Abstract
Poly(thionine)-Au, a novel multifunctional substrate with excellent redox signal, enzyme-like activity, and easy antibody immobilisation, was synthesised using HAuCl4 as the oxidising agent and thionine as the monomer. The prepared poly(thionine)-Au composite exhibited an admirable electrochemical redox signal at −0.15 V and excellent H2O2 catalytic ability. In addition, gold nanoparticles in this composite were found to directly immobilise antibodies and further improve conductivity. In addition, a label-free electrochemical immunosensor was developed using poly(thionine)-Au as the sensing substrate for ultrasensitive detection of cytokeratin antigen 21-1 (CYFRA 21-1), an immunoassay found in human serum. The prepared immunosensor showed a wide liner range from 100 ng mL−1 to 10 fg mL−1 and an ultralow detection limit of 4.6 fg mL−1 (the ratio of signal to noise (S/N) = 3). Additionally, this method was used to analyse human serum samples and yielded results consistency with those of ELISA, implying its potential application in clinical research. The poly(thionine)-Au composite can be easily extended to other polymer-based nanocomposites, which is significant for other electrochemical immunoassays.
Introduction
Early detection of cancer, one of the leading causes of death worldwide, is critical for successful treatment of the disease and to increase patient survival rates1–3. Tumour markers are chemical substances related to cancers, and their determination plays an important role in early detection4–11. To date, great efforts have been made to detect such markers, including enzyme-linked immunosorbent assay (ELISA), fluorescent immunoassay, and chemiluminescence enzyme immunoassay12–16. Although these methods offer some advantages, there are numerous inevitable drawbacks, such as enzyme deactivation and time-consuming processes. Thus, considerable attention has been devoted to develop a label-free electrochemical immunoassay, due to its desirable properties, including high sensitivity, efficiency, low cost, and user-friendly instrumentation17–24.
In a label-free electrochemical immunosensor, the following three aims are extraordinarily important for the sensing substrate: (1) adhering the redox species; (2) enhancing electrochemical signal; and (3) immobilising antibodies. Above all, redox species are indispensable for label-free electrochemical immunosensors and can be implemented in the following three ways25–33. One method is by adding the redox species into electrolyte solutions. However, high concentrations of redox species potentially decrease the bioactivity of antibodies or antigens. Redox species can also be modified directly on the substrate by chemical bonds. Yet, this will make the modification process of electrode complicated and tedious. Alternatively, redox species can be adsorbed onto an electrode and covered by a polymer film. The drawback here is the possibility of redox species leakage, which would affect the stability of the label-free electrochemical immunosensor. Although previous studies have introduced enzyme catalysis to achieve a higher electrochemical signal, this could increase the complication and expenses of the immunosensor preparation34–36. Considering the above situations, it is of great significance to develop a new type of multifunctional substrate that can provide a redox signal, amplify the signal, and immobilise antibodies for use in a label-free electrochemical immunosensor.
Herein, we synthesised a novel multifunctional poly(thionine)-Au nanocomposite using HAuCl4 as the oxidising agent and thionine as the monomer. The as-prepared nanocomposites displayed excellent redox activity and H2O2 catalytic ability, signal amplification, antibody immobilisation, and good conductivity with a strong single electrochemical redox signal at −0.15 V. Based on these outstanding properties, the poly(thionine)-Au nanocomposite was used as the sensing substrate to develop a label-free electrochemical immunosensor. Cytokeratin antigen 21-1 (CYFRA 21-1) was chosen as the model analyte to be detected. The proposed immunosensor exhibited superior performance, and the detection results were in good agreement with those of ELISA.
Results and Discussion
The TEM images were used to investigate the morphology of poly(thionine)-Au nanocomposite. In Fig. 1, the gold nanoparticles (AuNPs) are uniformly distributed on poly(thionine). The chemical composition of poly(thionine)-Au was analysed by XPS, and the overview spectrum in Figure S1A reveals the presence of C, N, O, S, and Au atoms in the composite. The carbon component is attributed to the backbone of the benzene ring, and the N is ascribed to secondary amine groups or tertiary amino groups in the composite. Further, the Au 4 f doublet (84.1 and 87.8 eV) in Figure S1B is consistent with the Au° state. These results demonstrate that poly(thionine)-Au was successfully formed.
Figure 1.
