Abstract
Coinfection of microorganisms is a common phenomenon in humans and animals. In order to further our understanding of the progress of coinfection and the possible interaction between different pathogens, we have built a coinfection mouse model with Schistosoma japonicum and Salmonella typhimurium, and used this model to investigate the systemic metabolic and immune responses using NMR-based metabonomics and immunological techniques. Our results show that Salmonella typhimurium (ATCC14028) infection reduces the number of adult schistosomal worms and eggs, relieves symptoms of schistosomiasis and also abates the mortality of mice infected by Schistosoma japonicum. In addition, Salmonella typhimurium infection counteracts the metabolic disturbances associated with schistosomiasis, which was reflected by the reverted levels of metabolites in coinfected mice, compared with the Schistosoma japonicum infected mice. Furthermore, immune analyses also indicate that shift of the immune response to different pathogens is a result of indirect interactions between Schistosoma japonicum and Salmonella typhimurium within the host. Salmonella typhimurium infection can ameliorate Schistosoma japonicum-caused schistosomiasis in BALB/c mice, which is most likely due to inverse immune polarization. Our work provides an insight into coinfection between Schistosoma japonicum and Salmonella typhimurium, and may further contribute to the development of new tools for controlling Schistosoma japonicum-associated diseases.
Introduction
Coinfection with diverse pathogens (e.g. virus, bacterium, fungus, protozoan and helminth) is a common occurrence in humans1–4, and this is estimated to exceed one sixth of the global population5. People infected with multiple helminths reached over 800 million6, mostly of which occurred in developing countries7. Other microorganisms, including Salmonellae 8, Mycobacterium tuberculosis 9, Helicobacter pylori 10, human immunodeficiency virus11, hepatitis C virus12, dengue virus13 etc, are also involved in coinfections. Compared to infection caused by one single pathogenic species, coinfection can often alter the pathogenicity of infectious diseases14, 15, availability of hosts16, clinical severity17, and thus influence the prevention and control of pathogen-associated diseases18. Therefore, further understanding on the mechanisms of coinfection is greatly needed.
Schistosomiasis is one of the most debilitating and widespread human diseases caused by infection with a parasitic blood fluke called Schistosome. Schistosome infection causes severe damage to the host and can eventually lead to death19. Coinfection of Schistosome and other pathogens is also frequent in epidemic areas20, 21 where people are exposed to helminths and other bacterial pathogens, such as Mycobacterium tuberculosis 22, or Salmonella 8. Although numerous studies have sought to uncover the interactions and mutual influences between these concurrent pathogens12, 23, 24, little is known regarding how the host response to these concurrent infections. Previously, we investigated the metabolic effects of hamsters coinfected with Schistosoma japonicum (S. japonicum) and Necator americanus 25. We found that coinfection, surprisingly, had no impact on the worm burden and that metabolic response of the host was sum of the two-single infections25. This could be due to both parasites being introduced to the host simultaneously, thereby removing any competition for energy resources or growth. It may also be possible that distinct immune responses stimulated by either helminth failed to suppress the other pathogen’s growth. Therefore in this study, we aimed to assess the impact of sequential infection of helminths and bacteria on the host. We chose the coinfection of Salmonella typhimurium (S. typhimurium) and S. japonicum as both pathogens are widespread in developing countries26, 27, therefore reflecting the high likelihood of concurrent Salmonellae and Schistosome infections28–31. Schistosome infection can result in a shift of host immune response from Th1 to Th2 polarization, which is in parallel with the progress of the schistosomiasis, including cercariae intruding, larvae migrating, the adult pairing and laying eggs19, whereas S. typhimurium infection only induces Th1 polarization32. Therefore immune interaction between these two species in the same host could be an important clinical consideration.
Evidence from previous investigations have shown that metabonomics is a robust tool for studying the metabolic responses to stimuli33, such as from a coinfection25. Metabonomics utilizes 1H nuclear magnetic resonance (NMR) spectroscopy or chromatography coupled to mass spectrometry, with multivariate statistical analysis for detecting metabolic changes of a system subjected to stress34. In this study, we have applied NMR-based metabonomics and immune techniques to investigate the impact of coinfection with S. typhimurium and S. japonicum on mice. We infected mice with S. japonicum, followed by S. typhimurium once schistosomiasis was fully established. We found that coinfection with S. typhimurium can ameliorate schistosomiasis in terms of worm burden and metabolic alterations associated with the infection, mostly due to host’s self-immune responses manipulated by the secondary bacterial infection. Our finding mirrored increasingly successful immunotherapy for treating cancers35, where the self-immune response of host plays a vital role. Our research provided important information about the impact of two infectious organisms on the host, which could provide an alternative avenue for treating schistosomiasis.
Results
Worm burden, egg burden in liver, animal survival rate and histopathology
A total of 19% of adult schistosomes were retrieved from the mice coinfected with S. japonicum and S. typhimurium (CI), which is significantly lower compared with 44% from mice with S. japonicum infection alone (SJ) (Fig. 1A). In addition, a reduction of S. japonicum eggs trapped in livers was also found from coinfected mice (Fig. 1B). This observation was confirmed by the histological examination of liver and intestinal tissues (Fig. 2). These data show that mice coinfected with S. japonicum and S. typhimurium were able to reduce the worm and egg burden of schistosomiasis and as a consequence, increase the survival rate of the coinfected mice. At the end of experiment, i.e. nine days after S. typhimurium ATCC14028 inoculation, approximately 70% of mice in the CI group survived and only about 40% survived in SJ group (Fig. 1C).
Figure 1.
