Abstract
Neuroendocrine tumors may present with pseudoallergic reactions like diarrhea and idiopathic anaphylaxis. Here we present the P-STS human ileal neuroendocrine cell line as a model cell line for these tumors. Neuroendocrine markers and changes in cytoplasmic calcium concentration ([Ca2+]i) in response to several possible activators of 5-hydroxytryptamine (5-HT) release were analyzed. P-STS cells still expressed chromogranin A and synaptophysin after 2 years of culture. Tryptophan hydroxylase 1 mRNA and a low amount of 5-HT were also detected. Acetylcholine (ACh) caused a rise in [Ca2+]i. Somatostatin inhibited, whereas histamine (HA) but not the HA receptor ligand betahistine enhanced activation by ACh. The [Ca2+]i response to ACh/HA was inhibited by the HA receptor H3 (H3R) agonist methimepip and by the antidepressant imipramine. Further [Ca2+]i response studies indicated the presence of H4Rs and of a functional calcium sensing receptor. High or low affinity IgE receptor protein or mRNA were not detected. Taken together, neuroendocrine markers and response to intestinal neurotransmitters approve the P-STS cell line as a valuable model for enterochromaffin cells. Enhancement of their ACh-induced pro-secretory response by HA, with a role for H3R and H4R, suggests an amplifying role of neuroendocrine cells in allergen-induced diarrhea or anaphylaxis.
Introduction
Enterochromaffin (EC) cells are dispersed throughout the intestinal mucosa and produce and store the largest pool of 5-HT (serotonin) in the body. They release 5-HT from basal vesicles to the serosal side upon signals like mechanical stimulation, acidic pH, nutrients or other chemical mediators1, 2. Animal models indicate that the crosstalk between EC and inflammatory cells via 5-HT determines intestinal inflammation3. Most of these cells have apical microvilli projecting into the lumen and are supposed to function as transepithelial sensory transducers, as no nerve fibers penetrate the intestinal epithelium4, 5. By binding to 5-HT4 receptors on presynaptic membranes of afferent vagal nerve synapses of the enteric nervous system, 5-HT is thought to augment neurotransmitter release and enhance gut secretory and motility reflexes in response to natural stimuli6–8. Accordingly, high 5-HT levels can cause diarrhea9 and a role of 5-HT in the pathology of inflammatory bowel disease and other disorders of gastrointestinal motility is discussed10, 11.
Jejuno-ileal neuroendocrine tumors are among the most common malignant neuroendocrine neoplasms of the gastrointestinal tract12. Although different types of enteroendocrine cells are present in this part of the intestine13, 14, neuroendocrine tumors arising from the jejuno-ileum almost exclusively show EC cell differentiation14, 15. The cell of origin of these tumors is thought to be a committed neuroendocrine progenitor cell14. Ileal neuroendocrine tumors are rare, slow-growing and often only detected when they have already metastasized16. They can cause symptoms like diarrhea17, flushes, bronchoconstriction or “idiopathic” anaphylaxis18, 19 caused by release of biogenic amines and peptides from the tumor cells20, 21. These symptoms sometimes occur in response to specific foods22 and can be alleviated by treatment with somatostatin (SST) receptor agonists in about 70% of the patients23. A model cell line could be a valuable tool to study the possible context to IgE-mediated hypersensitivities.
Human cell lines of small intestinal origin represent such useful experimental models but are scarce24. They may upon long-term cultivation lose their neuroendocrine features (e.g. CNDT225) or may be overgrown by genetically different cells present in the original culture26. Small numbers of Epstein Barr virus (EBV)-infected B cells transferred from the original tumor into cell culture easily outgrow slow-growing tumor cells27. The P-STS cell line26, 28, isolated from a poorly differentiated neuroendocrine tumor of the terminal ileum, grows with a stable genotype26. We aimed to definitely establish P-STS as a reliable 5-HT-producing EC cell line by showing stable expression of the neuroendocrine vesicle components chromogranin A (CgA) and synaptophysin and of tryptophan hydroxylase-1 (TPH1), the rate-limiting enzyme for synthesis of 5-HT expressed specifically in enteroendocrine cells1. Enteric 5-HT release is induced by muscarinic agonists (e.g. the endogenous agonist ACh) applied at the serosal side and involves influx of extracellular Ca2+ via voltage-gated L-type Ca2+ channels that is inhibited by SST1, 29–31.