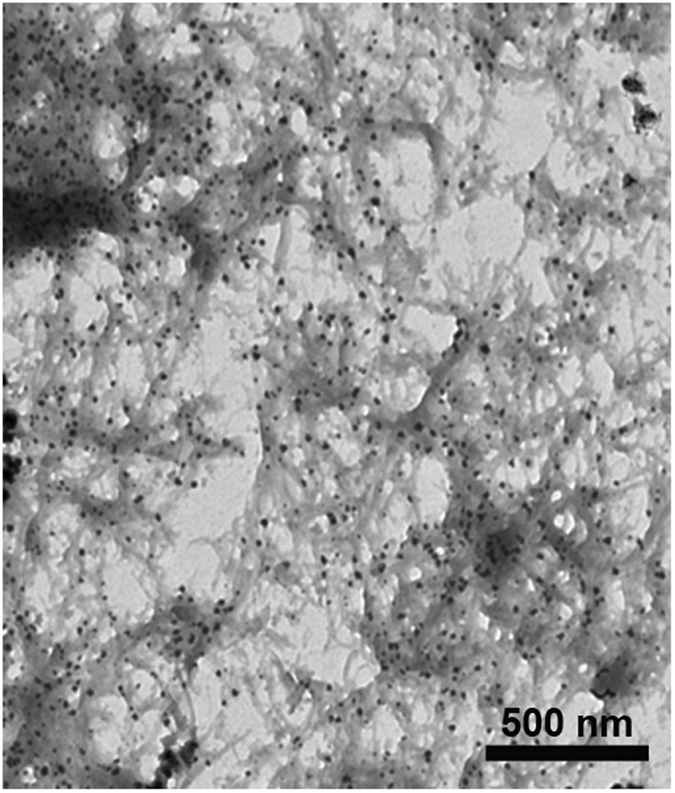
TEM images of poly(thionine)-Au nanocomposite.
In addition, the electrochemical redox activity, catalytic ability of poly(thionine)-Au toward H2O2, and its conductivity were investigated. As shown in Fig. 2A, poly(thionine)-Au exhibited a strong electrochemical signal at −0.15 V, suggesting that it is preconditioned for use as an immunosensing substrate in a label-free electrochemical immunosensor. In addition, amperometric i-t was conducted at −0.1 V in 30 mL PBS in which the current response increased after adding 5 mM H2O2 into electrolyte solution (Fig. 2B), which indicates that poly(thionine)-Au exhibited excellent H2O2 catalytic ability. Electrochemical impedance spectroscopy (EIS) was used to investigate the interfacial properties of the electrode modified with the poly(thionine)-Au composite. The Nyquist plot of the poly(thionine)-Au-modified glassy carbon electrode (GCE) (Fig. 2C, curve b) shows a semicircle with a smaller diameter compared to the bare GCE (Fig. 2C, curve a), verifying good conductivity of the nanocomposites.
Figure 2.
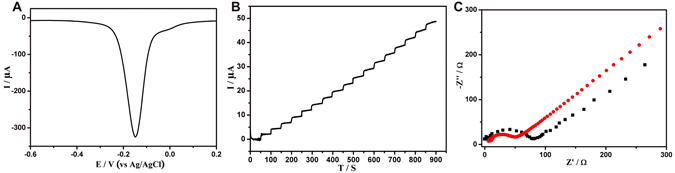
The poly(thionine)-Au nanocomposite exhibited strong redox activity at −0.15 V (A), ameperometic response of poly(thionine)-Au for successive addition of 5 mM H2O2 (B), and the EIS of bare GCE (curve a), GCE modified with poly(thionine)-Au (curve b) (C).
Poly(thionine) has been used in various electrochemical sensors due to its admirable electrochemical signal and good H2O2 catalytic ability, and thionine can be polymerised into poly(thionine)-Au using HAuCl4 as the oxidizing agent37, 38. According to our results, the composite possessed the following outstanding advantages: (1) poly(thionine) displayed strong current signal at −0.15 V and excellent H2O2 catalytic ability; (2) AuNPs can immobilise the antibody and further improve the electron transfer ability; and (3) the fabrication of this composite only requires an easy one-step method. Based on these merits, poly(thionine)-Au nanocomposite is a promising substrate for a label-free electrochemical immunoassay.