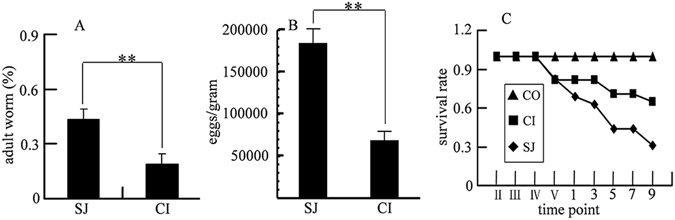
Worm burden, liver egg burden and animal survival rate. (A) Adult worm of S. japonicum obtained from mice infected with S. japonicum (SJ) and those coinfected with S. japonicum and S. typhimurium (CI); (B) S. japonicum eggs obtained from liver of mice infected with S. japonicum (SJ) and those coinfected with S. japonicum and S. typhimurium (CI); (C) Survival rate of mice in different groups. SJ, mice infected with 80 cercariae of S. japonicum only; CI, mice infected with 80 cercariae followed by S. typhimurium ATCC14028 infection 5 weeks later; CO, mice as uninfected control. Time points in Roman numerical mean corresponding weekly points, time points in Arabic numerical indicates days after S. typhimurium was introduced. The statistically significant differences were calculated using independent samples T test with SPSS 13.0 and error bars represent SEM, **p < 0.01.
Figure 2.
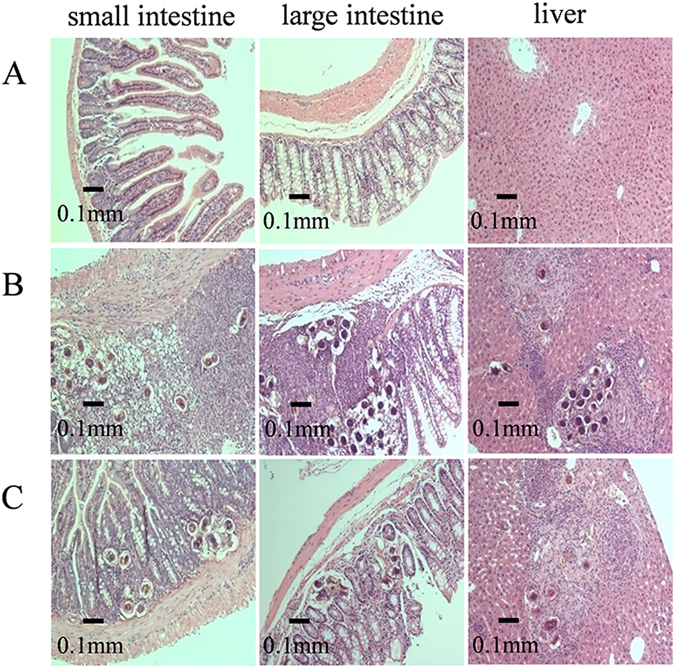
Histopathology examination of intestinal tissues and livers. The panel A was from control group, panels B and C were from single infection of S. japonicum and coinfection of S. japonicum and S. typhimurium respectively.
Metabolic changes in urine associated with coinfection
In order to identify metabolic changes associated with reduction in worm burden in coinfected mice, we investigated the metabolic profiles of urine, serum and liver extracts of mice from single infected and coinfected groups. A total of nearly 60 metabolites were identified from 1H NMR spectra of urine, serum and liver tissue extracts (Supplementary Fig. S1 and Table S1). Metabolic compositions of urine samples were mainly dominated by organic acids, whereas those of serum and liver extracts were dominated by lipids, amino acids, membrane metabolites, glucose and metabolites of RNA and DNA.
We compared urinary metabolic profiles of mice infected with S. japonicum and mice coinfected with S. japonicum followed by S. typhimurium, with control urine, at 5 days and 7 days after infection of S. typhimurium (i.e. 5 weeks + 5 days and 5 weeks + 7 days). The O-PLS-DA of the metabolic profiles showed that the levels of 13 metabolites had significantly changed after S. japonicum single infection (Fig. 3A and Supplementary Table S2) and almost identical changes were noted two days after (Fig. 3C and Supplementary Table S2). These were the elevated levels of 2-keto-isovalerate, N-methylnicotinamide (NMN), dimethylglycine (DMG), creatine, creatinine, taurine, 3-ureidopropionate (3-UP) and depleted levels of trigonelline, fumarate, trimethylamine-N-oxide (TMAO), hippurate and N-acetylglutamic acid. Considerable numbers of metabolites (including taurine, citrate, creatine and DMG) in the coinfected group reverted back to the levels comparable with uninfected control group (Fig. 3B,D and Supplementary Table S2) at both of the time points.
Figure 3.
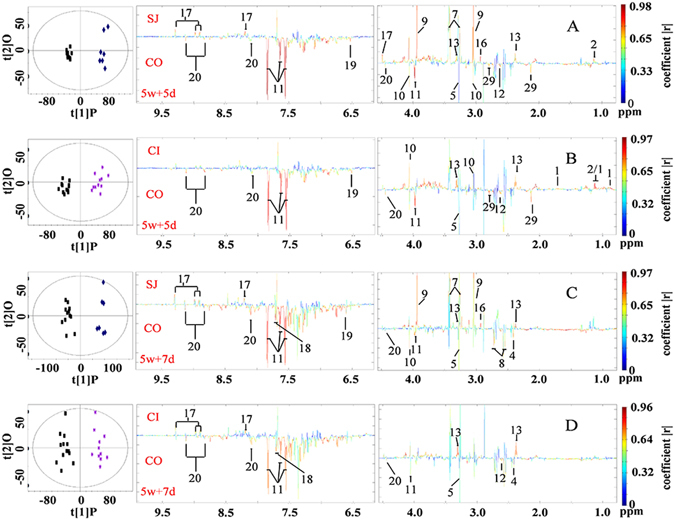
Scores plots and corresponding color-coded coefficient plots. These plots derived from O-PLS-DA of 1H NMR spectral data obtained from urine of uninfected control mice (CO), S. japonicum infected mice (SJ) and mice coinfected with S. japonicum and S. typhimurium (CI). Signals upwards indicate increase in the levels of metabolites compared with the control; signals downwards denote a decrease. 5w + 5d, time point, five days after coinfection; 5w + 7d, time point, seven days after coinfection. Keys: 1, 2-keto-3-methy-valerate; 2, 2-keto-isovalerate; 4, succinate; 5, trimethylamine-N-oxide (TMAO); 7, taurine; 8, citrate; 9, creatine; 10, creatinine; 11, hippurate; 12, 2-keto-isocaproate; 13, 3-ureidopropionate (3-UP); 16, dimethylglycine (DMG); 17, N-methylnicotinamide; 18, indoxyl sulfate; 19, fumarate; 20, trigonelline; 29, N-acetylglutamic acid.
