Abstract
Depression affects up to 30% of human immunodeficiency virus (HIV)-infected individuals. We estimated joint effects of antiretroviral therapy (ART) initiation and depressive symptoms on time to death using a joint marginal structural model and data from a cohort of HIV-infected women from the Women's Interagency HIV Study (conducted in the United States) from 1998–2011. Among 848 women contributing 6,721 years of follow-up, 194 participants died during follow-up, resulting in a crude mortality rate of 2.9 per 100 women-years. Cumulative mortality curves indicated greatest mortality for women who reported depressive symptoms and had not initiated ART. The hazard ratio for depressive symptoms was 3.38 (95% confidence interval (CI): 2.15, 5.33) and for ART was 0.47 (95% CI: 0.31, 0.70). Using a reference category of women without depressive symptoms who had initiated ART, the hazard ratio for women with depressive symptoms who had initiated ART was 3.60 (95% CI: 2.02, 6.43). For women without depressive symptoms who had not started ART, the hazard ratio was 2.36 (95% CI: 1.16, 4.81). Among women reporting depressive symptoms who had not started ART, the hazard ratio was 7.47 (95% CI: 3.91, 14.3). We found a protective effect of ART initiation on mortality, as well as a harmful effect of depressive symptoms, in a cohort of HIV-infected women.
Keywords: antiretroviral therapy, cohort studies, depression, HIV, marginal structural models, mortality, proportional hazards models, women
Depression is a chronic mental health condition that affects up to 30% of human immunodeficiency virus (HIV)-infected individuals (1–11). Major depressive disorder ordinarily is diagnosed through a combination of diagnostic criteria and clinical judgment (12, 13). Depression is rarely assessed in clinical studies. Depressive symptoms, a critical component of the diagnosis of depression, have been associated with higher mortality in many (14–20) but not all (21) studies of HIV-infected individuals. Lyketsos et al. (21) reported that depressive symptoms were not associated with risk of acquired immune deficiency syndrome (AIDS) or all-cause mortality among HIV-infected adult men in the Multicenter AIDS Cohort Study in the era before effective antiretroviral therapy (ART). However, more recent studies in the combined ART era noted strong effects of both ART and depressive symptoms on virologic and clinical outcomes including mortality, often in the context of investigations for racial disparities (14–16). Two studies found that depressive symptoms were associated with increased risk of mortality among HIV-infected adult men in the Multicenter AIDS Cohort Study (16) and adult women in the Women's Interagency HIV Study (WIHS) (15, 16). Murphy et al. (15) tested various domains of depressive symptoms (somatic, positive, negative, and interpersonal) and found each to be associated with AIDS mortality.
Depression and ART may interact with each other in influencing all-cause mortality, and the presence or absence of such an interaction may be important in guiding optimal care for individuals living with HIV (19, 22, 23). For example, the effect of depression on mortality might be attenuated among those who have initiated ART, especially in the modern treatment era with simpler and more robust regimens, as patients achieve improved physical health potentially leading to improved mental health (20, 24). If true, this might suggest that mental health treatment may be less important for reducing mortality in the current era of dramatically expanded ART treatment. Alternatively, the effect of depression on mortality may be comparable or even stronger among those initiating ART because ART initiation introduces a new pathway—ART adherence and care retention—through which depression influences mortality. Results supporting this conclusion would suggest that expanded depression treatment is equally important to or even more important than efforts to reduce overall mortality among HIV-infected adults.
In one important previous study, investigators examined the potential for interaction between depressive symptoms and adherence to ART and found no departures from multiplicative or additive effects (19). However, the investigators did not control for time-varying confounding over follow-up by variables such as CD4 cell count, and they examined depressive symptoms for only 1 year after ART initiation. We extend this work by focusing on a similar interaction but in a longitudinal context with time-varying depressive symptoms over a longer follow-up period, with time-varying confounding controlled through the use of a marginal structural model. Standard marginal structural model analyses have previously indicated a survival benefit of ART (25); yet, to our knowledge, no study has simultaneously examined the joint effects of time-varying ART and depressive symptoms on mortality. By joint effects, we mean the explicit estimation of the effects of multiple exposures on a single outcome, while adjusting for time-fixed and time-varying confounding for both exposures (26, 27). We estimated the joint effects of ART initiation and depressive symptoms on total mortality using data from 848 HIV-infected adult women followed from 1998 to 2011 in the WIHS.