Herein, the drop-coating method was used to apply the poly(thionine)-Au film onto the glassy carbon electrode (GCE) (Fig. 3). The film was found to catalyse H2O2 and enhance the current signal at −0.15 V. Following that, the modified electrode was used to adsorb anti-CYFRA 21-1, and excessive antibodies were removed by ultrapure water. After blocking with BSA, a label-free electrochemical immunosensor for CYFRA 21-1 was obtained. The current responses at −0.15 V were proportionate to the logarithm values of CYFRA21-1 concentrations.
Figure 3.
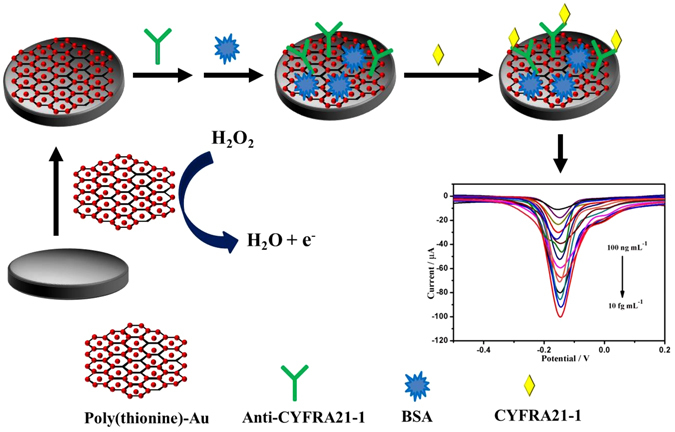
Schematic illustration of the fabrication process of the immunosensing interface.
Square wave voltammetry (SWV) measurements were used to monitor the electrochemical behaviour of the modification procedure after each step in a phosphate buffer saline (PBS) containing 5 mM H2O2. In Fig. 4, the bare GCE (curve a) did not display an electrochemical signal, but a strong current response of −0.15 V was obtained after modifying the electrode with poly(thionine)-Au (curve b). Subsequently, the loading of anti-CYFRA 21-1 led to an obvious decrease in the peak current (curve c), owing to the formation of an electron-blocking layer. The current response further decreased after the immunosensor was blocked with BSA (curve d) and incubated in a solution of 0.5 ng mL−1 CYFRA 21-1 (curve e). This originated from the insulating layers of BSA and CYFRA 21-1 protein on the electrode, which retarded the electron transfer.
Figure 4.
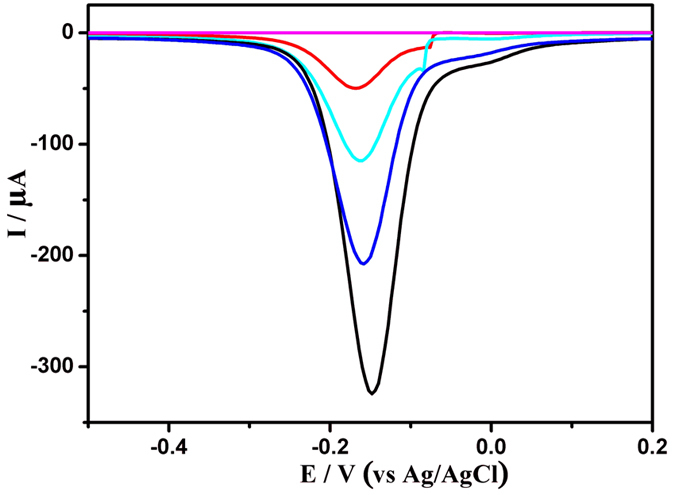
SWV responses of the modified procedure of electrodes in 0.1 M PBS and 5 mM H2O2 (pH 7.0). (a) bare GCE; (b) poly(thionine)-Au nanocomposite modified GCE; (c) anti-CYFRA21-1/poly(thionine)-Au nanocomposite modified GCE; (d) blocked with 1% BSA; (e) modified glassy carbon electrode after incubation with 0.5 ng mL−1 CYFRA21-1.
Considering that incubation time directly influences the combination of antibody and antigen, the effect of the incubation time on the immune reaction was investigated. As shown in Figure S2A, the peak current increased with the increase in incubation time, and then kept constant after 50 min. In this case, 50 min was used as the incubation time for the immunoassay. In order to optimise the incubation pH, the effect of pH on the immune reaction was also studied. As shown in Figure S2B, the varied current (∆I) increased when the pH value was decreased to less than 7.0 and decreased when the pH value increased to greater than 7.0. Thus, a pH value of 7.0 was used in subsequent experiments.