Metabolic changes in liver associated with coinfection
We analyzed the metabolic characteristics of the liver obtained from all infected mice. The levels of 23 metabolites significantly changed in liver tissues obtained from mice in the SJ group compared to those obtained from control mice (Fig. 4A and Supplementary Table S3), including an increase in the levels of a range of amino acids, TMAO, choline metabolites, uridine, adenine, inosine and uracil, and decreased levels of succinate, 3-hydroxybutyrate (3-HB), bile acid, GSSG, adenosine, niacinamide, AMP and ATP. Levels of 16 metabolites significantly changed in liver tissues of mice in the CI group compared to controls (Fig. 4B and Supplementary Table S3). The levels of several amino acids, TMAO, succinate and uridine in the coinfected group also reverted back to the levels comparable to controls (Fig. 4B).
Figure 4.
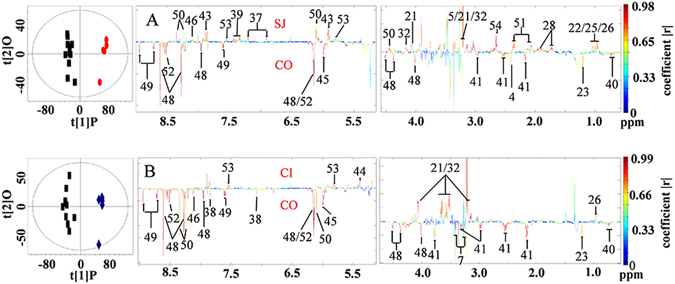
Scores plots and corresponding color-coded coefficient plots. These plots derived from O-PLS-DA of 1H NMR spectra of liver extracts obtained from uninfected control mice (CO), S. japonicum infected mice (SJ), S. japonicum and S. typhimurium ATCC14028coinfected mice (CI). Signals upwards denote increases in the level compared to the control; signals downwards indicate decrease in the levels compared to the control. Keys: 4, succinate; 5, trimethylamine-N-oxide (TMAO); 7, taurine; 21, choline; 22, valine; 23, 3-hydroxybutyrate (3-HB); 25, isoleucine; 26, leucine; 28, lysine; 32, phosphocholine; 37, tyrosine; 38, histidine; 39, phenylalanine; 40, bile acid; 41, oxidized glutathione (GSSG); 43, uridine; 44, glycogen; 45, adenosine; 46, adenine; 48, adenosine triphosphate (AMP); 49, niacinamide; 50, inosine; 51, glutamate; 52, ATP; 53, uracil; 54, methionine.
Metabolic changes in serum associated with coinfection
In serum, we found that elevated levels of a range of amino acids, O-acetylglycoproteins, lipids and decreased levels of citrate, malonate, fumarate and 3-HB, were associated with S. japonicum single infection (Fig. 5A and Supplementary Table S4). Metabolic alterations associated with coinfection (CI) were similar to those found in the single infection (SJ) and only two metabolites (fumarate, malonate) reverted back to the levels comparable to controls, which is fewer than the results obtained from urine and liver tissues (Fig. 5B and Supplementary Table S4), where many metabolites altered by single infection were returned to the level comparable to those of controls.
Figure 5.
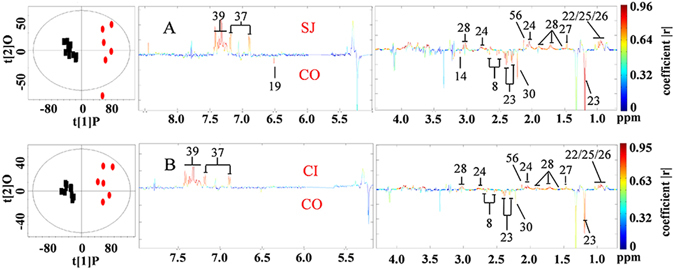
Scores plots and corresponding color-coded coefficient plots. These plots derived from O-PLS-DA of 1H NMR spectra obtained from serum of uninfected control mice (CO), S. japonicum infected mice (SJ), S. japonicum and S. typhimurium coinfected mice (CI). Signals upwards, increase compared with the control; signals downwards, decrease compared with the control. Keys: 8, citrate; 14, malonate; 19, fumarate; 22, valine; 23, 3-hydroxybutyrate; 24, lipids; 25, isoleucine; 26, leucine; 27, alanine; 28, lysine; 30, acetone; 37, tyrosine; 39, phenylalanine; 56, O-acetylglycoproteins.
IL-4 and IFN-γ in serum of coinfection
We further evaluated the impact of coinfection on cytokines in mice. Single infection with S. japonicum induced elevations of IFN-γ and coinfection further elevated the levels of IFN-γ (Fig. 6A). However, while a single infection of S. japonicum resulted in increased level of IL-4, a decrease in IL-4 was observed in mice of the CI group (Fig. 6B).
Figure 6.