METHODS
Study sample
A full description of the WIHS cohort is provided elsewhere (28, 29). In brief, the WIHS is a prospective cohort study with semiannual follow-up on key measures of HIV disease history and progression, including self-reported medication use, laboratory results, and self-reported behavioral characteristics. Between 1994 and 2013, the WIHS enrolled 4,346 women from 10 sites across the United States; 3,232 (74%) were HIV-infected at enrollment. We used a new-user cohort design (30), which limits the potential for bias due to women surviving a previous period of ART therapy. To examine a time period during which modern ART was the predominant therapy, for this study we started follow-up at WIHS visits beginning on April 1, 1998, and ended the study period on December 31, 2011. A total of 583 women had no visits during the study period, of whom 338 women had died prior to April 1, 1998. We further excluded 1,761 prevalent users of combination ART and 40 women who did not have any information on CD4 cell count, HIV RNA viral load, or Center for Epidemiologic Studies Depression Scale (CES-D) score within 1 year of their first study visit after April 1, 1998. Our analysis cohort included 848 HIV-infected women who participated in the WIHS between April 1, 1998, and December 31, 2011, and who had not initiated ART by April 1, 1998. All participants provided written informed consent, and local institutional review boards reviewed all study protocols.
Exposure assessment
The definition of ART was guided by the Department of Health and Human Services/Kaiser Foundation Panel guidelines (31). HIV-infected women in WIHS report use of all antiretroviral drugs during the previous 6 months at each study visit. We assumed that participants remain on ART after initiation until they died, were lost to follow-up, or were administratively censored, making our results analogous to an intention-to-treat analysis in a randomized controlled trial (32). We examined this assumption in a sensitivity analysis where we allowed ART use to vary over time. Given the interval design of our cohort, we set the first date of ART as the midpoint between the first date reporting ART and the last date not reporting ART. In the 8 cases where the difference between these 2 dates was greater than 4 years, we set the first date of ART as the first reported date of ART minus 2 years.
Depressive symptoms were defined by scores from the CES-D. The CES-D has been extensively validated and performs well in comparison to longer clinical surveys of depressive symptoms (33, 34). The CES-D score has a range from 0–60, and a score of 16 or higher was classified as having depressive symptoms; a score below 16 was classified as not having depressive symptoms. WIHS participants reported depressive symptoms at each semiannual visit. We allowed depressive symptoms to vary over time. Furthermore, some authors have noted the potential for overlap between symptoms of somatic depression and HIV (15, 35). We investigated this with a sensitivity analysis in which we removed the somatic items from the CES-D scale when determining depressive symptoms.
Outcome ascertainment
Incident deaths were continuously ascertained through medical record abstraction, health-care providers, personal contacts, and contacts with local health departments. Additionally, for women lost to follow-up or known to have died, National Death Index searches were performed on a yearly basis through December 31, 2011. Administrative censoring occurred at the last available National Death Index search date.
Statistical analysis
Let , where is the time, measured in days, from study entry to death, and is the time to administrative censoring or loss to follow-up, for participant . Women were considered lost to follow-up after 2 consecutive missed visits, and they did not reenter the risk set if they came back to the study at a later date. Let and be binary indicators of ART initiation and depressive symptoms, respectively, at day . Let be a vector denoting the levels of confounders of and , while is the vector subset of corresponding to time-fixed confounders measured at study entry. Define analogous vectors , , , and , which correspond to levels of time-varying and time-fixed confounders of and and of and , respectively. Overbars represent the history of a given random variable from study entry, or . Then, let represent the time to death for participant had she been assigned ART initiation history and depressive symptom history during the study period, rather than her observed histories and . Finally, let .
A marginal structural Cox proportional hazards model for the joint effects of and on is where , is the baseline hazard function and is the hazard function for . We adjust for time-varying confounders using inverse probability weights, which we describe below. The parameter is the hazard ratio for the effect of ART initiation on with no depressive symptoms at time , is the hazard ratio for the effect of depressive symptoms at time on prior to ART initiation, and is the ratio of hazard ratios for the joint effect of ART initiation and depressive symptoms on . Let .
The joint marginal structural model above is fit in a two-part process using inverse probability weights. Conditional on the estimated weights, the weighted maximum partial likelihood estimates for are obtained by maximizing , where is an indicator that participant i is at risk at distinct ordered event time t, the inverse probability weights , and as defined above. Given necessary assumptions stated below, are consistent asymptotically normal estimates of the joint effects of ART initiation and depressive symptoms on time to death accounting for time-varying confounding as well as selection bias due to censoring informed by measured covariates. Therefore, can be used to assess the presence of multiplicative interaction at time . To assess interaction on the additive scale, we calculated the relative excess risk due to interaction.
The stabilized weights can be used to fit the joint marginal structural model above, and have been previously described (36). The denominator for the ART initiation weights are estimated from a pooled logistic regression model , where . are time-specific intercepts, , and is a vector of log hazard ratios for the effects of on . The numerator for the weights can be estimated from a pooled logistic regression model with replacing in . A similar approach was taken to estimate the censoring weights and depressive symptom weights .
The assumptions necessary for asymptotically consistent estimation of α are treatment-variation irrelevance (essentially consistency) (37), no interference (38), conditional exchangeability, positivity, and correct model specification (36, 39).