The analytical performance of this immunosensor was also tested by SWV. In this experiment, 5 mM H2O2 was added into the electrolyte solution for signal amplification. The current response of the immunosensor decreased with increasing concentrations of CYFRA 21-1 (Fig. 5A). The immunosensor displayed good linear relation ranging from 100 ng mL−1 to 10 fg mL−1 with an ultralow detection limit of 4.6 fg mL−1 (S/N = 3). Compared with the previous reports (Table S1), it was found that the poly(thionine)-Au-based immunosensor exhibited higher sensitivity, a wider linear range, and lower detection limit. The exceptionally fast response and high sensitivity are attributed to the strong electrochemical signals, excellent H2O2 catalytic ability, and further improvement in the conductivity due to AuNPs.
Figure 5.
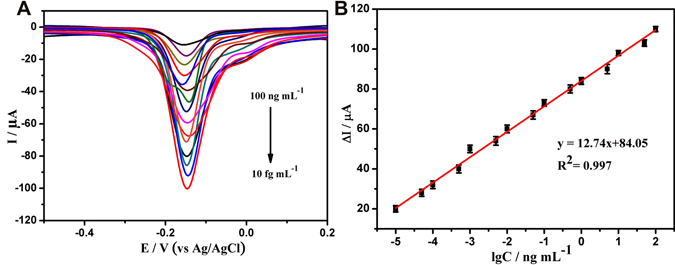
SWV responses of electrochemical immunoassay in 0.1 M PBS and 5 mM H2O2 (pH 7.0), curves a–i correspond to CYFRA21-1 at the concentrations from 100 ng mL−1 to 10 fg mL−1. (B) The calibration plot between the SWV peak current and the logarithm values of CYFRA21-1 concentrations. Error bars represent standard deviation, n = 3.
To investigate the reliability and accuracy of the above linear relationship, the CYFRA 21-1 content of twenty human serum samples (20 μL each sample) was measured three times with both this immunoassay and ELISA, and the results are shown in Table 1. It can be seen that the relative standard error was less than 8%, indicating good reliability and accuracy of this method.
Table 1.
Assay results of clinical serum samples using the proposed method and ELISA.
| Sample | Proposed immunosensor (ng mL−1) | ELISA (ng mL−1) | Relative error (%) |
|---|---|---|---|
| 1 | 0.80 | 0.81 | −1.23 |
| 2 | 1.30 | 1.27 | 2.36 |
| 3 | 0.60 | 0.65 | −7.69 |
| 4 | 0.60 | 0.61 | −1.64 |
| 5 | 0.60 | 0.61 | −1.64 |
| 6 | 0.75 | 0.74 | 1.35 |
| 7 | 0.80 | 0.82 | −2.44 |
| 8 | 0.75 | 0.74 | 1.35 |
| 9 | 0.90 | 0.90 | 0.00 |
| 10 | 0.90 | 0.85 | 5.89 |
| 11 | 0.90 | 0.91 | −1.10 |
| 12 | 0.95 | 0.90 | 5.96 |
| 13 | 1.05 | 1.03 | 1.94 |
| 14 | 0.95 | 0.91 | 4.34 |
| 15 | 1.00 | 0.99 | 1.01 |
| 16 | 0.75 | 0.74 | 1.35 |
| 17 | 0.75 | 0.77 | −2.50 |
| 18 | 0.65 | 0.65 | 0.00 |
| 19 | 0.70 | 0.74 | −5.40 |
| 20 | 0.60 | 0.61 | −1.64 |
Selectivity is an important characteristic and it is necessary to test it for the present immunoassay. Numerous analytes, NSE, AFP, BSA, IgG, PSA, AA, and CEA, were used to further test the selectivity of the immunosensor. When the immunosensor was incubated with 10 ng mL−1 NSE, AFP, BSA, IgG, PSA, AA, and CEA solution, no obvious changes in the current were observed compared to the blank test (no target analyte) in the same testing conditions, as shown in Fig. 6. However, when CYFRA 21-1 coexisted with interferences, the electrochemical responses were almost the same as that with only CYFRA 21-1. All results indicate that the proposed immunosensor has good specificity for CYFRA 21-1. To test the reproducibility of the immunosensor, five electrodes were prepared for the detection of 0.5 ng mL−1 CYFRA 21-1. The relative standard deviation (RSD) of the five electrodes was determined to be 5.3%, suggesting that the reproducibility of the proposed immunosensor is adequate. To further examine the stability of the immunoassay, the immunosensor was stored at 4 °C for about four weeks, and then its electrochemical property was measured. The change in current responses was less than 10%, revealing that the stability of the immunosensor is acceptable (Figure S3).