In addition to these known features of EC cells, we investigated the response of P-STS cells to other intestinal neurotransmitters (the β-adrenergic agonist isoproterenol, γ-aminobutyric acid (GABA) and 5-HT) and to histamine (HA), a consumed or endogenously generated molecule implicated in food intolerance and allergic reactions. We also screened for the presence of IgE receptors that might contribute to diarrhea, flushes or anaphylaxis associated with neuroendocrine tumors via immunoglobulin-mediated mechanisms of vesicle release. As a further step of characterization we investigated whether a [Ca2+]i rise is evoked by ligands of the calcium sensing receptor (CaSR) which plays an important role in intestinal secretion and nutrient sensing32–34.
Results
P-STS cells express neuroendocrine markers and are free of EBV
P-STS cells were growing semi-adherently (Fig. 1A) with a doubling time of about one week. Immunofluorescence staining showed expression of CgA and synaptophysin as expected for neuroendocrine cells35 after 6 months of continuous cultivation (Fig. 1B). In the cell lysate of P-STS cells 5-HT (10.7 ± 6.8 ng per 106 cells or 41 ± 26 ng mg−1 cell protein) was detected by ELISA (Fig. 1C). In comparison, the 5-HT content of native human ileal EC cells after several purification steps certainly causing 5-HT release from these touch-sensitive cells was 180 ng/mg cell protein36. Real-time PCR analysis confirmed the expression of CgA, synaptophysin and TPH1, the rate-limiting enzyme for 5-HT synthesis, on the mRNA level, while TAC1, another neuroendocrine marker mRNA, was not detected (Fig. 1D). Importantly, no EBV nucleic acids were detected by real-time PCR in about 105 P-STS cells, while in accordance with their known status of infection according to ATCC, in a similar number of Raji B cells 1.2 × 106 copies of EBV nucleic acid were detected. This shows that the P-STS neuroendocrine cells have not been overgrown by co-isolated EBV-infected lymphocytes during cell line establishment.
Figure 1.

Expression of neuroendocrine markers in P-STS cells. (A) Light microscopy picture of P-STS cells adhering to the culture dish, (B) Immunofluorescence staining with anti-CgA (Cy3-labeled secondary antibody) and anti-synaptophysin (Alexa 488-labeled secondary antibody) after 6 months of continuous cultivation, (C) 5-HT in the supernatant after 1 h of incubation of adherent P-STS cells (continuously cultivated for 10 months) in serum-free medium containing 5% BSA at room temperature and in the cell lysate as determined by ELISA. *p < 0.05 as calculated by the unpaired two-tailed Student’s t-test assuming equal variances. (D) Expression of neuroendocrine marker genes in P-STS cells assessed by real-time PCR analysis. Real-time PCR analysis was performed using the ΔCt method for relative quantification. Expression mRNA levels of the target gene were normalized to the average of the HKGs. Shown are genes encoding neuroendocrine markers such as CHGA (encoding chromogranin A), SYP (encoding synaptophysin), TPH1 (encoding tryptophan hydroxylase 1), and TAC1 (encoding the tachykinin peptide hormone family, substance P and neurokinin A, as well as the related peptides). Results are shown as mean values in one experiment ± SD and are representative of two independent experiments.
