Abstract
Attention-deficit/hyperactivity disorder (ADHD) has an uncertain etiology, with potential contributions from different risk factors such as prenatal environmental exposure to organochlorines and metals, social risk factors, and genetics. The degree to which geographic variability in ADHD is independent of, or explained by, risk factors may provide etiological insight. We investigated determinants of geographic variation in ADHD-related behaviors among children living near the polychlorinated biphenyl–contaminated New Bedford Harbor (NBH) Superfund site in Massachusetts. Participants were 573 children recruited at birth (1993–1998) who were born to mothers residing near the NBH site. We assessed ADHD-related behaviors at age 8 years using Conners’ Teacher Rating Scale–Revised: Long Version. Adjusted generalized additive models were used to smooth the association of pregnancy residence with ADHD-related behaviors and assess whether prenatal organochlorine or metal exposures, sociodemographic factors, or other factors explained spatial patterns. Models that adjusted for child's age and sex displayed significantly increased ADHD-related behavior among children whose mothers resided west of the NBH site during pregnancy. These spatial patterns persisted after adjusting for prenatal exposure to organochlorines and metals but were no longer significant after controlling for sociodemographic factors. The findings underscore the value of spatial analysis in identifying high-risk subpopulations and evaluating candidate risk factors.
Keywords: attention-deficit/hyperactivity disorder, environmental exposures, geographic analysis, sociodemographic risk factors
Attention-deficit/hyperactivity disorder (ADHD) reflects deficits in attention and impulse control. Its etiology is uncertain, although genetic and environmental factors are likely to play important roles (1). Associations with prenatal maternal cigarette smoking, pregnancy complications, low socioeconomic status, and lead exposure have been observed (2–4). In a birth cohort residing in New Bedford, Massachusetts, and communities adjacent to the polychlorinated biphenyl (PCB)–contaminated New Bedford Harbor (NBH) Superfund site (Figure 1), ADHD-related behaviors at age 8 years have been associated with prenatal exposures to PCBs, mercury, and ρ,ρ′-dichlorodiphenyl dichloroethylene (ρ,ρ′-DDE), as well as with prenatal smoking, paternal education, household income, home environment, and other factors (5–9).
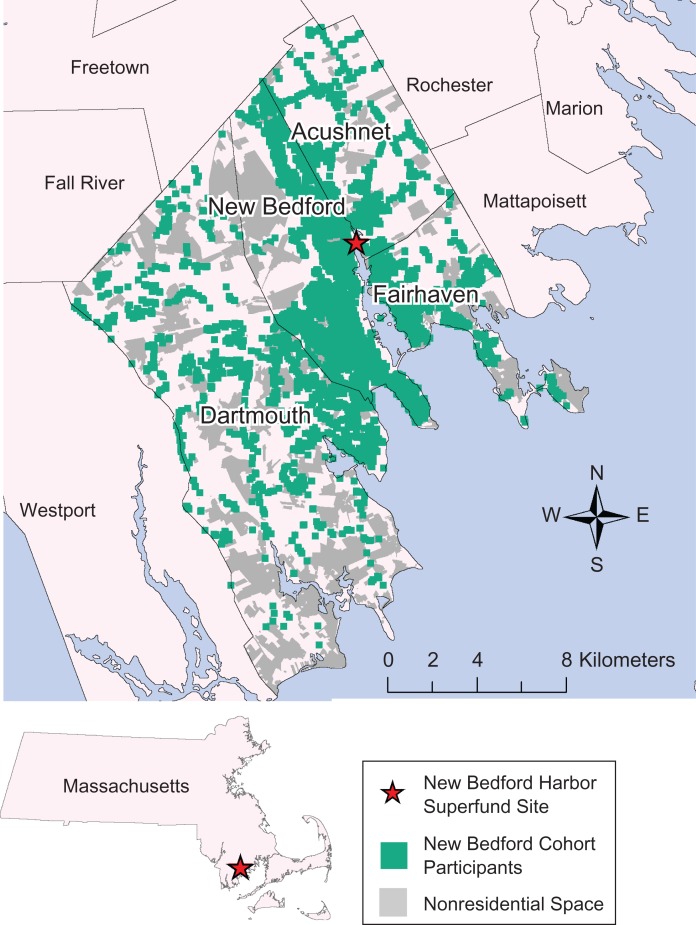
Figure 1.
Birth addresses of participants in the New Bedford Cohort, who were born in 1993–1998 and whose mothers were living in 4 towns neighboring the New Bedford Harbor Superfund site (New Bedford, Dartmouth, Acushnet, and Fairhaven, Massachusetts) during pregnancy. The population (shown in green) is distributed around primarily nonresidential open space (shown in gray).
NBH was listed as a Superfund site by the Environmental Protection Agency in 1982 because of contamination by PCB-laden waste released by local capacitor manufacturers from the 1940s until 1977. In addition, the New Bedford area is heavily industrialized, with other hazardous waste sites beyond NBH; thus, residential proximity to various contaminated sites may act as a proxy for chemical exposures. In this context, spatial analyses can provide insight into ADHD risks that may be related to geographic patterns of previously unidentified environmental exposures or other factors. For example, geographic studies have linked higher prevalence of ADHD with living at lower altitudes (10) and living in areas with lower solar intensity (11). At a community level, ADHD risk was found to be higher among children living further from a park in Milwaukee County, Wisconsin, suggesting that the built environment may partially explain the uneven geographic distribution of ADHD (12). In Cape Cod, Massachusetts, a community to the east of New Bedford, Hoffman et al. (13) observed decreasing odds of ADHD from north to south after adjusting for known risk factors.