We included the following time-fixed covariates for the 3 weight equations: age at enrollment, white race, CD4 cell count at enrollment, and HIV RNA viral load at enrollment. Time-varying covariates included CD4 cell count, HIV RNA viral load, and, for the model for time-varying depressive symptoms only, depressive symptoms at the previous visit. As a sensitivity analysis, we further included substance abuse, defined as a time-varying covariate for the use of either injected or noninjected drugs, in our weight equations. Missing values for time-varying covariates were replaced by values carried forward from the previous visit. Continuous variables were modeled using restricted cubic splines, with 3 knots (at the 5th, 50th, and 95th percentiles). We explored truncation at various percentiles and values of the weight distribution (36) as well as a marginal structural model without a term for interaction between ART initiation and depressive symptoms. In addition to the joint marginal structural Cox proportional hazards model, we also fitted unadjusted Cox proportional hazards models. All data analysis and model fitting was performed using SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
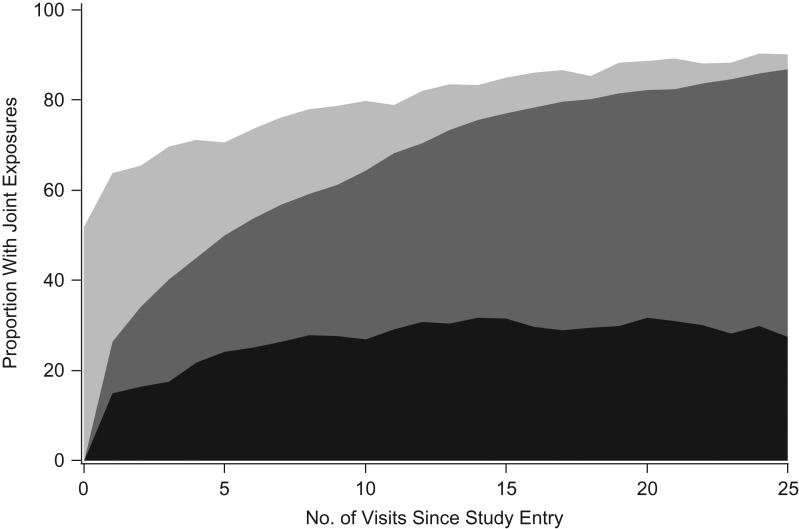
Table 1 presents characteristics at entry and over follow-up for the 848 women, who contributed a total of 6,721 years of follow-up; 66% were black, and 38% had less than 12 years of education. At study entry, the median age was 39, and the median CES-D score was 16. The median length of follow-up was 9.3 years, and the maximum was 13.7 years. A total of 189 women were lost to follow-up, and 397 women were administratively censored at the last date at which National Death Index searches were available. A total of 194 participants died during follow-up, resulting in a mortality rate of 2.9 per 100 women-years. Averaged over the 6,721 years of follow-up, the median CES-D score was 12, and 60% of women-years were after ART initiation. The joint exposure status of the cohort over successive visits is shown in Figure 1. The proportion of women who were experiencing no depressive symptoms and had started ART increased over time from 37% after approximately 5 years to 50% after approximately 10 years. The proportion of women who experienced depressive symptoms and had initiated ART increased to approximately 27% after approximately 5 years, but remained relatively constant around 30% afterwards. The most prevalent regimens over follow-up were protease-inhibitor-based regimens (43%) and nonnucleoside reverse transcriptase inhibitor-based regimens (41%). We had complete data on ART initiation, age, and race. Over the period of analysis, 77% of CES-D scores were available, and 91% of CD4 cell count and HIV RNA viral load values were available.
Table 1.
Characteristics of the 848 Included HIV-Infected Women at Study Entry and During Follow-up, Women's Interagency HIV Study, United States, 1998–2011
| Characteristic | At Entry (n = 848 Participants) | During Follow-up (n = 6,721 Women-Years)a | ||||
|---|---|---|---|---|---|---|
| Median (IQR) | No. | % | Median (IQR) | No. | % | |
| Age | 39 (33–45) | 43 (37–50) | ||||
| ART use | 4,028 | 60 | ||||
| CES-D scoreb | 16 (7–27) | 12 (4–24) | ||||
| Mono- or dual-therapyc | 263 | 31 | 939 | 14 | ||
| CD4 cell count | 440 (264–649) | 444 (283–640) | ||||
| Log10 HIV RNA | 3.5 (2.7–4.3) | 2.8 (2.3–4.0) | ||||
| Race | ||||||
| White | 162 | 19 | ||||
| Black | 558 | 66 | ||||
| Other | 128 | 15 | ||||
| Education, yearsd | ||||||
| <12 | 325 | 38 | ||||
| 12 | 264 | 31 | ||||
| >12 | 257 | 30 | ||||
Abbreviations: ART, antiretroviral therapy; CES-D, Center for Epidemiologic Studies Depression Scale; HIV, human immunodeficiency virus; IQR, interquartile range.
a Median follow-up: 9.1 years; maximum follow-up: 13.7 years.
b Possible scores: 0–60.
c Mono- or dual-therapy is classified as any use of antiretroviral medications that does not qualify as ART (use of 3 or more antiretroviral drugs, including at least 1 of the following: a protease inhibitor, a nonnucleoside reverse transcriptase inhibitor, one of the nucleoside reverse transcriptase inhibitors abacavir or tenofovir, an entry inhibitor, or an integrase inhibitor).
d Two women were missing information on their educational history.