P-STS cells show a [Ca2+]i response to ACh and CaSR agonists
P-STS cells were further characterized by their reaction to putative agonists of vesicle release. First it was established that the Ca2+ ionophore A23187 as well as membrane depolarization by 40 mM KCl evoked an immediate rise in [Ca2+]i (Fig. 2B). Then the effect of several intestinal neurotransmitters or related substances (ACh, the β-adrenergic activator isoproterenol, GABA, 5-HT, and HA37) and of CaSR agonists (Ca2+, tryptophan (Trp) combined with Ca2+, neomycin and the calcimimetic AC-265347)34, 38 on [Ca2+]i was evaluated in a screening experiment. Of all neurotransmitters tested, only ACh induced a rise in [Ca2+]i significantly exceeding that induced by addition of pure medium alone (Fig. 2A and B). As expected, the [Ca2+]i rise induced by ACh was inhibited by SST (p ≤ 0.065, Fig. 2C). Despite the strong activating effect of 10 µM ACh, neither 5-HT nor CgA were detectable by ELISA in the supernatants of cells grown to final density in 24-well dishes after treatment with this neurotransmitter (data not shown). This is in accordance with the low 5-HT content of P-STS cells. It has been observed previously that treatment of rabbit duodenal tissue with ACh releases only about 2% total tissue 5-HT assumed to be contained in EC cells30. In our experiments this amounts to a release of about 0.2 ng 5-HT per 106 cells. With a final cell density of 0.6 × 106 cells per well in 200 µl of medium the 5-HT concentration achieved following release of 2% of total 5-HT would correspond to the detection level of the ELISA. Among the CaSR receptor ligands, AC-265347 appeared to have some enhancing effect on [Ca2+]i, but only Trp in the presence of 6 mM Ca2+ provoked a rise in [Ca2+]i significantly higher than medium alone or 3 mM Ca2+ (Fig. 2B). In the presence of NPS-2143, an inhibitor of the CaSR39, this activating effect was lost. In contrast to Trp which in the presence of Ca2+ is an allosteric CaSR activator, arginine (Arg) is a Ca2+-dependent agonist of GPRC6A, another G-protein coupled receptor that might be present on EC cells40, but no CASR ligand. Substitution of Trp with Arg in the presence of 6 mM Ca2+ did not cause a significant rise in [Ca2+]i, however. As the reaction of the cells to Trp in the presence of 6 mM Ca2+ was weak, we retested the cells in a separate experiment which confirmed the presence of functional CaSRs (p ≤ 0.065, Fig. 2D, left panel). Upon further continuous cultivation the generation time of the cells became shorter (2–3 days) and cells cultivated for 2 years had largely lost their reaction to Trp in the presence of 6 mM Ca2+ (Fig. 2D, right panel). Immunofluorescence staining of P-STS cells for CaSR was positive in cells cultivated for 6 months and comparable to THP-1 cells used as staining control41 (Fig. 3A). In accordance with the functional assay CaSR staining was lost in almost all cells after 2 years of continuous cultivation while CgA and synaptophysin were still present (Fig. 3B).
Figure 2.
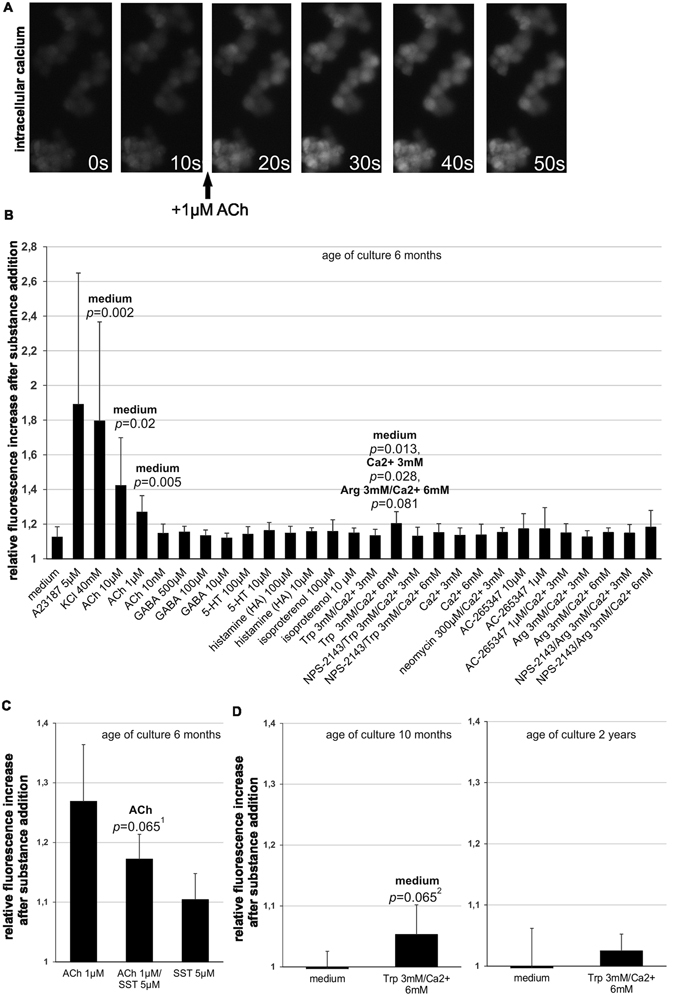
Intracellular [Ca2+]i response of P-STS to addition of neurotransmitters and CaSR ligands. (A) Time course of intracellular fluorescence of a group of Fluo-4 AM-stained P-STS cells after treatment with 1 µM ACh. (B) Screening experiment (see explanation in Methods) for fluorescence enhancement measured 10 s after the start of substance addition in relation to baseline fluorescence. Final substance concentrations are indicated. NPS-2143 (final concentration 2 µM) was added 1 min before taking the first time point for baseline determination. Mean values and standard deviations were calculated from 3–15 independent samples. Statistical significance compared to the treatment group indicated on top of the bar was calculated by univariate analysis with the Mann-Whitney U-test. (C) Inhibition of ACh-induced P-STS activation by somatostatin. Mean values and standard deviations were calculated from 6 experiments. 1 p = 0.059 when calculated from ranked data by the unpaired two-tailed t-test assuming unequal variances. (D) [Ca2+]i response to 3 mM Trp/6 mM Ca2+ after 10 months and 2 years of continuous cultivation. Mean values and standard deviations were calculated from 6 experiments. 2 p = 0.049 when calculated from ranked data by the unpaired two-tailed t-test assuming unequal variances.