Despite evidence of geographic differences in ADHD prevalence in other communities and likely spatial variability in environmental and social risk factors in the New Bedford area, no prior studies have assessed the potential for the risk of ADHD-related behavior to vary geographically in this community. The mapping of epidemiologic health data is now common, frequently leading to public demands for investigation of perceived “clusters” associated with environmental exposures. However, apparent localized increases in adverse health measures on these maps are often explained by confounding by sociodemographic risk factors. Generalized additive models (GAMs) provide a statistical approach with which to identify spatial patterns in ADHD risk and systematically determine predictors of the pattern. Our objectives in the present study were to examine geographic variation in ADHD-related behaviors in New Bedford and surrounding communities and to determine whether the geographic variation was explained by risk factors, including prenatal exposure to organochlorines or metals, sociodemographic factors, and/or lifestyle variables.
METHODS
Study population
We investigated the association between location of birth residence and ADHD-related behaviors using data from the New Bedford Cohort (NBC), an ongoing population-based prospective birth cohort study. The overall aim of the original study was to assess the relationship of prenatal organochlorine (PCBs and ρ,ρ′-DDE) and metal exposures (including lead and mercury) with subsequent neurobehavioral development, including ADHD-related behaviors, among children living near the NBH Superfund site (5). From 1993 to 1998, a total of 788 newborns from the 4 towns neighboring the NBH site (New Bedford, Acushnet, Fairhaven, and Dartmouth) whose mothers lived in those towns for at least the duration of pregnancy were enrolled in the study.
ADHD-related behavior and risk factor assessment
The NBC study includes biomarkers of prenatal pollutant exposures, questionnaire data, medical record abstraction, home assessments, and neurocognitive and behavioral assessments made in infancy and at ages 8 and 15 years. The home assessments use the Home Observation for Measurement of the Environment (HOME) Inventory to assess the quality of the home environment and parenting (14). The HOME Inventory is a standardized instrument that uses a combination of questionnaire items and observation to assess the support available to the child from the physical attributes of his/her home surroundings, as well as the cognitive stimulation and emotional support a child receives from exchanges with family and interactions with objects such as books and toys. Neurobehavioral evaluations made at age 8 years included Conners’ Teacher Rating Scale–Revised: Long Version (CTRS-R:L), in which the child's teacher reported the frequency of observed adverse behaviors related to ADHD. The CTRS-R:L provides 4 measures of ADHD-related behavior: Conners’ ADHD Index and 3 Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), ADHD symptom subscales (Inattentive, Hyperactive-Impulsive, and Total (i.e., Inattentive and Hyperactive-Impulsive symptom subscales combined)) (15). In this study, we used the CTRS-R:L measures expressed as raw scores (total frequency of adverse behaviors), with a higher score indicating more frequent adverse behavior.
Of the 788 children enrolled in the cohort, 607 participated in the 8-year follow up and 590 had a CTRS-R:L evaluation; 17 were missing data on umbilical cord serum organochlorine levels, leaving a total of 573 NBC children for the current spatial analysis (the same subset studied in a previous aspatial analysis of ADHD-related behaviors (7)). Umbilical cord serum samples were analyzed for 51 individual PCB congeners and ρ,ρ′-DDE. In addition, peripartum maternal total hair mercury levels were measured, and data on 12- to 36-month child blood lead levels were available from routine pediatric lead exposure screening. Collection of biomarker data and analytical methods are described elsewhere (5, 7, 9).
We modeled continuous raw scores of each of the 4 ADHD-related measures as independent outcomes. We included child's age and sex in all models to standardize the raw scores. Notably, the NBC had more ADHD-related behaviors than did the CTRS-R:L standardization population, with, for example, a mean Conners’ ADHD Index value of 53 as compared with the reference mean of 50 (7).
Spatial analysis
GAMs are a type of statistical model that combines smoothing with the ability to analyze continuous individual-level outcome data and adjust for covariates (16–20). We predicted continuous raw scores for ADHD-related behaviors in the New Bedford area using GAMs to smooth geocoded birth address location while controlling for maternal and child characteristics. We modeled location, a potential surrogate measure of spatially varying risk factors, using a bivariate smoother of longitude and latitude. The raw scores were square-root-transformed based on diagnostics that showed the variance structure was correctly specified on this scale. We applied a “loess” smoother from the MapGAM R package (21) (R Foundation for Statistical Computing, Vienna, Austria) with an a priori determined smoothing span of 0.15, which adapts to changes in population density while allowing for localized patterns to be observed (16). As with ordinary linear regression, this method allows for covariate adjustment to examine the influence of risk factors on geographic variation. We predicted the underlying spatial pattern of ADHD-related behaviors using an initial model that included the smooth function for location and child's age at CTRS-R:L assessment and sex. We then examined spatial patterns adjusted for umbilical cord serum measures for the sum of 4 prevalent PCB congeners (congeners 118, 138, 153, and 180; ∑PCB4) and ρ,ρ′-DDE, peripartum maternal hair mercury level, and peak 12- to 36-month blood lead levels to assess whether these chemical exposures could explain underlying geographic associations. Exposures were modeled independently using both a linear term and a loess smoother.