Figure 1.
Joint exposure status of antiretroviral therapy (ART) initiation and depressive symptoms in the Women's Interagency HIV Study, United States, 1998–2011. The proportion of women with depressive symptoms who had initiated ART is represented by the black shaded area, women without depressive symptoms who had initiated ART by dark gray, women with depressive symptoms who had not initiated ART by light gray, and women without depressive symptoms who had not initiated ART by white.
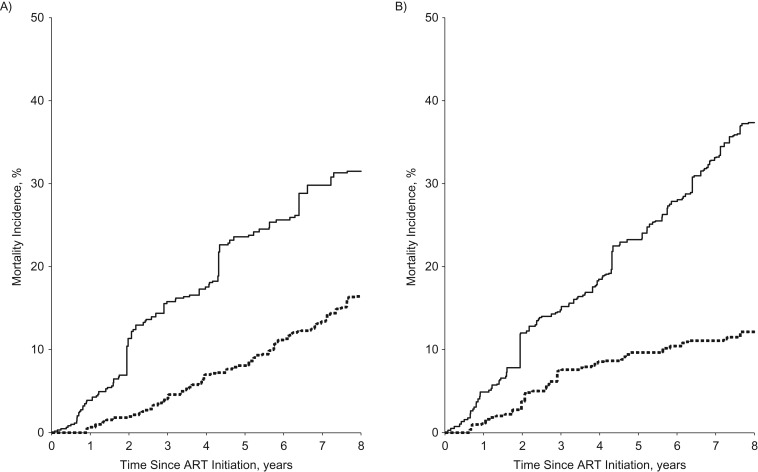
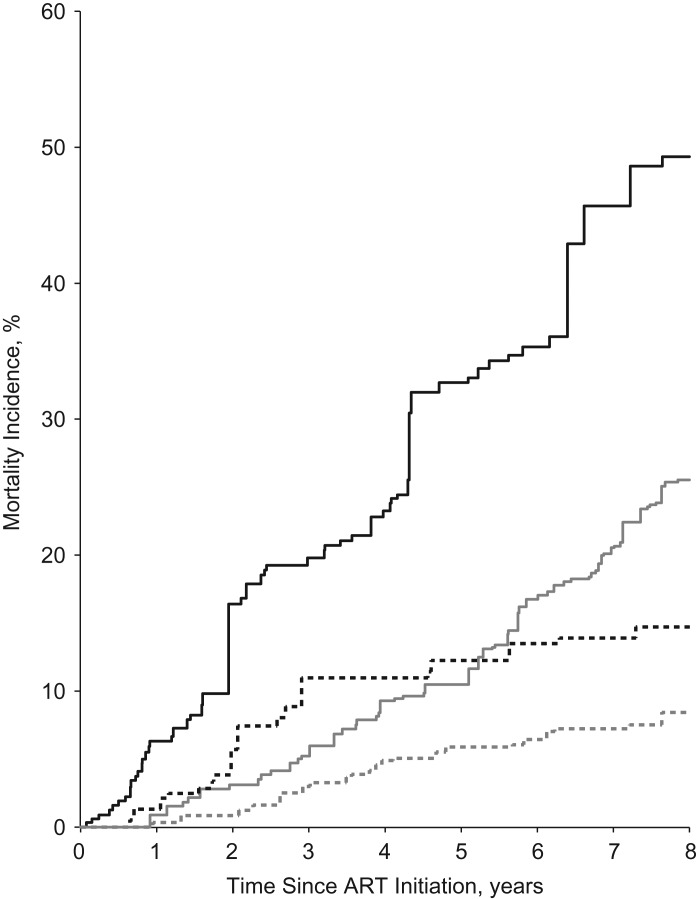
We present 8-year cumulative mortality curves for ART initiation, depressive symptoms, and the joint classification of ART and depressive symptoms in Figure 2. These curves are based upon the weighted marginal structural model results presented in Table 2. Cumulative mortality was higher in participants who had depressive symptoms and had not initiated ART (Figure 2). In the joint classification of ART and depressive symptoms (Figure 3), participants who reported depressive symptoms and had not initiated ART had the highest cumulative mortality, while those women without depressive symptoms who had initiated ART had the lowest cumulative mortality over 8 years. We also present unadjusted cumulative mortality curves (Web Figure 1, available at http://aje.oxfordjournals.org/); in the unadjusted results, cumulative mortality was higher in both groups with depressive symptoms.
Figure 2.