Figure 3.
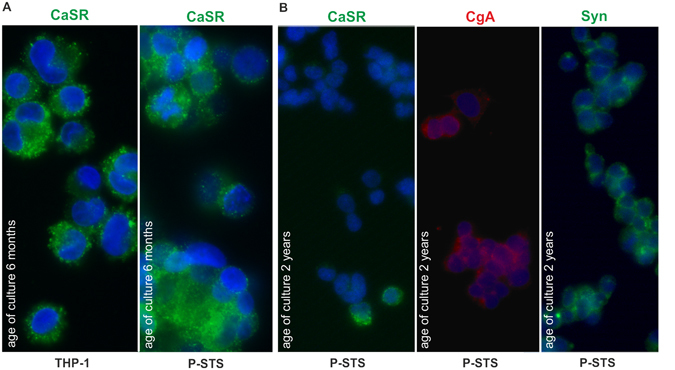
Expression of CaSR by P-STS cells. (A) Immunofluorescence staining with anti-CASR (Alexa 488-labeled secondary antibody) of THP-1 cells grown with 200 nM PMA for 2 d (control) and P-STS cells after 6 months of continuous cultivation. (B) Immunofluorescence staining with anti-CASR (Alexa 488-labeled secondary antibody), anti-CgA (Cy3-labeled secondary antibody) and anti-synaptophysin (Alexa 488-labeled secondary antibody) of P-STS cells after 2 years of continuous cultivation.
HA enhances the [Ca2+]i response to Ach
In a second set of [Ca2+]i response experiments compounds not eliciting a significant response when added to P-STS cells were screened for their capability to enhance or inhibit the response to 1 µM ACh (Fig. 4A). Again 100 µM GABA, 10 µM 5-HT, 10 µM of the β-adrenergic agonist isoproterenol and Trp or Arg in the presence of 3 mM Ca2+ were without significant effect compared to medium alone. When added simultaneously with ACh, 10 µM isoproterenol appeared to have an inhibitory effect on the [Ca2+]i response evoked by ACh (p = 0.106) in this screening experiment. This effect was, however, not reproducible when the cells were tested again (not shown). HA added together with ACh significantly enhanced the [Ca2+]i response elicited by medium to which only ACh was added. Further experiments showed that lowering the HA concentration from 10 to 1 µM abolished this enhancing effect, while an increase to 100 µM did not result in further acceleration of the [Ca2+]i response compared to 10 µM HA (Fig. 4B).
Figure 4.