Figure 6.
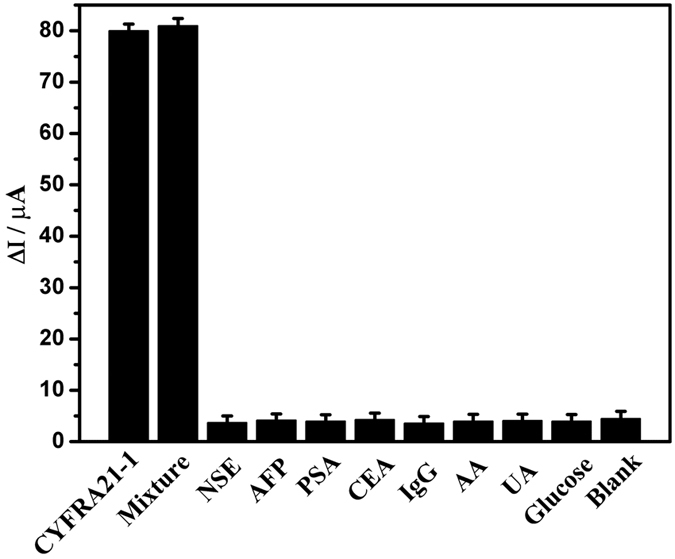
Anti-reference ability of the immunoassay (The error bars are standard deviations for n = 3). The concentrations of IgG, AA, UA, glucose, CEA, PSA, NSE and AFP were 10 ng mL−1. The mixture contains IgG (10 ng mL−1), AA (10 ng mL−1), UA (10 ng mL−1), glucose (10 ng mL−1), CEA (10 ng mL−1), PSA (10 ng mL−1), NSE (10 ng mL−1) AFP (10 ng mL−1), and CYFRA21-1 (0.5 ng mL−1).
Conclusion
In this work, a multifunctional substrate was synthesised using HAuCl4 as the oxidising agent and thionine as the monomer. The as-prepared composite exhibited admirable electrochemical redox activity at −0.15 V, excellent H2O2 catalytic ability, and easy antibody immobilisation. The poly(thionine)-Au nanocomposite was used as sensing substrate to fabricate a label-free electrochemical immunosensor, and the ultrasensitive detection of CYFRA 21-1 was realised. This immunoassay displayed good sensitivity in a wide detection range from 100 ng mL−1 to 10 fg mL−1 and an ultralow detection limit of 4.6 fg mL−1 (S/N = 3). Further, the immunosensor produced consistent results with those of ELISA for detection of human serum samples. The present method is significant for preparing and utilising other label-free amperometric immunosensors using poly(thionine)-Au.
Methods
Materials
Ascorbic acid (AA), and hydrogen tetrachloroaurate hydrate (HAuCl4·xH2O, 99.9%) were purchased from Alfa Aesar China (Tianjin). Mouse anti human monoclonal antibody to cytokeratins antigen 21-1 (anti-CYFRA21-1), CYFRA21-1, neuron-specific enolase (NSE), carcinoembryonic antigen (CEA), alpha fetoprotein (AFP), prostate specific antigen (PSA), were obtained from Shanghai Linc-Bio Science Co., Human immunoglobulin G (IgG), was obtained from Chengwen Biological Company (Beijing, China). Bovine serum albumin (BSA), thionine, hydrochloric acid (HCl, 36.0–38.0%), KCl, NaH2PO4, Na2HPO4, K3Fe(CN)6, K4Fe(CN)6, were purchased from Beijing Chemical Reagents Company (Beijing, China). Clinical human serum samples were obtained from Capital Normal University Hospital (Beijing, China). All other reagents were of analytical grade and used without further purification. Ultrapure water was used in all experiments (resistivity = 18 MΩ).
