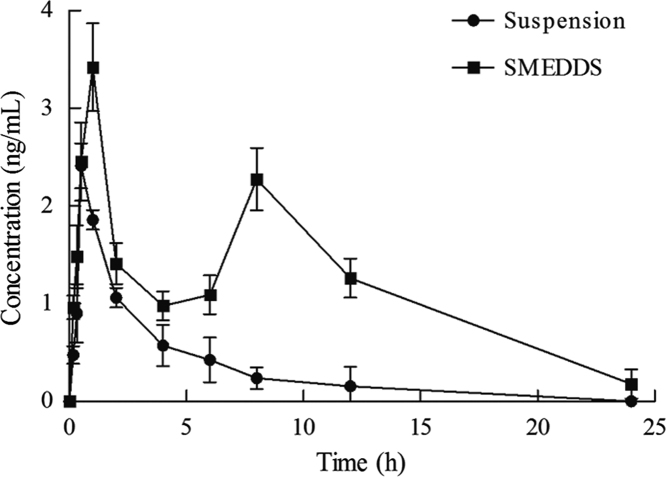
The value of Cmax of Hup-A SMEDDS was 1.42-fold (P<0.05, Table 1) greater than that of Hup-A suspension. In particular, the value of AUC of Hup-A SMEDDS was 21.07±4.59 ng·h/mL and Hup-A suspension was 10.05±2.70 ng·h/mL (P<0.01, Table 1). There was a significant improvement in the AUC of Hup-A SMEDDS compared with the Hup-A suspension. In the plasma concentration–time profile of Hup-A SMEDDS, a double-peak phenomenon was obtained, as shown in Fig. 2. It might be because Hup-A was excreted in the bile and underwent hepato-enteric circulation, which could lead to reabsorption in the intestine. In this study, the SMEDDS formulation had the smaller emulsion droplet, thus has faster drug release and the higher permeability in the intestine, which could enhance absorption in the intestine28. Moreover, when the emulsion was excreted in the bile, it may have an even smaller droplet29. For the Hup-A SMEDDS, the initial peak presumably was caused by the initial absorption of Hup-A in the gastrointestinal tract, and the later peak was caused by the hepato-enteric circulation and reabsorption of Hup-A. Therefore, the SMEDDS formulation can be used to improve the oral bioavailability of Hup-A.
Table 1.
Pharmacokinetic parameters of Hup-A in normal, saline- and cycloheximide-treated rats after oral administration of suspension and SMEDDS.
| Group | Pharmacokinetic parameter |
|||
|---|---|---|---|---|
| Cmax (ng/mL) | Tmax (h) | AUC (ng·h/mL) | ||
| Normal ratsa | SMEDDS | 3.42±0.45* | 1.07±0.16 | 21.07±4.59** |
| Suspension | 2.41±0.39 | 0.51±0.11 | 10.05±2.70 | |
| Saline-treated ratsb | SMEDDS | 3.45±0.42 | 1.03±0.54 | 20.16±4.42&& |
| Suspension | 2.44±0.31 | 0.49±0.10 | 9.97±2.65 | |
| Cycloheximide- treated ratsb | SMEDDS | 2.18±0.28# | 1.01±0.37 | 12.09±3.06## |
| Suspension | 2.16±0.23 | 0.50±0.11 | 9.50±2.25 | |
Data are expressed as mean±SD, n=6.
Bioavailability study.
Lymphatic transport study.
P<0.05,
P<0.01 versus suspension in rats;
P<0.05,
P<0.01 versus SMEDDS in saline-treated rats as the control;
P<0.01 versus suspension in saline-treated rats.
Figure 2.
The plasma drug concentration–time profiles of huperzine A in rats after oral administration of suspension and SMEDDS. Data are expressed as mean±SD, n=6.