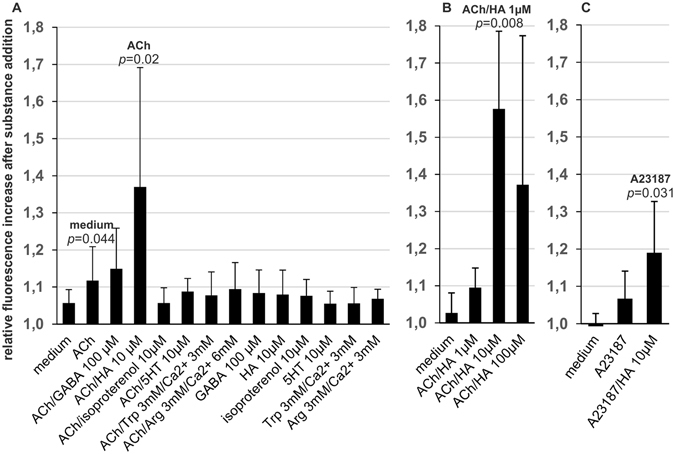
Screening for putative activators or inhibitors of the [Ca2+]i response to ACh and the Ca2+ ionophor A23187. Fluorescence enhancement was measured 10 s after addition of 200 µl medium containing ACh or A23187 (final concentrations 1 and 0.1 µM, respectively) and the putative activating/inhibiting substance in relation to baseline fluorescence. Final substance concentrations are indicated. Statistical significance compared to the treatment group indicated on top of the bar was calculated by univariate analysis with the Mann-Whitney U-test. (A) Screening test (see explanation in Methods) for P-STS activation by putative activators/inhibitors of [Ca2+]i response to ACh (age of culture 10 months). Mean values and standard deviations were calculated from 6–14 independent samples. (B) Dependence of the [Ca2+]i response to ACh on HA concentration (age of culture > 1 year). Mean values and standard deviations were calculated from 5 experiments testing. (C) Influence of 10 µM HA on the [Ca2+]i rise caused by A23187 (age of culture > 1 year). Mean values and standard deviations were calculated from 9 experiments.
HA causes a strong [Ca2+]i response in the presence of the Ca2+ ionophore A23187
As a first step to elucidate the mechanism behind the apparent synergism between ACh and HA, ACh was substituted by the Ca2+ ionophore A23187. An amount of A23187 causing only a slight increase in [Ca2+]i when added alone was sufficient to generate a strong [Ca2+]i response in the presence of 10 µM HA (Fig. 4C). This suggests that the primary contribution of ACh to the observed effect might be to cause an increase in [Ca2+]i.
Effect of HA receptor antagonists
Preincubation of the cells with selective HA receptor antagonists42, 43 before simultaneous addition of 1 µM ACh and 10 µM HA (ACh/HA) had little effect on the [Ca2+]i response (Fig. 5A, left panel). In accordance with published receptor affinities and inhibitory/effective concentrations, the H1R antagonist mepyramine with a pKi of ~8.843 at the H1R (as compared to the HA pEC50 of ~742) and the H2R antagonist ranitidine with a pKi of 6.7 at the H2R (while the pEC50 of HA is 5.843) were added at concentrations of 1 and 10 µM, respectively. Considering their receptor binding affinities42, 43 these concentrations were supposed to substantially inhibit the effect of 10 µM HA without inhibiting ACh receptors or ion channels42. BF2.649 has an almost 100-fold higher affinity to the human H3R than HA44 and a concentration of 1 µM was supposed to antagonize the effect of 10 µM HA at a putative H3R without causing unwanted side effects. The lack of inhibitory effects of H1R, H2R as well as H3R antagonists suggests that neither of these receptors is significantly involved in the enhancing effect of HA. Concerning the H4R, the binding affinities of HA and the specific antagonist JNJ-777712042, 45 are similar. 1 µM of this antagonist nevertheless was previously able to inhibit 10 µM HA by 50% in a functional assay45. No significant inhibition was observed in our experiment (Fig. 5A, left panel). Likewise, addition of 10 µM JNJ-7777120 simultaneously with 10 µM HA (e.g. without preincubation) did not have any significant effect on the [Ca2+]i response to ACh/HA (Fig. 5A, middle panel).
Figure 5.