Nonchemical risk factors may also contribute to the underlying spatial patterns of ADHD-related behaviors if they are not evenly distributed throughout the study area. Spatial confounders must be associated with both location (our exposure of interest) and the outcome. Potential confounders were chosen a priori based on risk factors for ADHD-related behaviors identified in previously published studies on the same cohort (5, 7, 9), and we included each spatially varying covariate individually in the underlying risk model. Variables that changed the underlying risk pattern by 10% were included as spatial confounders in the final adjusted analysis: maternal age at child's birth (in years), marital status at child's birth and at school age (age 8 years) (married/other), maternal and paternal education at child's birth and age 8 years (less than 12th grade, high school graduation, or any college), smoking status (yes/no) during pregnancy, average number of cigarettes smoked per day during pregnancy, annual household income at child's birth and at age 8 years (<$20,000, $20,000–$39,999, or ≥$40,000), and HOME score at age 8 years.
We also considered maternal race/ethnicity (Cape Verdean, Latino, non-Hispanic white, non-Hispanic black, Asian, Native American, or other), alcohol consumption during pregnancy, seafood intake during pregnancy (servings/day), illicit drug use in the year before the study child's birth, intelligence quotient, depression symptoms when the child was aged 8 years, and parity, as well as primary language spoken in the home (English/non-English) and child's elementary school type (public/other), but these covariates did not vary spatially in this population. We imputed missing covariate information (including missing mercury or lead data) using fully conditional specification (with location and all other covariates described above) implemented by the “mice” package in R to generate 5 imputed data sets (22–24). We applied the spatial GAM models to each data set and mapped both the individual results and the average of the pointwise predictions. All covariates were modeled parametrically. We used the R package MapGAM (21) to run the spatial analyses and create the maps (R software, version 3.0.2). We used the gam.exact function in the “gam” R package to determine the coefficients and standard errors for the parametric covariates (25).
The models were used to predict ADHD-related behaviors for a grid of evenly spaced points covering parts of New Bedford, Acushnet, and Fairhaven. Because GAMs may exhibit biased behavior at the edges of the data distribution, we did not predict outcomes in Dartmouth, where there was low data density, or along the geographic edges of our study area. However, all of the data were used to fit the spatial GAM model. For comparison, results for each outcome were mapped using the score range of the initial age- and sex-adjusted model predicted for a boy of median age (8.2 years). Additional covariates were held fixed so that the spatial surface represented predictions at the reference level for categorical covariates and the median value for continuous covariates (Table 1). To calculate a global P value for the significance of location in the models, we implemented a permutation test of the null hypothesis that ADHD-related behaviors do not depend on the geographic location of participants, adjusting for other risk factors (19, 20).
Table 1.
Characteristics of 573 Mother-Child Pairs (Children Born in 1993–1998) in the New Bedford Cohort (Massachusetts) and Their Univariate Associations With Square-Root-Transformed Scores on the DSM-IV ADHD Total Subscale
| Characteristic | No. of Pairs | % | DSM-IV ADHD Total Subscale Score | Change in DSM-IV ADHD Total Subscale Scorea | ||
|---|---|---|---|---|---|---|
| Median | Mean (SD) | β | 95% CI | |||
| Child's age at examination, years | 8.2 | 8.3 (0.7) | 0.17 | −0.05, 0.40 | ||
| Child's sex | ||||||
| Male | 293 | 51.1 | 1.04 | 0.75, 1.32 | ||
| Female | 280 | 48.9 | 0 | Referent | ||
| Maternal age at child's birth, years | 26.6 | 26.7 (5.3) | −0.08 | −0.10, −0.05 | ||
| Marital status at child's birth | ||||||
| Never married/divorced/widowed | 238 | 41.5 | 0.83 | 0.53, 1.12 | ||
| Married | 335 | 58.5 | 0 | Referent | ||
| Marital status at child's school age (age 8 years) | ||||||
| Never married/divorced/widowed | 239 | 41.7 | 0.83 | 0.53, 1.13 | ||
| Married | 334 | 58.3 | 0 | Referent | ||
| Annual household income at child's birth (3 values missing) | ||||||
| <$20,000 | 194 | 33.9 | 0.97 | 0.61, 1.32 | ||
| $20,000–$39,999 | 188 | 32.8 | 0.56 | 0.20, 0.92 | ||
| ≥$40,000 | 188 | 32.8 | 0 | Referent | ||
| Annual household income at child's school age (8 values missing) | ||||||
| <$20,000 | 117 | 20.4 | 0.82 | 0.44, 1.20 | ||
| $20,000–$39,999 | 157 | 27.4 | 0.60 | 0.25, 0.94 | ||
| ≥$40,000 | 291 | 50.8 | 0 | Referent | ||
| Mother's education at child's birth (2 values missing) | ||||||
| Less than 12th grade | 95 | 16.6 | 0.90 | 0.48, 1.32 | ||