3.3. Single-pass intestinal perfusion studies
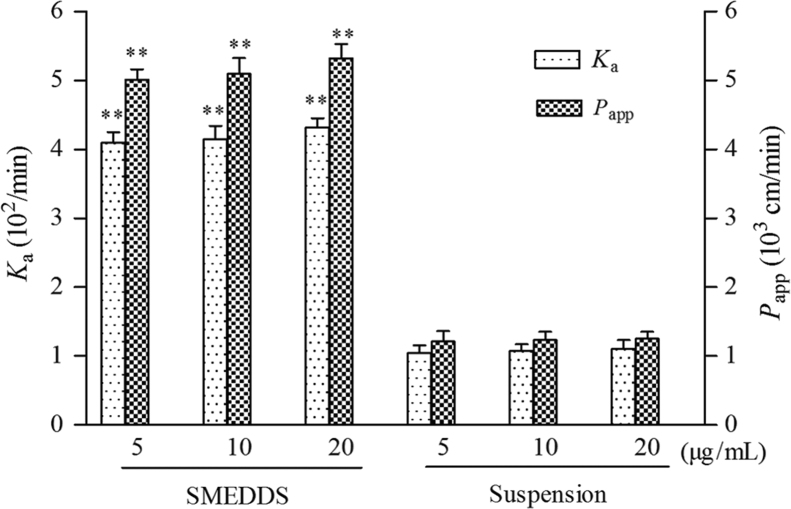
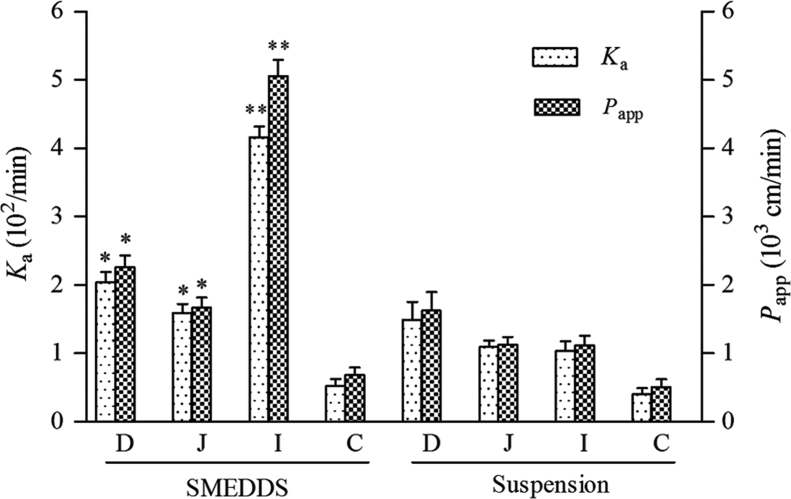
The results showed a concentration-independent absorption of two perfusion solutions (P>0.05), as shown in Fig. 3, and this indicated that the absorption mechanism of Hup-A was passive transport. In addition, the Ka and Papp of Hup-A SMEDDS were significantly greater than those for the Hup-A suspension (P<0.01, Fig. 3). This result may because the microemulsion has a smaller size (<50 nm), leading to an increase in contact area of drug with the gastrointestinal wall. In addition, because the microemulsion has smaller surface tension, it can easily contact intestinal epithelial cells, resulting in enhancing the intestinal absorption of drug30. The values of Ka and Papp of Hup-A SMEDDS in the ileum were significantly greater than those of other intestinal segments (P<0.05, Fig. 4).
Figure 3.
Comparison of Ka and Papp of huperzine A solutions at different concentrations determined by single-pass intestinal perfusion study in rat ileum. Data are expressed as mean±SD, n=6. **P<0.01 versus the same concentration of suspension.
Figure 4.
The Ka and Papp obtained for the huperzine A suspension and huperzine A SMEDDS using the single-pass intestinal perfusion technique in four different intestinal segments. Data are expressed as mean±SD, n=6. *P<0.05 versus the suspension in duodenum and Jejunum, respectively; **P<0.01 versus the suspension in ileum. D, duodenum; J, jejunum; I, ileum; C, colon.