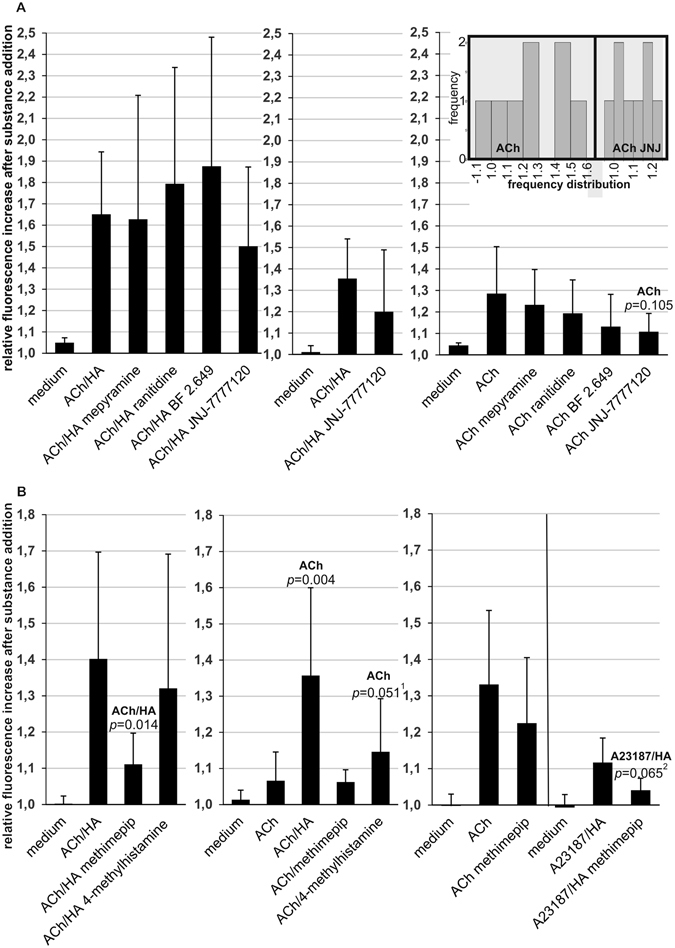
Effects of HA antagonists/inverse agonists and HA agonists on the [Ca2+]i response to ACh/HA, ACh or A23187/HA (age of culture > 1 year). Fluorescence enhancement was measured 10 s after addition of 200 µl medium containing ACh or A23187 (final concentrations as described below) with or without HA, methimepip, 4-methylhistamine or JNJ-7777120 (all added to final concentrations of 10 µM if not indicated otherwise). For preincubations with HA antagonists/inverse agonists the indicated substances or medium alone were added in 50 µl volume 2 min before taking the first time point for determination of baseline fluorescence, e.g. 10 s before addition of ACh or ACh/HA. In this case mepyramine, BF2.649, JNJ-7777120 were added to final concentrations of 1 µM and ranitidine to a final concentration of 10 µM. Statistical significance compared to the treatment group indicated on top of the bar was calculated by univariate analysis with the Mann-Whitney U-test. Mean values and standard deviations were calculated from at least 4 experiments. (A) Left panel: Inhibition of the [Ca2+]i response to ACh/HA (final concentrations 1 and 10 µM, respectively) by preincubation with HA receptor antagonists/inverse agonists (6 experiments), middle panel: effect of a higher concentration (10 µM) of JNJ-7777120 simultaneously added with ACh/HA (concentrations as in left panel, 6 experiments), right panel: inverse agonist effects of preincubation with HA receptor antagonists/inverse agonists on the [Ca2+]i response evoked by ACh (final concentration 2 µM, 8 experiments). The inset calculated with IBM SPSS Statistics shows the frequency distribution of the magnitude of [Ca2+]i response to treatment with ACh with and without preincubation with JNJ-7777120 derived from the same data. (B) Left panel: effect of methimepip and 4-methylhistamine on the [Ca2+]i response evoked by ACh/HA (final concentrations 1 and 10 µM, respectively, 4 experiments), middle panel: effect of methimepip and 4-methylhistamine on the [Ca2+]i response to ACh (final concentration 1 µM, 6 experiments), right panel: effect of methimepip on the [Ca2+]i response to ACh (final concentration 2 µM, 9 experiments) and A23187/HA (final concentrations 0.1 and 10 µM, respectively, 6 experiments). 1,2 p = 0.035 (1) and p = 0.049 (2) when calculated from ranked data by the unpaired two-tailed t-test assuming unequal variances.