Effects of antiretroviral therapy (ART) initiation and depressive symptoms on cumulative mortality in the Women's Interagency HIV Study, United States, 1998–2011. Women who had not initiated ART are represented by the solid line and women who had initiated ART by the dashed line (A). Women experiencing depressive symptoms are represented by the solid line and women not experiencing depressive symptoms by the dashed line (B). Weighted models adjusted for age at enrollment, race, CD4 cell count, log10 HIV RNA, baseline CD4 cell count, and baseline log10 HIV RNA. Weights for depressive symptoms also adjusted for depressive symptoms at the previous visit.
Table 2.
Unadjusted and Weighted Joint Effects of ART Initiation and Depressive Symptoms on All-Cause Mortality in the Women's Interagency HIV Study, United States, 1998–2011
| Model and ART/Depressive Symptom Status | Deaths | Women-Years | IRa | HR | 95% CI | P for Interactionb |
|---|---|---|---|---|---|---|
| Unadjusted | ||||||
| ART, no depressive symptoms | 36 | 2,341 | 1.54 | 1 | 0.80 | |
| ART, depressive symptoms | 86 | 1,686 | 5.10 | 3.35 | 2.26, 4.96 | |
| No ART, no depressive symptoms | 19 | 1,506 | 1.26 | 0.88 | 0.50, 1.54 | |
| No ART, depressive symptoms | 53 | 1,187 | 4.46 | 3.20 | 2.06, 4.97 | |
| Weightedc | ||||||
| ART, no depressive symptoms | 34 | 2,548 | 1.35 | 1 | 0.78 | |
| ART, depressive symptoms | 86 | 1,968 | 4.39 | 3.60 | 2.02, 6.43 | |
| No ART, no depressive symptoms | 33 | 1,356 | 2.46 | 2.36 | 1.16, 4.81 | |
| No ART, depressive symptoms | 86 | 1,136 | 7.55 | 7.47 | 3.91, 14.3 | |
| Truncatedd | ||||||
| ART, no depressive symptoms | 34 | 2,517 | 1.36 | 1 | 0.78 | |
| ART, depressive symptoms | 80 | 1,877 | 4.27 | 3.32 | 1.99, 5.54 | |
| No ART, no depressive symptoms | 33 | 1,348 | 2.47 | 2.25 | 1.14, 4.43 | |
| No ART, depressive symptoms | 80 | 1,129 | 7.06 | 6.64 | 3.69, 11.9 | |
| Weightede | ||||||
| ART, no depressive symptoms | 49 | 2,813 | 1.73 | 1 | 0.92 | |
| ART, depressive symptoms | 86 | 2,037 | 4.21 | 3.23 | 1.61, 6.47 | |
| No ART, no depressive symptoms | 32 | 1,366 | 2.36 | 1.94 | 0.91, 4.15 | |
| No ART, depressive symptoms | 87 | 1,133 | 7.64 | 6.58 | 3.28, 13.2 | |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; IR, incidence rate.
a Incidence rate per 100 women-years.
bP value for the term for interaction between the 2 joint exposures, ART initiation and depressive symptoms.
c Weighted models adjusted for age at enrollment, race, CD4 cell count, log10 HIV RNA, baseline CD4 cell count, and baseline log10 HIV RNA. Weights for depressive symptoms also adjusted for depressive symptoms at the previous visit.
d Weights truncated at 0.1 and 10.
e Weighted models further adjusted for substance abuse.
Figure 3.
Joint effects of antiretroviral therapy (ART) initiation and depressive symptoms on cumulative mortality in the Women's Interagency HIV Study, United States, 1998–2011. Women with depressive symptoms who had not initiated ART are represented by the solid black line, women with depressive symptoms who had initiated ART by the solid gray line; women without depressive symptoms who had not initiated ART by the dashed black line, and women without depressive symptoms who had initiated ART by the dashed gray line. Weighted models adjusted for age at enrollment, race, CD4 cell count, log10 HIV RNA, baseline CD4 cell count, and baseline log10 HIV RNA. Weights for depressive symptoms also adjusted for depressive symptoms at the previous visit.
Table 2 presents the crude and weighted incidence rates and hazard ratios. The overall marginal effect of ART initiation on mortality was 0.47 (95% confidence interval (CI): 0.31, 0.70), and the marginal effect of depressive symptoms on mortality was 3.38 (95% CI: 2.15, 5.33). Setting women who did not report depressive symptoms and had initiated ART as a reference category, we found a strong harmful effect of other combinations of these 2 exposures on mortality. Women who reported depressive symptoms and had initiated ART had a hazard ratio of 3.60 (95% CI: 2.02, 6.43). The hazard ratio for women without depressive symptoms who had not initiated ART was 2.36 (95% CI: 1.16, 4.81), which was subject to substantial time-varying confounding. For those women with depressive symptoms who had not initiated ART, the hazard ratio was 7.47 (95% CI: 3.91, 14.3). The Wald test P value for the term for interaction between ART initiation and depressive symptoms in the joint marginal structural weighted model was 0.78, indicating no strong evidence for departure from multiplicative effects. We also found no significant deviation from additivity, with a relative excess risk of interaction of −1.06 (95% CI: −2.90, 0.78).