Gut-associated lymphoid tissues (GALT) are found throughout the intestine in the form of isolated lymphoid follicles and organized follicular clusters such as ileal Peyer׳s patches (PPs)31. PPs are formed by groups of lymphoid follicles among the finger-like villi, which are covered by enterocytes. Microfold cells (M cells) are epithelium cells on the surfaces of lymphoid follicles31. Particles which reach the luminal surface of M cells are taken up by pinocytosis, carried in vesicles, and released into the M cells.
As previously reported, there are more M cells and PPs in the ileum than in other intestinal segments32. There have been also some studies reporting that drugs loaded in nanoparticles or microparticles were easily uptaken by PPs33. The microemulsion droplets may be absorbed via uptake into M cells, but this needs to be confirmed by further research. Thus, the significant differences in absorption between the ileum and the other three intestinal segments could be explained by the anatomical and physiological differences between rat ileum and the three other intestinal segments. A previously reported result gained further support in this study by ligating the PPs in the ileum. The values of Ka and Papp of Hup-A SMEDDS obtained by in situ intestinal perfusion with ligated PPs in the ileum are presented in Table 2. The values of Ka and Papp of Hup-A SMEDDS in the PPs ligatured ileum were significantly lower (P<0.05, Table 2) than those of the PPs without ligatured ileum. This experimental result indicates that PPs in the ileum significantly influence the ileal absorption of the SMEDDS formulation. Although the method of ligature of PPs may need further study, it at least provides a means to research the effect of PPs on drug intestinal absorption.
Table 2.
Influence of ligature of PPs on the ileal absorption of Hup-A SMEDDS.
| Group | Ka (102/min) | Papp (103 cm/min) |
|---|---|---|
| Ligature of PPs | 2.91±0.11 | 3.14±0.13 |
| Without ligature of PPs | 4.16±0.16* | 5.06±0.24* |
| The percentage of Ka values decreased (%) | 30.05 | – |
| The percentage of Papp values decreased (%) | – | 37.93 |
Data are expressed as mean±SD, n=6.
− Not applicable.
P<0.05 versus ligature of PPs.
3.4. In vivo drug concentration assessment in mesenteric lymph nodes
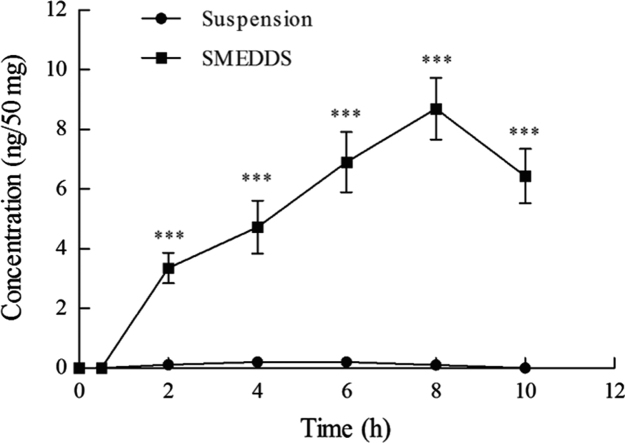
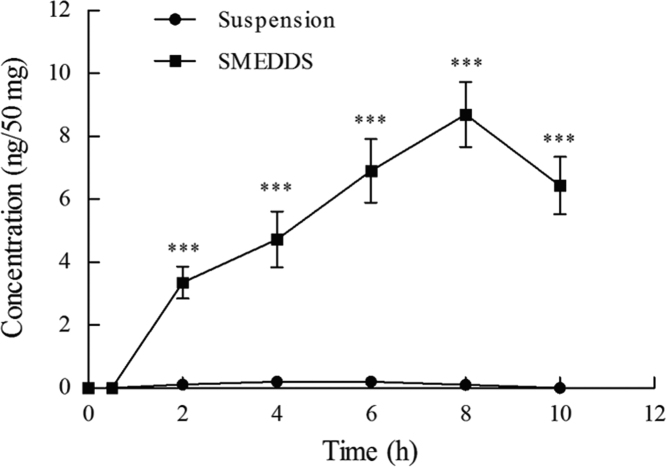
After the rats were administered the Hup-A suspension, a low concentration of Hup-A was found in the lymph nodes in all groups (Fig. 5). For the Hup-A SMEDDS preparation, a high concentration of Hup-A was found after 2 h, and an even higher value (8.70±1.03 ng/50 mg) was detected at 8 h (Fig. 5). After 10 h, the concentration of Hup-A begin to decline. These results affirm that the absorption of Hup-A SMEDDS is mainly via the intestinal lymphatic system whereas portal uptake is the main route for uptake of the Hup-A suspension.