Apparatus
All electrochemical measurements were carried out on a CHI832 electrochemical workstation (Chenhua Instruments Co., Shanghai, China). Transmission electron microscopy (TEM) images were obtained from a JEOL-100CX electron microscope under 80 kV accelerating voltage (H7650, Hitachi, Japan). X-ray photoelectron spectroscopy (XPS) analysis was obtained from an ESCALAB 250 X-ray photoelectron spectroscope (Thermofisher, American). Ultrapure water used in all procedures was purified through an Olst ultrapure K8 apparatus (Olst, Ltd.). A three electrochemical system in the experiment was composed of a glassy carbon electrode (GCE) (4 mm in diameter) as the working electrode, a platinum wire and an Ag/AgCl electrode as counter electrode and reference electrode, respectively.
Synthesis of poly(thionine)-Au
To synthesise the poly(thionine)-Au nanocomposite, thionine aqueous solution (1%, w/w) and HAuCl4 (4%, w/w) were mixed and stirred vigorously for 3 hours at 26 °C. The resulting mixture was then collected by centrifugation at 16000 rpm for 15 min and washed three times with ultrapure water.
Fabrication of immunosensor
Prior to the functionalisation procedure, a GCE with a diameter of 4 mm was polished using 0.05 μm alumina slurry and followed by ultrasonic cleaning several times in deionized-distilled water. After that, 15 μL dispersion solution of poly(thionine)-Au was dropped onto the surface of the pretreated GCE and allowed to form a thin homogeneous film for 30 min. Next, 80 μL anti-CYFRA21-1 (200 μg mL−1) was incubated on the modified electrode at 37 °C overnight. Finally, the resulting modified electrode was further incubated with a solution of BSA (1%, w/w) for 1 h at 37 °C to block the remaining active sites from non-specific absorption. The modified electrode was thoroughly rinsed with ultrapure water, and the desired immunosensor was finally obtained and stored at 4 °C prior to use.
Electronic supplementary material
Acknowledgements
This research was financed by grants from the National Natural Science Foundation of China (21273153, 21673143), Natural Science Foundation of Beijing Municipality (2172016, 2132008), and the Project of the Construction of Scientific Research Base by the Beijing Municipal Education Commission.
Author Contributions
Z.M. supervised the project. The experiments were carried out by H.W. and X.G. and H.W. wrote the main manuscript text and discussed the results and experimental conditions. All authors have given approval to the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01250-0
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang MH, Gong SQ. Immunosensor for the detection of cancer biomarker based on percolated graphene thin film. Chem. Commun. 2010;46:5796–5798. doi: 10.1039/c0cc00675k. [DOI] [PubMed] [Google Scholar]
- 2.Barak V, Goike H, Panaretakis KW, Einarsson R. Clinical utility of cytokeratins as tumor markers. Clin. Biochem. 2004;37:529–540. doi: 10.1016/j.clinbiochem.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Ogaidi IA, Aguilar ZP, Suri S, Gou HL, Wu NQ. Dual detection of cancer biomarker CA125 using absorbance and electrochemical methods. Analyst. 2013;138:5647–5653. doi: 10.1039/c3an00668a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu N, Wang ZF, Ma ZF. Platinum porous nanoparticles for the detection of cancer biomarkers: what are the advantages over existing techniques? Bioanalysis. 2014;6:903–905. doi: 10.4155/bio.14.32. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Zhong ZY, Chai YQ, Song ZJ, Zhuo Y, Su HL, Liu SM, Wang D, Yuan R. Simultaneous electrochemical immunoassay of three liver cancerbiomarkers using distinguishable redox probes as signal tags andgold nanoparticles coated carbon nanotubes as signal enhancers. Chem. Commun. 2012;48:537–539. doi: 10.1039/C1CC14886A. [DOI] [PubMed] [Google Scholar]
- 6.Duan RX, Zuo XL, Wang ST, Quan XY, Chen DL, Chen ZF, Jiang L, Fan CH, Xia F. Lab in a Tube: Ultrasensitive detection of microRNAs at the single-Cell level and in breast cancer patients using quadratic isothermal amplification. J. Am. Chem. Soc. 2013;135:4604–4607. doi: 10.1021/ja311313b. [DOI] [PubMed] [Google Scholar]