In our sensitivity analysis allowing ART use to vary over time, results were similar to those of our primary analysis, with some attenuation towards the null value. The hazard ratio for women with depressive symptoms who had initiated ART was 2.34 (95% CI: 1.08, 5.08). For women without depressive symptoms who had not initiated ART, the hazard ratio was 1.64 (95% CI: 0.67, 4.01). For women with depressive symptoms who had not initiated ART, the hazard ratio was 4.27 (95% CI: 1.88, 9.70). Finally, when removing somatic items from the calculation of the CES-D score, the results were further attenuated. Women with depressive symptoms who had not initiated ART had a hazard ratio of 3.32 (95% CI: 1.53, 7.20).
DISCUSSION
Using a joint marginal structural model, we found a strong protective effect of ART initiation, and a strong harmful effect of depressive symptoms on all-cause mortality in a cohort of HIV-infected women. In a joint model, mortality among women who either reported depressive symptoms or had not initiated ART was greatly increased compared with women who did not report depressive symptoms and had initiated ART. Strikingly, the harmful effect on mortality of depressive symptoms alone (hazard ratio = 3.60) was greater in magnitude than the effect on mortality of failing to initiate ART alone (hazard ratio = 2.36). The effect of the combined exposures was particularly strong in the scenario of no ART initiation with depressive symptoms: Although the product term was not statistically significant, the hazard of death for these women was over 7 times that of women without depressive symptoms who had initiated ART. Notably, our findings echoed previous research in a British Columbia cohort, where the hazard ratio for the effect of depressive symptoms among patients with lower adherence to ART was 5.90 (19).
These findings tend to support the importance of effective treatment of depression among women with HIV infection. Conversely—and as expected—among patients with or without depressive symptoms, ART initiation and adherence is clearly critical. Notably, we did not find evidence of interaction between these 2 common exposures on either the multiplicative or additive scale, in accordance with previous work (19). By assessing these very common exposures jointly, we provide evidence that they do not combine beyond what would be expected in a multiplicative or additive fashion.
Prior research has shown a strong protective effect of ART initiation on mortality; our mortality hazard ratio of 0.47 (95% CI: 0.31, 0.70) was similar to results from the Multicenter AIDS Cohort Study/WIHS (25), and the HIV-CAUSAL Collaboration (40). Although we cannot preclude the possibility of interaction between these 2 exposures, the protective effect of ART was similar to prior estimates when examined jointly with depressive symptoms. The effect of depressive symptoms on mortality is less clearly demonstrated in the literature. Results prior to the advent of modern combination ART indicated no effect of depressive symptoms on mortality (21); yet more recent studies have shown a harmful effect (14–20). Our results similarly indicate a harmful effect of depressive symptoms on mortality.
The risk of major depression in HIV-infected women is much higher than in HIV-infected men and is associated with disease progression (6, 20). Depression is associated with impaired immunity among HIV-infected women (6, 20, 41, 42). Depression reduces natural killer cell activity and increases CD8 T-lymphocyte activity and viral load, which is one possible mechanism through which depression may increase mortality (6, 20, 41, 42).
A causal interpretation of our results requires the assumptions of treatment-variation irrelevance (essentially consistency), no interference, conditional exchangeability, positivity, and correct model specification. Treatment-variation irrelevance is the assumption that the means through which a treatment or exposure is delivered is irrelevant to the potential outcome (37). More informally, treatment-variation irrelevance requires that there is either a single version of treatment, or that the multiple versions are exchangeable. Many versions of ART existed throughout the study period; our assumption of treatment-variation irrelevance is strong but a necessary simplifying assumption given the size of our cohort and number of regimens. Additionally, the dichotomization of depressive symptoms may be a violation of treatment-variation irrelevance, but it is the typical interpretation of the CES-D in the existing literature and is necessary for stable estimates in these data. The assumption of no interference between subjects requires that an individual's outcome is independent of the treatment received by other individuals, which seems a reasonable assumption in this cohort (38). Conditional exchangeability occurs when potential outcomes are independent of the treatment received given measured confounders (36, 39). This is often informally stated as “no unmeasured confounding and no informative censoring.” As with most observational studies, unmeasured confounding is a possible source of bias in our study. Positivity requires that for all combinations of confounders, there is a nonzero probability of receiving the treatments (36). If treatment is not possible for a particular confounding level, then the positivity assumption is violated. Given our relatively limited adjustment set and well-behaved weights, we consider any violations of the positivity assumption to be minor. Our weights also provide some support for the assumption of correct model specification.