Testing of inverse agonist activities of HA receptor antagonists indicates H4R activity in P-STS cells
For all antagonists used in this work inverse agonist activity, e.g. an inhibitory effect on a putative constitutive receptor activity in the absence of HA, has been observed43, 46–48. Therefore the effect of preincubation with these substances on the [Ca2+]i response to 2 µM ACh in the absence of HA was tested (Fig. 5A, right panel). Again, mepyramine and ranitidine were without significant effect giving no indication for the presence of functional H1R or H2R receptors. The H3R inverse agonist/antagonist BF 2649 appeared to be inhibitory, but no significance was obtained in 8 experiments. JNJ-7777120 at a concentration of 1 µM was the only inverse agonist/antagonist with an effect on the ACh-evoked [Ca2+]i response that came near to reaching statistical significance (p = 0.105). The inset in Fig. 5A illustrates that the extended apparently bimodal frequency distribution of the magnitude of the [Ca2+]i response obtained with ACh substantially narrows after preincubation with JNJ-7777120. At a concentration of 1 µM side effects of JNJ-7777120 on other cellular components are unlikely45. Consequently the inhibition of the ACh-evoked [Ca2+]i response by this low concentration of JNJ-7777120 indicates that P-STS cells express functional H4Rs. It furthermore suggests that H4Rs positively contribute to the cellular response to ACh by displaying constitutive activity. Inhibition of this constitutive activity by an H4R inverse agonist/antagonist reducing the responsiveness to ACh may explain the effect observed with JNJ-7777120.
Effect of H3R and H4R agonists
H3R agonists inhibit the release of neurotransmitters in the nervous system46. In P-STS cells, the selective H3R agonist methimepip43, 49 (10 µM) strongly inhibited the [Ca2+]i response to ACh/HA when added simultaneously (Fig. 5B, left panel). As methimepip was unable to significantly reduce the [Ca2+]i response to ACh in the absence of HA (Fig. 5B, middle panel), it appears unlikely that the observed inhibitiory effect on the [Ca2+]i response to ACh/HA was due to interaction of methimepip with the ACh receptor. Furthermore, in accordance with competition at the H3R, methimepip also inhibited the rise in [Ca2+]i caused by HA in the presence of the Ca2+ ionophore A23187 (p ≤ 0.065, Fig. 5B, right panel).
The selective H4R agonist 4-methylhistamine43 had a significant enhancing effect on the [Ca2+]i response to ACh (p ≤ 0.051, Fig. 5B, middle panel). Despite similar binding affinity to H4R43 4-methylhistamine had a weaker enhancing effect than seen with equimolar HA in our experiment. Nevertheless, our observations denote H4R as a candidate for mediating the enhancement of the cellular response to ACh by HA, as there is no strict correlation between binding affinities of HA receptor agonists or antagonists and activities in functional assays50.
Effect of the antipsychotic betahistine and the tricyclic antidepressant imipramine
Betahistine, a H1R agonist, has a pEC50 at H1R of ~6, e.g. about ten times lower than HA42. It did not enhance the [Ca2+]i response to 1 µM ACh at concentrations about three and ten times higher than HA in our experiments (e.g. 30 and 100 µM, Fig. 6A). This again indicates that H1R does not play an important role in the [Ca2+]i response to ACh. Imipramine, another psychoactive drug with high affinitiy to the H1R but also to H2R42, was strongly inhibitory at a concentration of 30 µM (Fig. 6B). Imipramine showed equally strong inhibition of the [Ca2+]i response to ACh alone, however. It therefore appears that its inhibitory effect was primarily due to its known antimuscarinic effect51.
Figure 6.
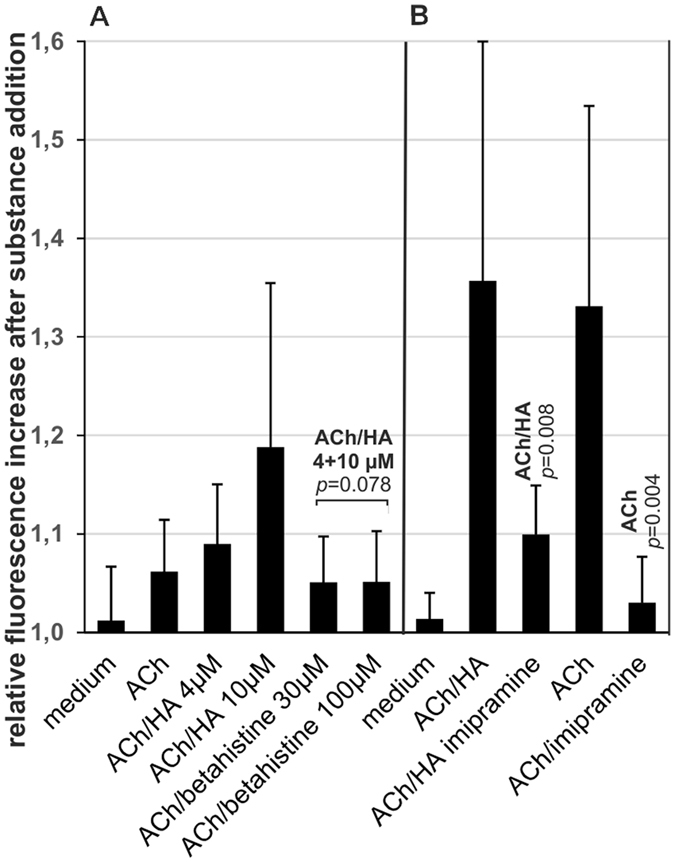