Figure 5.
Huperzine A concentration in mesenteric lymph node of rats after oral administration of suspension and SMEDDS. Data are expressed as mean±SD, n=6. ***P<0.001 versus the suspension.
3.5. Lymphatic transport of the Hup-A SMEDDS
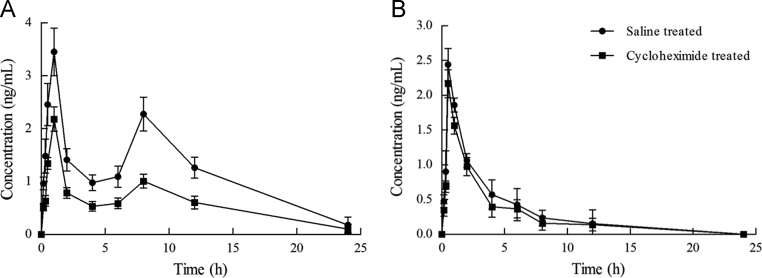
For Hup-A SMEDDS, as compared with the control model, the values of AUC and Cmax in the blocking model significantly decreased (P<0.05, Fig. 6A and Table 1). For the Hup-A suspension, the results indicate that there were no significant differences in AUC and Cmax between the control model and the blocking model (P>0.05, Fig. 6B and Table 1). Based on previously reported results, the proportion of lymphatic pathway transport can be calculated by subtracting the proportion delivered to the systemic circulation in rats pretreated with cycloheximide from the total bioavailability in rats pretreated with saline, and then dividing by the total bioavailability18, 19. The percentage of lymphatic pathway transport of Hup-A SMEDDS and Hup-A suspension were about 40% and 5%, respectively. The results of the chylomicron flow-blocking experiments confirmed that Hup-A SMEDDS was absorbed through the lymphatic route.
Figure 6.
(A) The plasma drug concentration–time profiles of huperzine A in rats treated with cycloheximide or saline after oral administration of SMEDDS. Data are expressed as mean±SD, n=6. (B) The plasma drug concentration–time profiles of huperzine A in rats treated with cycloheximide or saline after oral administration of suspension. Data are expressed as mean±SD, n=6.
Drugs absorbed via the intestinal lymph seem to enter into the lymphatic system by three routes: via the paracellular route by means of absorption enhancers; via the M cells and GALT; and via a transcellular route in association with the triglyceride core of the chylomicrons2. Although the exact mechanisms of lymphatic transport have not been fully elucidated, the third route was historically thought to be the major mechanism of lymphatic delivery of lipophilic drugs formulated with lipid-based vehicles2.
According to the results of the single pass perfusion studies, PPs play an important role in intestinal absorption of Hup-A SMEDDS. Does cycloheximide influence the lymphatic transport via the M cells except for its blocking chylomicron flow in enterocyte? Phagocytosis has been known to involve the remodeling of the actin cytoskeleton and is also required for local membrane exocytosis34, 35, 36. In addition, a labile protein was essential for endocytosis37. Cycloheximide is a non-specific protein synthesis inhibitor. Results of the amoebae study showed that phagocytosis was sensitive to cycloheximide and cell motility was blocked by cycloheximide38. Therefore, it can be speculated that cycloheximide can also inhibit the phagocytic activity of M cells and further block the pathway of lymphatic transport via M cells. In other words, cycloheximide can block lymphatic transport by both blocking chylomicron flow in enterocytes and inhibiting the phagocytic activity of M cells.