Some aspects of the study design may limit our inferences. Of note, the sample size of the study was relatively small, as indicated by modest precision of our estimates. We considered the restriction of our study sample to women without prevalent ART use to be important in preventing prevalent-user bias (30), but this restriction did limit the sample and resulting precision. Our assumption that ART use was constant after ART initiation is optimistic. Although women are counseled on the importance of adherence to therapy, interruptions of therapy occur and may be the result of depressive symptoms (42). However, we interrogated this assumption with a sensitivity analysis where we allowed ART use to vary over time. We also used a measurement for depression (depressive symptoms) that is based upon a dichotomization of a continuous, imperfect assessment. The CES-D, used to measure depressive symptoms, may be limited as a mortality predictor in a population experiencing high morbidity due to overlap with somatic symptoms such as poor appetite and fatigue. Finally, as with all models, misspecification is a concern. While our relatively well-behaved weights provide circumstantial evidence against model misspecification, the potential for bias due to model misspecification remains.
Our study addressed the joint effects of ART initiation and depressive symptoms in a population of HIV-infected women. We highlight the need for continued attention by HIV providers to the mental health, including specific conditions such as depression, of their patients. We report a strong protective effect of ART initiation on mortality in HIV-infected women and a harmful effect of depressive symptoms on mortality.
ACKNOWLEDGMENTS
Author affiliations: Gillings School of Global Public Health, Department of Epidemiology, University of North Carolina, Chapel Hill, North Carolina (Jonathan V. Todd, Stephen R. Cole, Brian W. Pence, Catherine R. Lesko, Adaora A. Adimora); Institute for Global Health and Infectious Diseases, University of North Carolina, Chapel Hill, North Carolina (Jonathan V. Todd, Stephen R. Cole, Adaora A. Adimora); Department of Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, California (Peter Bacchetti); Department of Medicine, Cook County Health and Hospital System, Chicago, Illinois (Mardge H. Cohen); Department of Medicine, Rush University, Chicago, Illinois (Mardge H. Cohen); Division of Biostatistics, Department of Public Health Sciences, Miller School of Medicine, University of Miami, Miami, Florida (Daniel J. Feaster); Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (Catherine R. Lesko, Stephen Gange); Center of Biostatistics and Bioinformatics, University of Mississippi Medical Center, Jackson, Mississippi (Michael E. Griswold); Division of Biostatistics, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California (Wendy Mack); Department of Behavioral Sciences and Health Education, Rollins School of Public Health, Emory University, Atlanta, Georgia (Anna Rubtsova); Women's Interagency HIV Study, Georgetown University Medical Center, Washington, DC (Cuiwei Wang); Department of Epidemiology and Biostatistics, Downstate Medical Center School of Public Health, State University of New York, Brooklyn, New York (Jeremy Weedon); and Einstein Global Health Center, Albert Einstein College of Medicine, Bronx, New York (Kathryn Anastos).
This work was supported by the National Institutes of Health (grants U01AI103390 (WIHS-NC), R01AI100654, and P30AI50410 (CFAR)). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Mental Health. Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Deafness and other Communication Disorders, and the National Institutes of Health Office of Research on Women's Health. WIHS data collection is also supported by the University of California, San Francisco, CTSA (grant UL1-TR000004) and Atlanta CTSA (grant UL1-TR000454).
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health. WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Mary Young), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV).
We thank Dr. Chanelle Howe and Dr. Andrew Edmonds for expert advice. We also thank the principal investigators, co-investigators, and research staff at participating Women's Interagency HIV Study sites.
Conflicts of interest: C.R.L. reports personal fees from Gilead Sciences. K.A. reports personal fees from Miriam Hospital of Rhode Island and personal fees from Bristol Myers Squibb. A.A.A. reports personal fees from Viiv and grants from Gilead Sciences. The other authors report no conflicts.