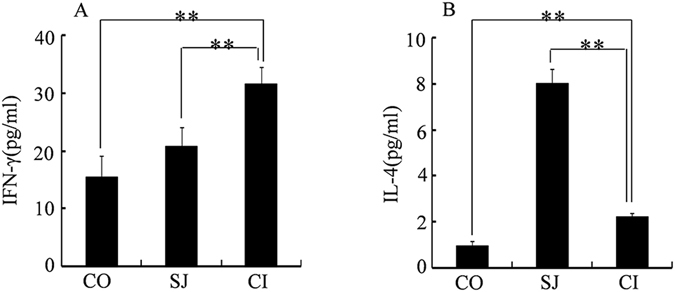
Concentrations (pg/mL) of IFN-γ (A), IL-4 (B) in serum. CO, control mice; SJ, mice infected with S. japonicum and CI, mice coinfected with S. japonicum and S. typhimurium. The statistically significant differences were calculated using independent samples T test with SPSS 13.0 and error bars represent SEM, **p < 0.01.
Discussion
In contrast to laboratory animals, humans and other animals in nature are often simultaneously exposed to a large number of acute or chronic infections caused by viruses (HIV, HBV, influenza virus)36, 37, bacteria (Haemophilus influenzae, S. typhimurium, Staphylococcus aureus)38 or helminths (Plasmodium falciparum, S. japonicum)39. Many of these pathogens could be present in the same host concurrently, thereby affecting severity of the infection and disrupting normal metabolism of the host. For this reason, investigation on coinfection is of great importance.
One of the most important findings of the current investigation is that coinfection with S. typhimurium (ATCC14028) reduces worm burden and improves the survival rate of S. japonicum infected mice (Fig. 1). This is surprising since wild-type S. typhimurium (ATCC14028) is generally lethal to rodents40, but when infected in mice previously inoculated with S. japonicum, it can be beneficial to the host. The improved survival rate is likely due to reduction in the numbers of adult schistosomes and eggs accumulated in the liver and intestinal tissues (Figs 1A,B and 2). The pathogenesis of chronic schistosomiasis is mostly due to the eggs laid by paired adult schistosome that is trapped in the liver41. In normal cases, a pair of adult S. japonicum produces approximately 3000 eggs per day, which will be transported into the liver and intestine where they inhabit42. The soluble proteinase secreted by the eggs initiates host immune responses and provokes the typical eosinophilic inflammatory and subsequent granulomatous reaction, and eventually leads to liver fibrosis19. This process in normal circumstances is irreversible even with drug treatment since drugs only kill adult worms but cannot remove eggs that have already deposited in tissues. However, our results showed that co-infecting mice with S. typhimurium could potentially reverse the process of schistosomiasis by not only reducing number of adult worms but also reducing the numbers of eggs trapped in liver and intestinal tissues.
The complex immune interaction between the two pathogens within the host could play an important role in the observed phenomenon. The early lifecycle of S. japonicum induces the host to develop Th1-polarized cellular immunity in response to S. japonicum antigens43. After 3 weeks, when paired adult S. japonicum worms begin to lay eggs, egg antigens trigger the host immune response towards Th2-mediated humoral immunity44. In contrast, the immune response to S. typhimurium infection is predominantly Th1-polarized45, 46. In our investigation, S. typhimurium infection was introduced after five weeks of S. japonicum infection, during which time the host’s immune response was Th2-polarized. However, the level of Th2-type cytokine IL-4 in serum decreased sharply while the Th1-type cytokine IFN-γ increased (Fig. 6) nine days after coinfection with S. typhimurium. This indicates that S. typhimurium infection had stimulated the host immune response to re-establish Th1 polarization. This immune transition coincided with clearance of adult worms and schistosome eggs, indicating that re-establishment of Th1 polarized immune response in host mice could ameliorate schistosomiasis, and most importantly, is associated with reduced organ damage caused by schistosome eggs (Fig. 7). Th1 polarized immune response induced by malaria infection was used for treating neurosyphilis at early twenty century by Julius Wagner-Jauregg who was awarded to the Nobel Prize47, 48. Previous studies have found that attenuated S. typhimurium carrying Schistosome-antigen can provide high levels of protection against Schistosome infection in a mouse model49. The mechanism of the protection effect could be partly similar to our current investigation. The coinfection associated amelioration effects to organ and system, which could be indicated by the restoration of these metabolite levels in the coinfected mice.
Figure 7.
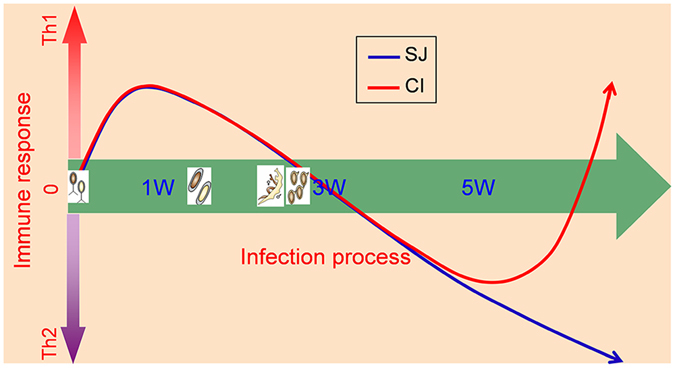
Schematic graph showing the change of immune response of mice to different infections. SJ, immune response of mice infected by S. japonicum only; CI, immune response of mice infected by S. japonicum firstly then by S. typhimurium ATCC14028 five weeks later. Th1, type 1-T helper cell polarized immune response; Th2, type-2 T helper cell polarized immune response. 1 W, 3 W, 5 W, different time points after infection.