4. Conclusions
The SMEDDS formulation can enhance the oral bioavailability and intestinal absorption of Hup-A. According to the detection of Hup-A concentration in mesenteric lymph nodes and the results of the chylomicron flow-blocking experiments, we confirmed that Hup-A SMEDDS was absorbed through the lymphatic route. These results obtained in this study highlight the importance of lymphatic uptake on the enhanced oral bioavailability of Hup-A. Moreover, the transcellular route was historically thought to be the major mechanism of lymphatic transport of lipophilic drugs formulated in SMEDDS. However, the results of our research indicate that the route via M cells and GALT (PPs) might be another important route for lymphatic uptake of SMEDDS, in addition to the transcellular route. More studies are needed to determine the precise mechanism of lymphatic uptake of SMEDDS.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81274100; 81573615), Natural Science Foundation of Anhui Province of China (Grant No. 1408085QH189), Key Project for the Excellent Higher Education of Anhui Province of China (Grant No. 2013SQRL019ZD) and Research Project for the Science and Technology of Bozhou city of China (Grant No. BK2015005).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Porter C.J., Pouton C.W., Cuine J.F., Charman W.N. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv Drug Deliv Rev. 2008;60:673–691. doi: 10.1016/j.addr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 2.O׳Driscoll C.M. Lipid-based formulations for intestinal lymphatic delivery. Eur J Pharm Sci. 2002;15:405–415. doi: 10.1016/s0928-0987(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 3.O׳Driscoll C.M., Griffin B.T. Biopharmaceutical challenges associated with drugs with low aqueous solubility—the potential impact of lipid-based formulations. Adv Drug Deliv Rev. 2008;60:617–624. doi: 10.1016/j.addr.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Trevaskis N.L., Charman W.N., Porter C.J. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliv Rev. 2008;60:702–716. doi: 10.1016/j.addr.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trevaskis N.L., Caliph S.M., Nguyen G., Tso P., Charman W.N., Porter C.J. A mouse model to evaluate the impact of species, sex, and lipid load on lymphatic drug transport. Pharm Res. 2013;30:3254–3270. doi: 10.1007/s11095-013-1000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lespine A., Chanoit G., Bousquet-Melou A., Lallemand E., Bassissi F.M., Alvinerie M. Contribution of lymphatic transport to the systemic exposure of orally administered moxidectin in conscious lymph duct−cannulated dogs. Eur J Pharm Sci. 2006;27:37–43. doi: 10.1016/j.ejps.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Gershkovich P., Hoffman A. Effect of a high-fat meal on absorption and disposition of lipophilic compounds: the importance of degree of association with triglyceride-rich lipoproteins. Eur J Pharm Sci. 2007;32:24–32. doi: 10.1016/j.ejps.2007.05.109. [DOI] [PubMed] [Google Scholar]
- 8.Caliph S.M., Cao E., Bulitta J.B., Hu L., Han S., Porter C.J. The impact of lymphatic transport on the systemic disposition of lipophilic drugs. J Pharm Sci. 2013;102:2395–2408. doi: 10.1002/jps.23597. [DOI] [PubMed] [Google Scholar]
- 9.Sha X., Wu J., Chen Y., Fang X. Self-microemulsifying drug-delivery system for improved oral bioavailability of probucol: preparation and evaluation. Int J Nanomed. 2012;7:705–712. doi: 10.2147/IJN.S28052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawless E., Griffin B.T., O׳Mahony A., O׳Driscoll C.M. Exploring the impact of drug properties on the extent of intestinal lymphatic transport—in vitro and in vivo studies. Pharm Res. 2015;32:1817–1829. doi: 10.1007/s11095-014-1578-x. [DOI] [PubMed] [Google Scholar]