- 7.Zhao B, Yan J, Wang DF, Ge ZL, He SJ, He DN, Song SP, Fan CH. Carbon nanotubes multifunctionalized by rolling circle amplification and their application for highly sensitive detection of cancer markers. Small. 2013;9:2595–2601. doi: 10.1002/smll.201202957. [DOI] [PubMed] [Google Scholar]
- 8.Liu YQ, Zhang M, Yin BC, Ye BC. Attomolar ultrasensitive microRNA detection by dNA-Scaffolded silver-Nanocluster probe based on isothermal amplification. Anal. Chem. 2012;84:5165–5169. doi: 10.1021/ac300483f. [DOI] [PubMed] [Google Scholar]
- 9.Wang CY, Hou F, Ma YC. Simultaneous quantitative detection of multiple tumor markers with a rapid and sensitive multicolor quantum dots based immunochromatographic test strip. Biosens. Bioelectron. 2015;68:156–162. doi: 10.1016/j.bios.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 10.Lai GS, Wu J, Ju HX, Yan F. Streptavidin-functionalized silver-nanoparticle-enriched carbon nanotube tag for ultrasensitive multiplexed detection of tumor markers. Adv. Funct. Mater. 2011;21:2938–2943. doi: 10.1002/adfm.201100396. [DOI] [Google Scholar]
- 11.Zhao YJ, Zhao XW, Hu J, Xu M, Zhao WT, Sun LG, Zhu C, Xu H, Gu ZZ. Encoded porous Bbeads for label-free multiplex detection of tumor markers. Adv. Mater. 2009;21:569–572. doi: 10.1002/adma.200802339. [DOI] [PubMed] [Google Scholar]
- 12.Chikkaveeraiah BV, Bhirde AA, Morgan NY, Eden HS, Chen XY. Electrochemical Immunosensors for Detection of Cancer Protein Biomarkers. ACS Nano. 2012;6:6546–6561. doi: 10.1021/nn3023969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacBeath G. Protein microarrays and proteomics. Nat. Genet. 2002;32:526–532. doi: 10.1038/ng1037. [DOI] [PubMed] [Google Scholar]
- 14.Gooneilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. 2007;33:266–270. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Gu BX, Xu CX, Yang C, Liu SQ, Wang ML. ZnO quantum dot labeled immunosensor for carbohydrate antigen 19-9. Biosens. Bioelectron. 2011;26:2720–2723. doi: 10.1016/j.bios.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Angenendt P, Glokler J, Konthur Z, Lehrach H, Cahill DJ. 3D Protein Microarrays: Performing Multiplex Immunoassays on a Single Chip. Anal. Chem. 2003;75:4368–4372. doi: 10.1021/ac034260l. [DOI] [PubMed] [Google Scholar]
- 17.Li JP, Li SH, Yang CF. Electrochemical biosensors for cancer biomarker detection. Electroanalysis. 2012;24:2213–2229. doi: 10.1002/elan.201200447. [DOI] [Google Scholar]
- 18.Liao F, Yang SW, Li XB, Yang LJ, Xie ZH, Hu CS, Yan S, Ren TY, Liu ZD. Preparation of heteroatom doped poly(o-phenylenediamine) fluorescent nanospheres: Tunable fluorescent spectrum and sensing performance. Syn. Met. 2014;189:126–134. doi: 10.1016/j.synthmet.2014.01.008. [DOI] [Google Scholar]
- 19.Lin JH, Wei ZJ, Mao CM. A label-free immunosensor based on modified mesoporous silica for simultaneous determination of tumor markers. Biosens. Bioelectron. 2011;29:40–45. doi: 10.1016/j.bios.2011.07.063. [DOI] [PubMed] [Google Scholar]
- 20.Liu BQ, Cui YL, Tang DP, Yang HH, Chen GN. Au(III)-assisted core-shell iron oxide@poly(o-phenylenediamine)nanostructures for ultrasensitive electrochemical aptasensors based on DNase I-catalyzed target recycling. Chem. Commun. 2012;48:2624–2626. doi: 10.1039/c2cc17790k. [DOI] [PubMed] [Google Scholar]
- 21.Liu ZM, Ma ZF. Fabrication of an ultrasensitive electrochemical immunosensor for CEA based on conducting long-chain polythiols. Biosens. Bioelectron. 2013;46:1–7. doi: 10.1016/j.bios.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Liu ZM, Rong QF, Ma ZF, Han HL. One-step synthesis of redox-active polymer/AU nanocomposites for electrochemical immunoassay of multiplexed tumor markers. Biosens. Bioelectron. 2014;65:307–313. doi: 10.1016/j.bios.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Jia XL, Liu ZM, Liu N, Ma ZF. A label-free immunosensor based on graphene nanocomposites for simultaneous multiplexed electrochemical determination of tumor markers. Biosens. Bioelectron. 2014;53:160–166. doi: 10.1016/j.bios.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 24.Kong FY, Xu BY, Du Y, Xu JJ, Chen HY. A branched electrode based electrochemical platform: towards new label-free and reagentless simultaneous detection of two biomarkers. Chem. Commun. 2013;49:1052–1054. doi: 10.1039/C2CC37675J. [DOI] [PubMed] [Google Scholar]