The liver is one target organ of S. japonicum. In our investigation, we observed significant changes in levels of metabonomes within the liver of mice with S. japonicum infection, in particular, the accumulated amino acids, which has been frequently documented50–53. Accumulation of amino acids in the liver indicates an S. japonicum-associated liver injury. We showed that coinfection with S. typhimurium could ameliorate liver injury as suggested by several of amino acids being lapsed to the levels equivalent to controls (Fig. 4 and Supplementary Table S3). Regained function of amino acid metabolism of liver after coinfection could also been manifested in the increased levels of 2-keto-3-methy-valerate in urine of coinfected mice (Supplementary Table S2), a catabolic product of isoleucine. Another important metabolic alteration associated with S. japonicum is suppression of the TCA cycle52, 53. In our study, we observed decreased levels of succinate in the liver of S. japonicum infected mice as well as decreased levels of fumarate in serum of S. japonicum infected mice, which are consistent with previous investigations52, 53. The depressed TCA cycle has been reverted by introducing secondary infection with S. typhimurium. This notion is supported by the leveling of these TCA cycle intermediates observed in the coinfected mice (Supplementary Table S2 and Table S4). Suppressed pyrimidine metabolism has been previously associated with schistosomiasis52, which is consistent with our findings, since there were accumulated levels of uridine and 3-ureidopropionate measured in the liver and urine of S. japonicum infected mice, respectively. However, it was unclear whether coinfection of S. japonicum with S. typhimurium restored pyrimidine metabolism to levels comparable to control mice, since we only noted restored levels of uridine, but not 3-ureidopropionate in liver (Supplementary Table S2 and Table S3). Given that urine samples were collected on Day 5 and Day 7 post coinfection, whereas liver samples were obtained on Day 9 post infection, the time difference between the collections may have contributed towards the discrepancies observed.
In conclusion, our study presents strong evidence that coinfection of mice with established schistosomiasis with a secondary pathogen, S. typhimurium, could ameliorate the severity of schistosomiasis. The beneficial effects of coinfection were evident due to the reduced burden of S. japonicum worms, associated increased survival rate of mice, and restored metabolic function. These effects are likely associated with a shift from a Th2 to Th1 polarized immune response, likely stimulated by the additional S. typhimurium infection. Our findings have thus highlighted the importance and complexity of poly-pathogenic infections. Our results have further suggested that stimulating certain immune responses with a second pathogen maybe used for treating certain debilitating diseases, such as schistosomiasis, which could potentially open an alternative avenue for disease control.
Methods
Animal experimental procedure
S. typhimurium ATCC14028 was cultured aerobically at 37 °C in Luria-Bertani (LB) broth (Sigma Aldrich) overnight and recovered by centrifugation at 10,000 g for 1 minute. The recovered cells were washed twice with sterile saline and re-suspended to the final concentration (109 CFU/mL).
Oncomelanias carrying S. japonicum cercariae were purchased from Jiangsu Institute of Parasitic Diseases. S. japonicum cercariae were released from oncomelanias, which were immersed in dechlorinated water under fluorescent lamp at 26 °C for 6 hours. The numbers of cercariae were counted in small water drops on cover slips under a dissecting microscope.
Six-week old specific pathogen free (SPF) female BALB/c mice were purchased from Vital River Laboratory (Beijing, China) and three weeks of acclimation were allowed before experiments were performed. Mice were housed in well ventilated plastic cages under controlled conditions with free access to rodent food and water (temperature, 22 °C; humidity, 60%; light-dark cycle, 12 h-12 h in SPF animal experimental facility of Wuhan Institute of Physics and Mathematics, CAS).
A total of 36 mice were randomly divided into three groups with each group of 12 mice: control group (CO), single infected group with S. japonicum (SJ) and co-infected group with S. japonicum followed with S. typhimurium (CI). Mice in single infected group with S. japonicum alone and co-infected group were inoculated with eighty S. japonicum cercariae each via abdominal skin, and mice in the CI group were further infected with S. typhimurium ATCC14028 (8 × 104CFU/mL, 0.3 mL per mouse) via gavage after five weeks of S. japonicum infection52, 54. Mice were sacrificed at 9 days after the second infection. Mice in control group and single infected cases were terminated at the same time as mice in the co-infected group. A urine sample was collected from each mouse at one day before and after inoculation, and at different time intervals (day 1, 3, 5, 7) after the second infection. Urine sample collections were carried out between 8:30–12:00 to avoid potential metabolic variation owning to diurnal rhythm. Adult schistosomes were collected after sacrifice of animals by perfusing the vasculature of mice with ice cold saline solution containing sodium heparin (25 IU/mL)55. In addition, worms resident in mesenteric veins were isolated. The collected worms were placed in ice cold saline solution and counted. Schistosome eggs trapped in liver tissues were also counted after digesting liver tissues with 4% KOH at 37 °C overnight49. Serum and liver tissue samples were collected at the same time. All samples were immersed in liquid nitrogen immediately after collection and stored at −80 °C until further analysis.
Ethics statement
All animal experimental procedures were performed in strict accordance with the National Guidelines for Experimental Animal Welfare (People’s Republic of China, 2006) and were approved by the Animal Welfare Committee of Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences (Permission No. S-051-10-04-OU).
Measurement of IL-4 and IFN-γ in serum and histopathology
The concentrations of IL-4 and IFN-γ in serum were quantified by IL-4 high sensitive ELISA kits (BMS613HS, eBioscience) and IFN-γ immunoassay kits (MIF00, R&D Systems Inc.) respectively. All measurements were performed according to the respective manufacturer’s protocol.
For histological examination, liver and intestine samples were fixed in 10% buffered neutral formalin, embedded in paraffin, and serially sectioned. Tissue sections were stained with hematoxylin and eosin. Images were captured using a Nikon E100 upright microscope.