| High school graduation | 227 | 39.6 | 0.59 | 0.27, 0.91 | ||
| Any college | 249 | 43.5 | 0 | Referent | ||
| Father's education at child's birth (12 values missing) | ||||||
| Less than 12th grade | 142 | 24.8 | 1.22 | 0.84, 1.61 | ||
| High school graduation | 247 | 43.1 | 0.44 | 0.10, 0.79 | ||
| Any college | 172 | 30.0 | 0 | Referent | ||
| HOME scoreb (12 values missing) | 46.0 | 45.6 (5.3) | −0.11 | −0.14, −0.08 | ||
| Smoking status during pregnancy (41 values missing) | ||||||
| Yes | 158 | 27.6 | 0.83 | 0.51, 1.15 | ||
| No | 374 | 65.3 | 0 | Referent | ||
| Average smoking level during pregnancy (41 values missing), cigarettes/day | 0.0 | 2.5 (5.5) | 0.06 | 0.04, 0.09 | ||
| Exposure measuresc | ||||||
| ∑PCB4, ng/g | 0.2 | 0.3 (0.3) | ||||
| ρ,ρ′-DDE, ng/g | 0.3 | 0.5 (1.0) | ||||
| 12- to 36-month peak blood lead level (24 values missing), μg/dL | 6.0 | 6.7 (3.9) | ||||
| Peripartum maternal hair mercury level (176 values missing), μg/g | 0.4 | 0.6 (0.5) | ||||
| Townc | ||||||
| Acushnet | 29 | 5.1 | ||||
| Dartmouth | 62 | 10.8 | ||||
| Fairhaven | 60 | 10.5 | ||||
| New Bedford | 422 | 73.6 | ||||
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CI, confidence interval; ρ,ρ′-DDE, ρ,ρ′-dichlorodiphenyl dichloroethylene; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; HOME, Home Observation for Measurement of the Environment; PCB, polychlorinated biphenyl; ∑PCB4, sum of 4 prevalent PCB congeners (congeners 118, 138, 153, and 180); SD, standard deviation.
a Association between the covariate and square-root-transformed DSM-IV ADHD Total subscale score in a model with spatial smoothing and the single covariate.
b The HOME Inventory (14) is a standardized instrument that uses a combination of questionnaire items and observation to assess the support available to the child from the physical attributes of his/her home surroundings, as well as the cognitive stimulation and emotional support a child receives from exchanges with family and interactions with objects such as books and toys. Scores can range from 0 to 59, where a higher score indicates a better home environment.
c Exposure variables and town were not included in the final spatial model.
The institutional review boards of the University of California at Irvine (Irvine, California) and Boston University Medical Center and Brigham and Women's Hospital (Boston, Massachusetts) approved the research.
RESULTS
Characteristics of the 573 mother-child pairs included in the final spatial analyses and their unadjusted associations with the square-root-transformed DSM-IV Total score are displayed in Table 1. At the time of their child's birth, NBC mothers had a median age of 26.6 years, with a substantial portion being unmarried (42%) and living in households with an income under $20,000 per year (34%); almost one-third smoked during pregnancy (Table 1). Over 20% (n = 119) of mothers changed marital status between the child's birth and age 8 years (Spearman's correlation coefficient (rS) = 0.57). Of the 335 mothers who were married at the child's birth, 60 were no longer married at the child's school age (age 8 years); there were 59 mothers who were not married at the child's birth but were married at the child's school age. Education at birth was highly correlated with education at child's school age for both mothers (rS = 0.77) and fathers (rS = 0.83). As such, only measures of parental education at the child's birth were included in the spatial models. Umbilical cord serum PCB levels were low compared with those in other birth cohort studies with PCB measures (5, 26). Figure 1 shows the distribution of birth addresses; there were more NBC children in southern New Bedford. This reflects the overall population distribution, as, for example, northwestern New Bedford largely consists of an airport and a state reservation with few residences.
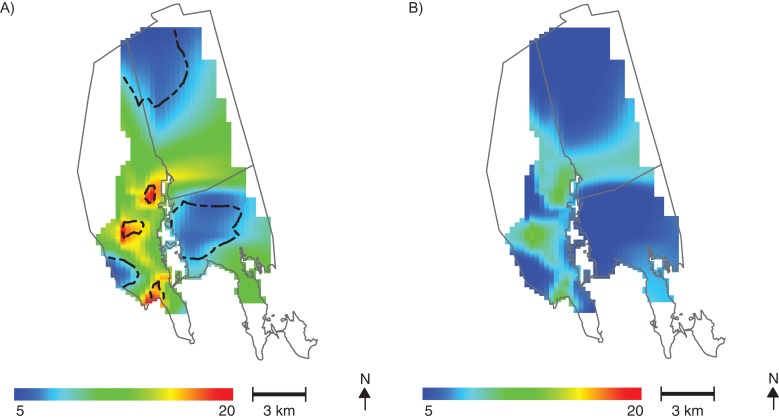
Results for the DSM-IV Total raw score analysis of a single imputed data set are presented in Figure 2. The underlying spatial pattern of ADHD-related behavior showed higher scores (i.e., a greater number of reported behavior problems) in 3 areas west of the NBH site (Figure 2A). The global test for the importance of location was significant (P = 0.003), with up to 15 points’ difference in predicted raw score (adjusted for child's age and sex) based on where the child lived. The DSM-IV Total raw scores in the data set ranged from 0 to 52, with a median of 9; as expected, smoothing of the data produced an averaging of scores over the study area at fixed covariate values, resulting in a much narrower range of predicted scores as compared with observed scores. The spatial patterns of predicted DSM-IV Total raw scores did not change after adjustment for cord serum ∑PCB4, cord serum ρ,ρ′-DDE, maternal hair mercury, or peak 12- to 36-month blood lead levels modeled with either a loess smoother or a linear term, and location remained highly significant (see Web Figure 1, available at http://aje.oxfordjournals.org/).