REFERENCES
- 1. Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–728. [DOI] [PubMed] [Google Scholar]
- 2. Orlando M, Burnam MA, Beckman R, et al. Re-estimating the prevalence of psychiatric disorders in a nationally representative sample of persons receiving care for HIV: results from the HIV Cost and Services Utilization Study. Int J Methods Psychiatr Res. 2002;11(2):75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pence BW, Miller WC, Whetten K, et al. Prevalence of DSM-IV-defined mood, anxiety, and substance use disorders in an HIV clinic in the Southeastern United States. J Acquir Immune Defic Syndr. 2006;42(3):298–306. [DOI] [PubMed] [Google Scholar]
- 4. Israelski DM, Prentiss DE, Lubega S, et al. Psychiatric co-morbidity in vulnerable populations receiving primary care for HIV/AIDS. AIDS Care. 2007;19(2):220–225. [DOI] [PubMed] [Google Scholar]
- 5. Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–730. [DOI] [PubMed] [Google Scholar]
- 6. Evans DL, Ten Have TR, Douglas SD, et al. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry. 2002;159(10):1752–1759. [DOI] [PubMed] [Google Scholar]
- 7. Atkinson JH, Heaton RK, Patterson TL, et al. Two-year prospective study of major depressive disorder in HIV-infected men. J Affect Disord. 2008;108(3):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perkins DO, Stern RA, Golden RN, et al. Mood disorders in HIV infection: prevalence and risk factors in a nonepicenter of the AIDS epidemic. Am J Psychiatry. 1994;151(2):233–236. [DOI] [PubMed] [Google Scholar]
- 9. Kelly B, Raphael B, Judd F, et al. Psychiatric disorder in HIV infection. Aust NZ J Psychiatry. 1998;32(3):441–453. [DOI] [PubMed] [Google Scholar]
- 10. Lipsitz JD, Williams JB, Rabkin JG, et al. Psychopathology in male and female intravenous drug users with and without HIV infection. Am J Psychiatry. 1994;151(11):1662–1668. [DOI] [PubMed] [Google Scholar]
- 11. Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5(4):163–171. [DOI] [PubMed] [Google Scholar]
- 12. Tsai AC. Reliability and validity of depression assessment among persons with HIV in sub-Saharan Africa: systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2014;66(5):503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 4th ed: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 14. Anastos K, Schneider MF, Gange SJ, et al. The association of race, sociodemographic, and behavioral characteristics with response to highly active antiretroviral therapy in women. J Acquir Immune Defic Syndr. 2005;39(5):537–544. [PubMed] [Google Scholar]
- 15. Murphy K, Hoover DR, Shi Q, et al. Association of self-reported race with AIDS death in continuous HAART users in a cohort of HIV-infected women in the United States. AIDS. 2013;27(15):2413–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wada N, Jacobson LP, Cohen M, et al. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol. 2013;177(2):116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. French AL, Gawel SH, Hershow R, et al. Trends in mortality and causes of death among women with HIV in the United States: a 10-year study. J Acquir Immune Defic Syndr. 2009;51(4):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cook JA, Grey D, Burke J, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94(7):1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lima VD, Geller J, Bangsberg DR, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21(9):1175–1183. [DOI] [PubMed] [Google Scholar]
- 20. Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–1474. [DOI] [PubMed] [Google Scholar]
- 21. Lyketsos CG, Hoover DR, Guccione M, et al. Depressive symptoms as predictors of medical outcomes in HIV infection. Multicenter AIDS Cohort Study. JAMA. 1993;270(21):2563–2567. [PubMed] [Google Scholar]
- 22. Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70(5):539–545. [DOI] [PubMed] [Google Scholar]
- 23. Arseniou S, Arvaniti A, Samakouri M. HIV infection and depression. Psychiatry Clin Neurosci. 2014;68(2):96–109. [DOI] [PubMed] [Google Scholar]
- 24. Wagner GJ, Ghosh-Dastidar B, Garnett J, et al. Impact of HIV antiretroviral therapy on depression and mental health among clients with HIV in Uganda. Psychosom Med. 2012;74(9):883–890. [DOI] [PubMed] [Google Scholar]
- 25. Cole SR, Hernán MA, Robins JM, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158(7):687–694. [DOI] [PubMed] [Google Scholar]
- 26. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the joint causal effect of nonrandomized treatments. J Am Stat Assoc. 2001;96(454):440–448. [Google Scholar]
- 27. Howe CJ, Cole SR, Mehta SH, et al. Estimating the effects of multiple time-varying exposures using joint marginal structural models: alcohol consumption, injection drug use, and HIV acquisition. Epidemiology. 2012;23(4):574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bacon MC, Von Wyl V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 30. Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. [DOI] [PubMed] [Google Scholar]
- 31. Panel on Clinical Practices for Treatment of HIV Infection, US Department of Health and Human Services and Henry J. Kaiser Family Foundation Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Bethesda, MD: AIDSinfo, National Institutes of Health, 2008. [Google Scholar]
- 32. Cole SR, Hernán MA, Margolick JB, et al. Marginal structural models for estimating the effect of highly active antiretroviral therapy initiation on CD4 cell count. Am J Epidemiol. 2005;162(5):471–478. [DOI] [PubMed] [Google Scholar]
- 33. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 34. Weissman MM, Sholomskas D, Pottenger M, et al. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–214. [DOI] [PubMed] [Google Scholar]
- 35. Kalichman SC, Rompa D, Cage M. Distinguishing between overlapping somatic symptoms of depression and HIV disease in people living with HIV-AIDS. J Nerv Ment Dis. 2000;188(10):662–670. [DOI] [PubMed] [Google Scholar]
- 36. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. VanderWeele TJ. Concerning the consistency assumption in causal inference. Epidemiology. 2009;20(6):880–883. [DOI] [PubMed] [Google Scholar]
- 38. Hudgens MG, Halloran ME. Toward causal inference with interference. J Am Stat Assoc. 2008;103(482):832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 40. Ray M, Logan R, Sterne JA, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24(1):123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cruess DG, Douglas SD, Petitto JM, et al. Association of resolution of major depression with increased natural killer cell activity among HIV-seropositive women. Am J Psychiatry. 2005;162(11):2125–2130. [DOI] [PubMed] [Google Scholar]
- 42. Gonzalez JS, Batchelder AW, Psaros C, et al. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]