1H NMR spectroscopy
100 µL urine sample was mixed with 400 µL water (10% D2O) and 50 µL phosphate buffer (1.5 M, pH = 7.4) containing 0.1% sodium 3-(trimethylsilyl) propionate-2,2,3,3-d 4 (TSP) as chemical shift reference and 0.1% NaN3 as aseptic agent56. After vortex and centrifugation at 16,000 g, 4 °C for 10 minutes, 500 µL supernatant was transferred into a 5 mm NMR tube.
Serum sample (200 µL) was mixed with 400 µL of phosphate buffer saline solution (45 mM, pH = 7.4, 50% D2O). The mixture was centrifuged at 11,000 g, 4 °C for 10 minutes and 550 µL supernatant was transferred into a 5 mm NMR tube.
Liver samples were extracted by mixing 50 mg tissue with 0.6 mL cold mixture of methanol and water (2:1, v:v), and homogenized by a tissuelyser for 90 seconds at 20 Hz followed by ultrasonication for three one-minute sessions with one minute interval. Supernatants were collected after centrifugation at 11,000 g, 4 °C for 10 minutes. The extraction procedure was repeated three times and supernatants from the same samples were combined. Methanol was removed by speed vacuum and extractions were freeze-dried. The resulting powder was reconstituted in 0.6 mL phosphate buffer (0.1 M, pH = 7.4, 50% D2O, 0.05% TSP and 0.1% NaN3) and 550 µL supernatant was transferred into a 5 mm NMR tube following centrifugation.
1H NMR spectra of urine and liver extracts were acquired at 298 K using Bruker AVIII 600 MHz NMR spectrometer (Bruker Biospin, Germany) with cryogenic probe, operating at proton frequency of 600.13 MHz. The first increment of NOESY pulse sequence (recycle delay-90°-t 1-90°-t m-90°-acquisition) with water suppression was employed. 1H NMR spectra of serum were acquired at 298 K on Bruker AVII 500 MHz NMR spectrometer with broad band inverse detection probe, operating at 500.13 MHz proton frequency. Spin-spin relaxation edited 1H NMR experiment using Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence with water saturation was performed for serum. Two-dimensional NMR spectra (1H-1H COSY and TOCSY, 1H-13C HSQC and HMBC) were acquired for selected samples to assist the spectral assignment.
NMR spectral data processing and multivariate pattern recognition analysis
1H NMR spectra were manually corrected for phase and baseline deformation using Topspin software package (V2.0, Bruker Biospin, Germany). Spectra of urine and liver extracts were referenced to TSP (δ, 0 ppm), whereas serum spectra were calibrated to the low field peak (δ, 5.233 ppm) of the doublets belonging to α-glucose. The spectra ranging from δ 0 to δ 10 ppm were integrated with an equal width of 0.002 ppm for urine and liver extracts and 0.004 ppm for serum using the AMIX package (V3.8, Bruker Biospin, Germany). Water regions were removed and normalization to total intensity of the spectrum was carried out for urinary and serum spectra, while normalization to the wet weight was performed for the spectra of liver extracts. Principal component analysis (PCA) and orthogonal-projection to latent structures discriminant analysis (O-PLS-DA) of spectral data were conducted using SIMCA-P+ software package (V12, Umetrics, Sweden)57, 58. Unit variance (UV) scaling for multivariate statistics was employed. The model quality was indicated by corresponding parameters, such as Q2and R2, denoting predictability and interpretability of the model, respectively. O-PLS-DA models were validated using a 7-fold cross-validation method, and further ensured with CV-ANOVA test59. The discrimination significance p values of given metabolites were set <0.05. For visualization, the loadings were back-transformed and plotted with color-coded coefficients for each variable using an in-house developed MATLAB script60.
Electronic supplementary material
Acknowledgements
We thank Prof. Guo (Wuhan University) for her providing the bacterial strain S. typhimurium ATCC14028. This work is funded by the National Natural Science Foundation of China (21375144, 21675169 and 91439102) and the Ministry of Science and Technology of China (2012CB934004).
Author Contributions
X.Y.Z., J.F.W., H.R.T. and Y.L.W. designed research. X.Y.Z., L.C. and J.F.W. performed research. X.Y.Z., H.R.T. and Y.L.W. analyzed data. X.Y.Z. and Y.L.W. wrote the paper. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-00992-1
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huiru Tang, Email: huiru_tang@fudan.edu.cn.
Yulan Wang, Email: yulan.wang@wipm.ac.cn.