Figure 2.
Spatial distribution of predicted raw scores on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) attention-deficit/hyperactivity disorder Total subscale for children in the New Bedford Cohort, born in 1993–1998. A) Spatial variability of DSM-IV Total raw scores among New Bedford Cohort children is predicted for an 8-year-old boy without adjustment for chemical and nonchemical risk factors. The global P value for the significance of location is 0.003. Contour lines denote areas of significantly increased and decreased scores. Adjustment for umbilical cord serum level of the sum of 4 prevalent polychlorinated biphenyl congeners (congeners 118, 138, 153, and 180), umbilical cord serum ρ,ρ′-dichlorodiphenyl dichloroethylene level, peripartum maternal hair mercury level, and child peak 12- to 36-month blood lead level does not change the results. B) Spatial variability in predicted DSM-IV Total scores is attenuated in an analysis adjusted for Home Observation for Measurement of the Environment (HOME) score (predicted at the 95th percentile, where a higher score is indicative of a better home environment and parenting), and location is no longer significant (P = 0.236). Adjustment for other sociodemographic factors produces similar results.
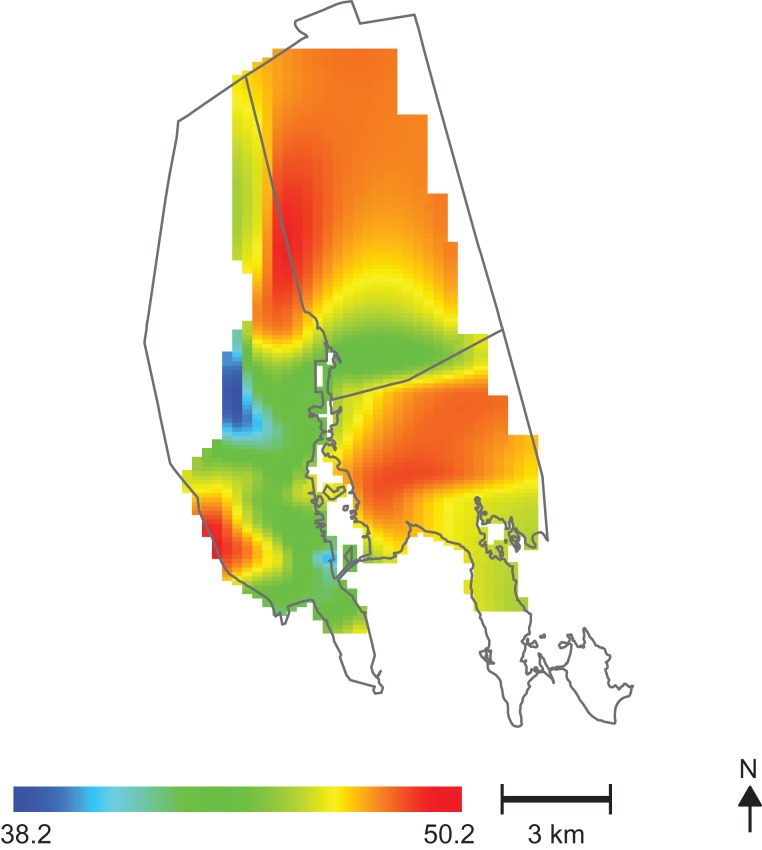
Changes in the spatial variability of predicted ADHD-related behaviors were observed after individually adjusting for maternal marital status, parental education, annual household income, HOME score, maternal age, and prenatal smoking. Figure 2B shows the results obtained after adjusting for HOME score, predicted at the 95th percentile. An increase in the HOME score from the fifth percentile (poor home environment) to the 95th percentile (better home environment) resulted in an average 11-point decrease in the age- and sex-adjusted DSM-IV Total raw score. The test for the importance of location in this analysis was no longer significant (P = 0.236). Areas of higher HOME score (better home environment) in the study population (Figure 3) spatially coincided with areas of decreased DSM-IV Total raw score (Figure 2A).
Figure 3.
Geographic patterns of Home Observation for Measurement of the Environment (HOME) scores for children in the New Bedford Cohort, born in 1993–1998. Areas with higher HOME scores (indicating a better home environment and parenting) are the same areas where predicted raw scores on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, attention-deficit/hyperactivity disorder Total subscale are lowest (less frequent adverse behavior) (see Figure 2A).