- 25.Shi WT, Ma ZF. A novel label-free amperometric immunosensor for carcinoembryonic antigen based on redox membrane. Biosens. Bioelectron. 2011;26:3068–3071. doi: 10.1016/j.bios.2010.11.048. [DOI] [PubMed] [Google Scholar]
- 26.Sun XB, Ma ZF. Highly stable electrochemical immunosensor for carcinoembryonic antigen. Biosens. Bioelectron. 2012;35:470–474. doi: 10.1016/j.bios.2012.02.061. [DOI] [PubMed] [Google Scholar]
- 27.Wang K. Flexible solid-state supercapacitors based on a conducting polymer hydrogel with enhanced electrochemical performance. J. Mater. Chem. A. 2014;2:19726–19732. doi: 10.1039/C4TA04924A. [DOI] [Google Scholar]
- 28.Xia L, Wei ZX, Wan MX. Conducting polymer nanostructures and their application in biosensors. J. Colloid Interface Sci. 2010;341:1–11. doi: 10.1016/j.jcis.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, Liu BR, Pan LJ, Yu GH. 3D nanostructured conductive polymer hydrogels for high-performance electrochemical devices. Energy Environ. Sci. 2013;6:2856–2870. doi: 10.1039/c3ee40997j. [DOI] [Google Scholar]
- 30.Rong QF, Han HL, Feng F, Ma ZF. Network nanostructured polyprrole hudrogel/Au composites as enhanced electrochemical biosensing platform. Sci. Rep. 2015;5:11440. doi: 10.1038/srep11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Feng F, Ma Z. Novel electrochemical redoxactive species: one-step synthesis of polyaniline derivative-Au/Pd and its application for multiplexed immunoassay. Sci. Rep. 2015;5:16855. doi: 10.1038/srep16855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charles PT. Fabrication and characterization of 3D hydrogel microarrays to measure antigenicity and antibody functionality for biosensor applications. Biosens. Bioelectron. 2004;20:753–764. doi: 10.1016/j.bios.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Jia XL, Chen X, Han JM, Ma J, Ma ZF. Triple signal amplification using gold nanoparticles, bienzyme and platinum nanoparticles functionalized graphene as enhancers for simultaneous multiple electrochemical immunoassay. Biosens. Bioelectron. 2014;53:65–70. doi: 10.1016/j.bios.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Q, Chai YQ, Zhuo Y, Yuan R. Ultrasensitive simultaneous detection of four biomarkers based on hybridization chain reaction and biotin-streptavidin signal amplification strategy. Biosens. Bioelectron. 2015;68:42–48. doi: 10.1016/j.bios.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Yuan YL, Xie SB, Chai YQ, Yuan R. Amplified amperometric aptasensor for selective detection of protein using catalase-functional DNA-PtNPs dendrimer as a synergetic signal amplification label. Biosens. Bioelectron. 2014;60:224–230. doi: 10.1016/j.bios.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 36.Fan HX. Ultrasensitive electrochemical immunosensor for carbohydrate antigen 72-4 based on dual signal amplification strategy of nanoporous gold and polyaniline-Au asymmetric multicomponent nanoparticles. Biosens. Bioelectron. 2015;64:51–54. doi: 10.1016/j.bios.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 37.Qin XL, Yin Y, Yu HJ, Guo WJ, Pei MS. A novel signal amplification strategy of an electrochemical aptasensor for kanamycin, based on thionine functionalized graphene and hierarchical nanoporous PtCu. Biosens. Bioelectron. 2016;77:752–758. doi: 10.1016/j.bios.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 38.Han JM, Ma J, Ma ZF. One-step synthesis of poly(thionine)-Au nano-network and nanowires and its application for non-enzyme biosensing of hydrogen peroxide. Electrochem. Commun. 2013;33:47–50. doi: 10.1016/j.elecom.2013.04.027. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