- 11.Faisal W., O׳Driscoll C.M., Griffin B.T. Bioavailability of lycopene in the rat: the role of intestinal lymphatic transport. J Pharm Pharmacol. 2010;62:323–331. doi: 10.1211/jpp.62.03.0006. [DOI] [PubMed] [Google Scholar]
- 12.Griffin B.T., O׳Driscoll C.M. A comparison of intestinal lymphatic transport and systemic bioavailability of saquinavir from three lipid-based formulations in the anaesthetised rat model. J Pharm Pharmacol. 2006;58:917–925. doi: 10.1211/jpp.58.7.0006. [DOI] [PubMed] [Google Scholar]
- 13.Tang T.T., Hu X.B., Liao D.H., Liu X.Y., Xiang D.X. Mechanisms of microemulsion enhancing the oral bioavailability of puerarin: comparison between oil-in-water and water-in-oil microemulsions using the single-pass intestinal perfusion method and a chylomicron flow blocking approach. Int J Nanomed. 2013;8:4415–4426. doi: 10.2147/IJN.S51469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou A., Lu T., Wang L., Lu C., Wang L., Wan M. Lymphatic transport of puerarin occurs after oral administration of different lipid-based formulations to unconscious lymph duct-cannulated rats. Pharm Dev Technol. 2014;19:743–747. doi: 10.3109/10837450.2013.829093. [DOI] [PubMed] [Google Scholar]
- 15.Dahan A., West B.T., Amidon G.L. Segmental-dependent membrane permeability along the intestine following oral drug administration: evaluation of a triple single-pass intestinal perfusion (TSPIP) approach in the rat. Eur J Pharm Sci. 2009;36:320–329. doi: 10.1016/j.ejps.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Lennernäs H. Animal data: the contributions of the using chamber and perfusion systems to predicting human oral drug delivery in vivo. Adv Drug Deliv Rev. 2007;59:1103–1120. doi: 10.1016/j.addr.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Dahan A., Hoffman A. Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur J Pharm Sci. 2005;24:381–388. doi: 10.1016/j.ejps.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Lind M.L., Jacobsen J., Holm R., Müllertz A. Intestinal lymphatic transport of halofantrine in rats assessed using a chylomicron flow blocking approach: the influence of polysorbate 60 and 80. Eur J Pharm Sci. 2008;35:211–218. doi: 10.1016/j.ejps.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Sun M., Zhai X., Xue K., Hu L., Yang X., Li G. Intestinal absorption and intestinal lymphatic transport of sirolimus from self-microemulsifying drug delivery systems assessed using the single-pass intestinal perfusion (SPIP) technique and a chylomicron flow blocking approach: linear correlation with oral bioavailabilities in rats. Eur J Pharm Sci. 2011;43:132–140. doi: 10.1016/j.ejps.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Sun D., Wei X., Xue X., Fang Z., Ren M., Lou H, et al. Enhanced oral absorption and therapeutic effect of acetylpuerarin based on D-α-tocopheryl polyethylene glycol 1000 succinate nanoemulsions. Int J Nanomed. 2014;9:3413–3423. doi: 10.2147/IJN.S63777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R., Tang X.C. Neuroprotective effects of huperzine A. A natural cholinesterase inhibitor for the treatment of Alzheimer׳s disease. Neurosignals. 2005;14:71–82. doi: 10.1159/000085387. [DOI] [PubMed] [Google Scholar]
- 22.Ye L., Hu R.F., Wang X.H., Tang J.H. Optimization of puerarin self-microemulsifying system using central composite design-response surface methodology. Chin Tra Pat Med. 2014;36:514–519. [Google Scholar]