Geographic patterns for a fully adjusted spatial analysis (Web Figure 2) including sociodemographic factors as well as prenatal smoking were similar to those of Figure 2B. Spatial patterns were similar for Conners’ ADHD Index, the DSM-IV Inattentive subscale, and the DSM-IV Hyperactive-Impulsive subscale (Web Figure 3). Although Conners’ ADHD Index and the DSM-IV Total subscale only have 3 assessment items in common, the rS values for correlations between the ADHD index and the 3 DSM-IV symptom subscales ranged from 0.63 to 0.96 (7). Results were also nearly identical across imputed data sets (results for other data sets not shown).
DISCUSSION
Spatial analyses have been used previously to identify disease risk factors that were not identified through traditional epidemiologic analyses. For example, results from spatial analyses have facilitated the identification of chemical exposure sources that may contribute to disease clusters (15, 27, 28). In the NBC, the NBH Superfund site and other local industries represent potential sources of population exposure to chemical contaminants associated with ADHD-related behaviors, including PCBs. Although residential distance to the PCB-contaminated NBH Superfund site was not associated with umbilical cord serum PCB levels in a previous analysis (29), residential distance to a contaminated site may reflect information beyond that captured by biomarkers of chemical exposure, potentially including unmeasured chemicals or sociodemographic patterns related to land use and property values. Our age- and sex-adjusted analyses identified several geographic areas of increased ADHD-related behavior among NBC participants living in New Bedford neighborhoods to the west of the NBH site (Figure 2). We did not identify high-risk areas in the other study towns. Most NBC study participants were in New Bedford (Table 1), so we had less statistical power to assess spatial correlates of ADHD-related behavior in the other study communities.
Results adjusted for cord serum ∑PCB4 levels, cord serum ρ,ρ′-DDE, peripartum maternal hair mercury, or peak 12- to 36-month blood lead levels were similar to those of the crude analysis. Data for all 4 biomarker measures were generally higher to the south (Web Figure 4), but this did not explain the significant spatial pattern of ADHD-related behavior. Although previous analyses have shown a relationship between PCB, ρ,ρ′-DDE, and mercury exposures and ADHD-related behaviors in this cohort (7–9), our results suggest that these exposures are not spatially clustered near the NBH Superfund site or other local sources of environmental contaminants. Such findings are probably attributable to the importance of food consumption as a PCB exposure pathway in the study populations. In the NBC, maternal consumption of organ meat was associated with significantly higher cord blood PCB levels (29), and cultural predictors of diet (race/ethnicity, primary language spoken in the home) did not vary spatially in this analysis. Although observed spatial patterns were not attenuated after adjustment for chemical exposures, supporting the aspatial nature of these risk factors, this does not preclude their importance in predicting ADHD-related behaviors. Because the spatial patterns were primarily explained by individual-level sociodemographic factors, we did not extend our analysis to include proximity-based measures for other industrial point sources.
The fact that the observed spatial patterns were largely attenuated after adjustment for marital status, parental education, annual household income, or HOME score supports the importance of sociodemographic risk factors for ADHD-related behavior (30–32). Although the mechanism whereby such factors may influence ADHD-related behavior is unknown, sociodemographic stressors such as poverty or low parental education are associated with childhood psychiatric disorders (33). In addition, socioeconomic and demographic characteristics may correlate with a number of ADHD risk factors, including chemical exposures from parental occupation, household or dietary factors that were not measured in our population, health-related behaviors that may not be fully captured by survey questions, and parenting behavior (34).
For example, indoor contaminants associated with adverse child behavioral health, such as pesticide residues, can be found at high levels in low-income urban housing (35). Indeed, household pesticide use and exposure vary by sociodemographic factors such as race/ethnicity and education (35, 36). Similarly, exposure to other substances correlated with adverse child behavioral development, such as bisphenol A or phthalates, can vary by income, with generally higher exposures being seen among lower-income households (37, 38). Associations of sociodemographic factors with ADHD-related behaviors could result from confounding by genetic risk factors, whereby parents with ADHD may have lower educational achievement and income and also be more likely to have children with ADHD-related behaviors (39).
Lastly, socioeconomic factors may enhance (or attenuate) the detrimental influence of neurotoxic exposures. For example, among 354 Dutch children, adverse associations of prenatal PCB exposures with childhood cognitive and motor abilities were evident only among children with suboptimal parental and home characteristics (40). Regardless of the mechanism of association, the ability of sociodemographic measures to explain spatial variability in ADHD-related behaviors in the NBC supports the potential for such measures to integrate complex exposure, lifestyle, parenting, and genetic risk factors for ADHD-related behaviors.
In summary, by combining individual-level data from a prospective cohort study with spatial statistical techniques, we were able to examine ADHD-related behaviors among children living near a Superfund site. We observed significant spatial heterogeneity that was largely explained by sociodemographic covariates with similar spatial patterning. Our methodological approach coupled with geocoded birth addresses provided insight that would not be available when using administrative data at a coarse geographic resolution.