References
- 1.Brogden KA, Guthmiller JM, Taylor CE. Human polymicrobial infections. Lancet. 2005;365:253–255. doi: 10.1016/S0140-6736(05)70155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rigaud T, Perrot-Minnot MJ, Brown MJF. Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proc. R. Soc. B. 2010;277:3693–3702. doi: 10.1098/rspb.2010.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox FEG. Concomitant infections, parasites and immune responses. Parasitology. 2001;122:S23–S38. doi: 10.1017/S003118200001698X. [DOI] [PubMed] [Google Scholar]
- 4.Hamm DM, et al. Coinfections with Schistosoma haematobium, Necator americanus, and Entamoeba histolytica/Entamoeba dispar in children: chemokine and cytokine responses and changes after antiparasite treatment. J. Infect. Dis. 2009;199:1583–1591. doi: 10.1086/598950. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths EC, Pedersen AB, Fenton A, Petchey OL. The nature and consequences of coinfection in humans. J. Infect. 2011;63:200–206. doi: 10.1016/j.jinf.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotez PJ, et al. Current concepts - Control of neglected tropical diseases. N. Engl. J. Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 7.Rollemberg CV, et al. Predicting frequency distribution and influence of sociodemographic and behavioral risk factors of Schistosoma mansoni infection and analysis of co-infection with intestinal parasites. Geospat Health. 2015;10:303. doi: 10.4081/gh.2015.303. [DOI] [PubMed] [Google Scholar]
- 8.Gordon MA, et al. Schistosomiasis does not contribute to death or recurrence of nontyphoid Salmonella bacteremia in human immunodeficiency virus-infected Malawian adults. Clin. Infect. Dis. 2003;37:E177–E179. doi: 10.1086/379828. [DOI] [PubMed] [Google Scholar]
- 9.Babu S, et al. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J. Infect. Dis. 2009;200:288–298. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellicano R, et al. Helicobacter pylori seroprevalence in hepatitis C virus positive patients with cirrhosis. J. Hepatol. 2000;33:648–650. doi: 10.1016/S0168-8278(00)80018-5. [DOI] [PubMed] [Google Scholar]
- 11.Mwinzi PNM, Karanja DMS, Colley DG, Orago ASS, Secor WE. Cellular immune responses of schistosomiasis patients are altered by human immunodeficiency virus type 1 coinfection. J. Infect. Dis. 2001;184:488–496. doi: 10.1086/322783. [DOI] [PubMed] [Google Scholar]
- 12.Kamal SM, et al. Acute hepatitis C without and with schistosomiasis: Correlation with hepatitis C-specific CD4(+) T-cell and cytokine response. Gastroenterology. 2001;121:646–656. doi: 10.1053/gast.2001.27024. [DOI] [PubMed] [Google Scholar]
- 13.Pancharoen C, Thisyakorn U. Coinfections in dengue patients. Pediatr. Infect. Dis. J. 1998;17:81–82. doi: 10.1097/00006454-199801000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Lello J, Boag B, Fenton A, Stevenson IR, Hudson PJ. Competition and mutualism among the gut helminths of a mammalian host. Nature. 2004;428:840–844. doi: 10.1038/nature02490. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohani P, Green CJ, Mantilla-Beniers NB, Grenfell BT. Ecological interference between fatal diseases. Nature. 2003;422:885–888. doi: 10.1038/nature01542. [DOI] [PubMed] [Google Scholar]
- 17.Sternberg ED, Lefevre T, Rawstern AH, de Roode JC. A virulent parasite can provide protection against a lethal parasitoid. Infect. Genet. Evol. 2011;11:399–406. doi: 10.1016/j.meegid.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Chiodini PL. Chemotherapy for patients with multiple parasitic infections. Parasitology. 2001;122:S83–S89. doi: 10.1017/S0031182000017674. [DOI] [PubMed] [Google Scholar]
- 19.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 20.Bustinduy AL, et al. Age-stratified profiles of serum IL-6, IL-10, and TNF-alpha cytokines among Kenyan children with Schistosoma haematobium, Plasmodium falciparum, and other chronic parasitic co-infections. Am. J. Trop. Med. Hyg. 2015;92:945–951. doi: 10.4269/ajtmh.14-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abruzzi A, Fried B, Alikhan SB. Coinfection of Schistosoma species with hepatitis B or hepatitis C viruses. Adv. Parasitol. 2016;91:111–231. doi: 10.1016/bs.apar.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Monin L, et al. Helminth-induced arginase-1 exacerbates lung inflammation and disease severity in tuberculosis. J. Clin. Invest. 2015;125:4699–4713. doi: 10.1172/JCI77378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porto AF, et al. HTLV-1 modifies the clinical and immunological response to schistosomiasis. Clin. Exp. Immunol. 2004;137:424–429. doi: 10.1111/j.1365-2249.2004.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lescano SA, Nakhle MC, Ribeiro MC, Chieffi PP. IgG antibody responses in mice coinfected with Toxocara canis and other helminths or protozoan parasites. Rev. Inst. Med. Trop. Sao Paulo. 2012;54:145–152. doi: 10.1590/S0036-46652012000300006. [DOI] [PubMed] [Google Scholar]
- 25.Wu JF, et al. Metabolic alterations in the hamster co-infected with Schistosoma japonicum and Necator americanus. Int. J. Parasitol. 2010;40:695–703. doi: 10.1016/j.ijpara.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Chen MG. Progress in schistosomiasis control in China. Chin. Med. J. 1999;112:930–933. [PubMed] [Google Scholar]
- 27.Roka M, Goni P, Rubio E, Clavel A. Prevalence of intestinal parasites in HIV-positive patients on the island of Bioko, Equatorial Guinea: Its relation to sanitary conditions and socioeconomic factors. Sci. Total Environ. 2012;432:404–411. doi: 10.1016/j.scitotenv.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 28.Abruzzi A, Fried B. Coinfection of schistosoma (Trematoda) with bacteria, protozoa and helminths. Adv. Parasitol. 2011;77:1–85. doi: 10.1016/B978-0-12-391429-3.00005-8. [DOI] [PubMed] [Google Scholar]
- 29.Gendrel D, et al. Schistosoma intercalatum and relapses of Salmonella infection in children. Am. J. Trop. Med. Hyg. 1984;33:1166–1169. doi: 10.4269/ajtmh.1984.33.1166. [DOI] [PubMed] [Google Scholar]
- 30.Hathout SED, Elghaffa Y, Awny AY. Salmonellosis complicating schistosomiasis in Egypt - a new clinical appreciation. Am. J. Trop. Med. Hyg. 1967;16:462–472. doi: 10.4269/ajtmh.1967.16.462. [DOI] [PubMed] [Google Scholar]