- 23.Li X., Yuan Q., Huang Y., Zhou Y., Liu Y. Development of silymarin self-microemulsifying drug delivery system with enhanced oral bioavailability. AAPS PharmSciTech. 2010;11:672–678. doi: 10.1208/s12249-010-9432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh A.K., Chaurasiya A., Singh M., Upadhyay S.C., Mukherjee R., Khar R.K. Exemestane loaded self-microemulsifying drug delivery system (SMEDDS): development and optimization. AAPS PharmSciTech. 2008;9:628–634. doi: 10.1208/s12249-008-9080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao B., Cui C., Ji H., Tang J., Wang Z., Liu H. Enhanced oral bioavailability of piperine by self-emulsifying drug delivery systems: in vitro, in vivo and in situ intestinal permeability studies. Drug Deliv. 2015;22:740–747. doi: 10.3109/10717544.2014.898109. [DOI] [PubMed] [Google Scholar]
- 26.Nie S.F., Pan W.S., Yang X.G., Liu H.F., Liu Z.D. Evaluation of gravimetry in the rat single-pass intestinal perfusion technique. Chin J New Drugs. 2005;14:1176–1179. [Google Scholar]
- 27.Zhai X.Z., Huang L.Q., Liu S., Sun M.H., Si L.Q. Study on the rat intestinal absorption of rapamycin formulated in self-microemulsifying drug delivery system by single-pass intestinal perfusion technique. Chin Hosp Pharm J. 2011;31:217–221. [Google Scholar]
- 28.Porter C.J., Trevaskis N.L., Charman W.N. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov. 2007;6:231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z., Gao F., Jiang S., Chen L., Liu Z., Yu H. Bile salts enhance the intestinal absorption of lipophilic drug loaded lipid nanocarriers: mechanism and effect in rats. Int J Pharm. 2013;452:374–381. doi: 10.1016/j.ijpharm.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence M.J., Rees G.D. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2012;64:175–193. doi: 10.1016/s0169-409x(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 31.Owen R.L. Uptake and transport of intestinal macromolecules and microorganisms by M cells in Peyer׳s patches—a personal and historical perspective. Semin Immunol. 1999;11:157–163. doi: 10.1006/smim.1999.0171. [DOI] [PubMed] [Google Scholar]
- 32.Sato S., Kaneto S., Shibata N., Takahashi Y., Okura H., Yuki Y. Transcription factor Spi-B-dependent and -independent pathways for the development of Peyer׳s patch M cells. Mucosal Immunol. 2013;6:838–846. doi: 10.1038/mi.2012.122. [DOI] [PubMed] [Google Scholar]
- 33.Shakweh M., Besnard M., Nicolas V., Fattal E. Poly(lactide-co-glycolide) particles of different physicochemical properties and their uptake by Peyer׳s patches in mice. Eur J Pharm Biopharm. 2005;61:1–13. doi: 10.1016/j.ejpb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Stossel T.P. Contractile proteins in phagocytosis: an example of cell surface-to-cytoplasm communication. Fed Proc. 1977;36:2181–2184. [PubMed] [Google Scholar]
- 35.Hackam D.J., Rotstein O.D., Sjolin C., Schreiber A.D., Trimble W.S., Grinstein S. V-SNARE--dependent secretion is required for phagocytosis. Proc Natl Acad Sci U S A. 1998;95:11691–11696. doi: 10.1073/pnas.95.20.11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di A., Nelson D.J., Bindokas V., Brown M.E., Libunao F., Palfrey H.C. Dynamin regulates focal exocytosis in phagocytosing macrophages. Mol Biol Cell. 2003;14:2016–2028. doi: 10.1091/mbc.E02-09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez C., Satre M. Endocytosis in Dictyostelium discoideum amoebae. Inhibition by cycloheximide and other inhibitors of protein synthesis. J Cell Sci. 1991;99:535–543. [Google Scholar]
- 38.Clotworthy M., Traynor D. On the effects of cycloheximide on cell motility and polarisation in Dictyostelium discoideum. BMC Cell Biol. 2006;7:5. doi: 10.1186/1471-2121-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]