Despite these strengths, our spatial analyses had some limitations. GAMs may exhibit biased behavior at the edges of the data distribution (41), so we chose to not estimate ADHD-related behavior scores along the study area edges with low data density. All NBC study mothers resided in one of the 4 towns adjacent to the NBH site for the duration of pregnancy; the residential address at birth was used in this analysis. However, we did not have information on residential histories during pregnancy or between birth and the age 8 assessments. Thus, birth address may not have been indicative of where chemical exposures occurred, particularly for postnatal blood lead levels. We did have information on child's address at age 8 years, but we did not include it in the analysis because of the possibility that a child's adverse behavior may have affected the location of the age 8 address (i.e., moving to be closer to family support or a different school).
The study was also limited by the small sample size and missing data, although there was relatively little missing data (<10%) except for data on maternal hair mercury level (31%), and characteristics for children with and without missing data were similar (7). More generally, the socioeconomic and demographic terms included in the model were proxies for a complex array of correlated risk factors, including unmeasured chemical exposures, and understanding their influence on spatial patterns does not provide insight regarding specific interventions that could reduce ADHD-related behaviors in the community. That being said, identification of high-risk or vulnerable subpopulations is an important first step in developing more specific insights about causal factors, and it can inform interventions designed to mitigate social stressors associated with ADHD-related behaviors, such as improving the quality of the home environment.
Our results demonstrate the importance of spatial analysis as a tool for investigating complex associations in epidemiologic data. Using GAMs, we generated maps of ADHD-related behaviors within a cohort study that included extensive data on potential chemical and nonchemical risk factors and birth location. We identified an area of increased ADHD-related behaviors to the west of the NBH Superfund site that was explained by geographic clustering of families with lower socioeconomic status in that same area, as reflected in lower household income, HOME scores, and parental education, as well as maternal marital status. Adjustment for cord serum ∑PCB4, cord serum ρ,ρ′-DDE, peripartum maternal hair mercury, and peak 12- to 36-month blood lead levels did not affect the geographic patterns observed, suggesting that these particular environmental exposures were not spatially clustered in a similar pattern. Our analysis is particularly useful for disentangling the contribution of spatially variable risk factors to observed clusters and identifying high-risk populations.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Program in Public Health, University of California, Irvine, California (Verónica M. Vieira); Department of Environmental Health, School of Public Health, Boston University, Boston, Massachusetts (Verónica M. Vieira, M. Patricia Fabian, Thomas F. Webster, Jonathan I. Levy); Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, Massachusetts (M. Patricia Fabian, Jonathan I. Levy, Susan A. Korrick); and Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Susan A. Korrick).
This work was supported by grants P42 ES007381, P42 ES005947, and R01 ES014864 from the National Institute of Environmental Health Sciences, National Institutes of Health.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the views of the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1. Faraone SV, Doyle AE. The nature and heritability of attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am. 2001;10(2):299–316. [PubMed] [Google Scholar]
- 2. Bellinger D, Leviton A, Allred E, et al. . Pre- and postnatal lead exposure and behavior problems in school-aged children. Environ Res. 1994;66(1):12–30. [DOI] [PubMed] [Google Scholar]
- 3. Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57(11):1215–1220. [DOI] [PubMed] [Google Scholar]
- 4. Millichap JG. Etiologic classification of attention deficit/hyperactivity disorder. Pediatrics. 2008;121(2):e358–e365. [DOI] [PubMed] [Google Scholar]
- 5. Korrick SA, Altshul LM, Tolbert PE, et al. . Measurement of PCBs, DDE, and hexachlorobenzene in cord blood from infants born in towns adjacent to a PCB-contaminated waste site. J Expo Anal Environ Epidemiol. 2000;10(6):743–754. [DOI] [PubMed] [Google Scholar]
- 6. Sagiv SK, Epstein JN, Bellinger DC, et al. . Pre- and postnatal risk factors for ADHD in a nonclinical pediatric population. J Atten Disord. 2013;17(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sagiv SK, Thurston SW, Bellinger DC, et al. . Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am J Epidemiol. 2010;171(5):595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sagiv SK, Thurston SW, Bellinger DC, et al. . Neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally to organochlorines. Environ Health Perspect. 2012;120(6):904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sagiv SK, Thurston SW, Bellinger DC, et al. . Prenatal exposure to mercury and fish consumption during pregnancy and attention-deficit/hyperactivity disorder-related behavior in children. Arch Pediatr Adolesc Med. 2012;166(12):1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huber RS, Kim TS, Kim N, et al. . Association between altitude and regional variation of ADHD in youth [published online ahead of print March 25, 2015]. J Atten Disord. (doi:10.1177/1087054715577137). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arns M, van der Heijden KB, Arnold LE, et al. . Geographic variation in the prevalence of attention-deficit/hyperactivity disorder: the sunny perspective. Biol Psychiatry. 2013;74(8):585–590. [DOI] [PubMed] [Google Scholar]