- 31.Neves J, Martins NRD. Long duration of septicaemic salmonellosis - 35 cases with 12 implicated species of Salmonella. Trans. R. Soc. Trop. Med. Hyg. 1967;61:541–&. doi: 10.1016/0035-9203(67)90105-8. [DOI] [PubMed] [Google Scholar]
- 32.O’Donnell H, McSorley SJ. Salmonella as a model for non-cognate Th1 cell stimulation. Front. Immunol. 2014;5:1–13. doi: 10.3389/fimmu.2014.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nat. Rev. Drug Discov. 2002;1:153–161. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- 35.Chodon T, Koya RC, Odunsi K. Active immunotherapy of cancer. Immunol. Invest. 2015;44:817–836. doi: 10.3109/08820139.2015.1096684. [DOI] [PubMed] [Google Scholar]
- 36.Brenner B, et al. Coinfection with hepatitis viruses and human immunodeficiency virus in multiply transfused patients. Isr. J. Med. Sci. 1994;30:886–890. [PubMed] [Google Scholar]
- 37.Borkow G, Teicher C, Bentwich Z. Helminth-HIV coinfection: Should we deworm? Plos Negl. Trop. Dis. 2007;1:e160. doi: 10.1371/journal.pntd.0000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mina MJ, Burke RM, Klugman KP. Estimating the prevalence of coinfection with influenza virus and the atypical bacteria Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1585–1589. doi: 10.1007/s10096-014-2120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hillier SD, et al. Plasmodium falciparum and helminth coinfection in a semi urban population of pregnant women in Uganda. J. Infect. Dis. 2008;198:920–927. doi: 10.1086/591183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tannock GW, Blumershine RV, Savage DC. Association of Salmonella typhimurium with, and its invasion of, ileal mucosa in mice. Infect. Immun. 1975;11:365–370. doi: 10.1128/iai.11.2.365-370.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warren KS. The pathology, pathobiology and pathogenesis of schistosomiasis. Nature. 1978;273:609–612. doi: 10.1038/273609a0. [DOI] [PubMed] [Google Scholar]
- 42.Xu B, Feng Z, Xu XJ, Hu W. Evaluation of Kato-Katz technique combined with stool hatching test in diagnosis of Schistosomiasis japonica. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2011;23:321–323. [PubMed] [Google Scholar]
- 43.Ricciuti C, et al. Immune response in schistosomiasis. Clin. Ter. 1987;120:489–494. [PubMed] [Google Scholar]
- 44.Zhang RL, et al. Vaccination with calpain induces a Th1-biased protective immune response against Schistosoma japonicum. Infect. Immun. 2001;69:386–391. doi: 10.1128/IAI.69.1.386-391.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mittrucker HW, Kaufmann SHE. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 2000;67:457–463. doi: 10.1002/jlb.67.4.457. [DOI] [PubMed] [Google Scholar]
- 46.Zinkernagel RM. Cell mediated immune response to Salmonella typhimurium infection in mice - Development of nonspecific bactericidal activity against Listeria monocytogenes. Infect. Immun. 1976;13:1069–1073. doi: 10.1128/iai.13.4.1069-1073.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chernin E. The malariatherapy of neurosyphilis. J. Parasitol. 1984;70:611–617. doi: 10.2307/3281739. [DOI] [PubMed] [Google Scholar]
- 48.Tsay CJ. Julius Wagner-Jauregg and the legacy of malarial therapy for the treatment of general paresis of the insane. Yale J. Biol. Med. 2013;86:245–254. [PMC free article] [PubMed] [Google Scholar]
- 49.Chen G, et al. Oral delivery of the Sj23LHD-GST antigen by Salmonella typhimurium type III secretion system protects against Schistosoma japonicum infection in mice. PLoS. Negl. Trop. Dis. 2011;5:e1313. doi: 10.1371/journal.pntd.0001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li JV, et al. Metabolic profiling of a Schistosoma mansoni infection in mouse tissues using magic angle spinning-nuclear magnetic resonance spectroscopy. Int. J. Parasitol. 2009;39:547–558. doi: 10.1016/j.ijpara.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Wang YL, et al. System level metabolic effects of a Schistosoma japonicum infection in the Syrian hamster. Mol. Biochem. Parasitol. 2006;146:1–9. doi: 10.1016/j.molbiopara.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Wu J, et al. Metabolic changes reveal the development of schistosomiasis in mice. PLoS Negl. Trop. Dis. 2010;4:e807. doi: 10.1371/journal.pntd.0000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, et al. Metabonomic investigations in mice infected with Schistosoma mansoni: an approach for biomarker identification. Proc. Natl. Acad. Sci. USA. 2004;101:12676–12681. doi: 10.1073/pnas.0404878101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barman M, et al. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect. Immun. 2008;76:907–915. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bazzone LE, et al. Coinfection with the intestinal nematode Heligmosomoides polygyrus markedly reduces hepatic egg-induced immunopathology and proinflammatory cytokines in mouse models of severe schistosomiasis. Infect. Immun. 2008;76:5164–5172. doi: 10.1128/IAI.00673-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao C, Hao F, Qin X, Wang Y, Tang H. An optimized buffer system for NMR-based urinary metabonomics with effective pH control, chemical shift consistency and dilution minimization. Analyst. 2009;134:916–925. doi: 10.1039/b818802e. [DOI] [PubMed] [Google Scholar]
- 57.Trygg J. O2-PLS for qualitative and quantitative analysis in multivariate calibration. J. Chemom. 2002;16:283–293. doi: 10.1002/cem.724. [DOI] [Google Scholar]
- 58.Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS) J. Chemom. 2002;16:119–128. doi: 10.1002/cem.695. [DOI] [Google Scholar]
- 59.Eriksson L, Trygg J, Wold S. CV-ANOVA for significance testing of PLS and OPLS (R) models. J. Chemom. 2008;22:594–600. doi: 10.1002/cem.1187. [DOI] [Google Scholar]
- 60.Cloarec O, et al. Evaluation of the orthogonal projection on latent structure model limitations caused by chemical shift variability and improved visualization of biomarker changes in H-1 NMR spectroscopic metabonomic studies. Anal. Chem. 2005;77:517–526. doi: 10.1021/ac048803i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