- 12. Baumgardner DJ, Schreiber AL, Havlena JA, et al. . Geographic analysis of diagnosis of attention-deficit/hyperactivity disorder in children: eastern Wisconsin, USA. Int J Psychiatry Med. 2010;40(4):363–382. [DOI] [PubMed] [Google Scholar]
- 13. Hoffman K, Webster TF, Weinberg JM, et al. . Spatial analysis of learning and developmental disorders in upper Cape Cod, Massachusetts using generalized additive models. Int J Health Geogr. 2010;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caldwell B, Bradley R. Home Observation for Measurement of the Environment—Revised Edition Little Rock, AR: University of Arkansas; 1984. [Google Scholar]
- 15. Conners CK. Conners’ Rating Scales—Revised Technical Manual North Tonawanda, NY: Multi-Health Systems, Inc.; 2000. [Google Scholar]
- 16. Hastie TJ, Tibshirani RJ. Generalized Additive Models. London, United Kingdom: Chapman & Hall Ltd.; 1990. [Google Scholar]
- 17. Kelsall JE, Diggle PJ. Spatial variation in risk of disease: a nonparametric binary regression approach. J R Stat Soc Ser C Appl Stat. 1998;47(4):559–573. [Google Scholar]
- 18. Wood SN. Generalized Additive Models: An Introduction with R. London, United Kingdom: Chapman & Hall Ltd.; 2006. [Google Scholar]
- 19. Vieira V, Webster T, Weinberg J, et al. . Spatial analysis of lung, colorectal, and breast cancer on Cape Cod: an application of generalized additive models to case-control data. Environ Health. 2005;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Webster T, Vieira V, Weinberg J, et al. . Method for mapping population-based case-control studies: an application using generalized additive models. Int J Health Geogr. 2006;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vieira VM, Bartell SM, Bliss RY. MapGAM: Mapping Smoothed Effect Estimates from Individual-Level Data (R package, version 0.7-5). Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 22. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 23. Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91(434):473–489. [Google Scholar]
- 24. Fichman M, Cummings JN. Multiple imputation for missing data: making the most of what you know. Organ Res Methods. 2003;6(3):282–308. [Google Scholar]
- 25. Hastie T. gam: Generalized Additive Models (R package, version 1.12). Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 26. Longnecker MP, Wolff MS, Gladen BC, et al. . Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ Health Perspect. 2003;111(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guarjardo OA, Oyana TJ. A critical assessment of geographic clusters of breast and lung cancer incidences among residents living near the Tittabawassee and Saginaw Rivers, Michigan, USA. J Environ Public Health. 2009;2009:316249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jacquez GM, Shi C, Meliker JR. Local bladder cancer clusters in southeastern Michigan accounting for risk factors, covariates and residential mobility. PLoS One. 2015;10(4):e0124516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi AL, Levy JI, Dockery DW, et al. . Does living near a Superfund site contribute to higher polychlorinated biphenyl (PCB) exposure. Environ Health Perspect. 2006;114(7):1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hjern A, Weitoft GR, Lindblad F. Social adversity predicts ADHD-medication in school children—a national cohort study. Acta Paediatr. 2009;99(6):920–924. [DOI] [PubMed] [Google Scholar]
- 31. Russell AE, Ford T, Russell G. Socioeconomic associations with ADHD: findings from a mediation analysis. PLoS One. 2015;10(6):e0128248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Russell AE, Ford T, Williams R, et al. . The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440–458. [DOI] [PubMed] [Google Scholar]
- 33. Carter AS, Wagmiller RJ, Gray SA, et al. . Prevalence of DSM-IV disorder in a representative, healthy birth cohort at school entry: sociodemographic risks and social adaptation. J Am Acad Child Adolesc Psychiatry. 2010;49(7):686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adamkiewicz G, Spengler JD, Harley AE, et al. . Environmental conditions in low-income urban housing: clustering and associations with self-reported health. Am J Public Health. 2014;104(9):1650–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McKelvey W, Jacobson JB, Kass D, et al. . Population-based biomonitoring of exposure to organophosphate and pyrethroid pesticides in New York City. Environ Health Perspect. 2013;121(11-12):1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu XM, Bennett DH, Ritz B, et al. . Residential insecticide usage in northern California homes with young children. J Expo Sci Environ Epidemiol. 2011;21(4):427–436. [DOI] [PubMed] [Google Scholar]
- 37. Larsson K, Ljung Björklund K, Palm B, et al. . Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environ Int. 2014;73:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Findlay LC, Kohen DE. Bisphenol A and child and youth behaviour: Canadian Health Measures Survey 2007 to 2011. Health Rep. 2015;26(8):3–9. [PubMed] [Google Scholar]
- 39. Russell G, Ford T, Rosenberg R, et al. . The association of attention deficit hyperactivity disorder with socioeconomic disadvantage: alternate explanations and evidence. J Child Psychol Psychiatry. 2014;55(5):436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vreugdenhil HJ, Lanting CI, Mulder PG, et al. . Effects of prenatal PCB and dioxin background exposure on cognitive and motor abilities in Dutch children at school age. J Pediatr. 2002;140(1):48–56. [DOI] [PubMed] [Google Scholar]
- 41. Vieira VM, Hart JE, Webster TF, et al. . Association between residences in US northern latitudes and rheumatoid arthritis: a spatial analysis of the Nurses’ Health Study. Environ Health Perspect. 2010;118(7):957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.