Effect of betahistine and imipramine on the [Ca2+]i response evoked by ACh/HA or ACh alone (age of culture > 1 year). Fluorescence enhancement was measured 10 s after addition of 200 µl medium containing ACh or ACh/HA and the indicated substances. Statistical significance compared to the treatment group indicated on top of the bar was calculated by univariate analysis with the Mann-Whitney U-test. Mean values and standard deviations were calculated from at least 6 experiments. (A) Comparison of the effects of betahistine and HA. Betahistine or HA were added to the cells simultaneously with ACh (final concentration 1 µM, 6 experiments) at the indicated final concentrations. As indicated by the bracket, in the Students’s t-test all betahistine samples, irrespective of betahistine concentration, were compared to the samples with HA added to a final concentration of 10 µM. (B) Effect of imipramine. Imipramine was added simultaneously with ACh/HA (final concentrations 1 and 10 µM, respectively, 6 experiments) or with ACh (final concentration 2 µM, 7 experiments) as indicated. Note that ACh in the absence of HA was used at a concentration of 2 µM to attain a similar response as in the presence of HA for better comparability of the inhibitory capacity of imipramine.
No IgE- or IgG-receptor expression was found in P-STS cells
To clarify whether P-STS cells have any receptors suitable for activation of vesicle release via an immunoglobulin-mediated mechanism, complexes of NIP-BSA (see methods section) with IgE specific for this model allergen (Fig. 7A) or heat-aggregated IgG (Fig. 7B) were incubated with the cells at 37 °C and binding or internalization of immunoglobulins was monitored by immunofluorescence microscopy. While IgE complexes clearly bound to RBL SX-38 cells expressing the human high affinity IgE receptor and heat-aggegated IgG was abundantly detected associated with phorbol 12-myristate 13-acetate (PMA)-differentiated THP-1 cells, no immunoglobulin staining was seen on P-STS cells despite treatment with IL-4 for potential induction of IgE receptors or lipopolysaccharides (LPS) for potential induction of IgG receptors, as shown for other cell types52, 53. In agreement with this lack of IgG binding by P-STS cells, [Ca2+]i imaging after addition of medium containing non-aggregated versus heat-aggregated human IgG (30 µg ml−1 final concentration) showed no difference between these treatments or when compared to medium without IgG (not shown). While IL-4 was able to induce expression of the low affinity IgE receptor CD23 on THP-1 cells (positive control for IL-4 integrity, not shown) and CD23 was stained on Raji cells as a positive control, no CD23 was detected on P-STS cells cultivated with IL-4 (Fig. 7C). In line with the results of immunofluorescence microscopy and and functional assays, negative results for high and low affinity IgE receptors were also obtained on the mRNA expression level assayed by real-time PCR analysis in comparison to Raji cells (Fig. 7D).
Figure 7.

No IgG- or IgE-receptor expression was found in P-STS cells (age of culture < 6 months). (A) RBL SX-38 cells or Il-4 treated P-STS cells were incubated for 1 h at 37 °C with JW8/5/13 cell supernatant, washed two times with PBS and incubated for 1 h at 37 °C with NIP-BSA (100 ng ml−1) in pure M199/Ham’s F12 medium. The cells were then prepared for immunofluorescence staining with FITC-labeled anti-IgE. (B) Heat-aggregated IgG (30 µg ml−1) was internalized for 15 min into THP-1 cells grown with 200 nM PMA for 2 d or P-STS cells grown for 3 d with 1 µg ml−1 LPS, cells were fixed and prepared for immunofluorescence staining with FITC-labeled anti-IgG. (C) Immunofluorescence staining of Raji cells or Il-4 treated P-STS cells (both not permeabilized) with anti-CD23 (Alexa 488-labeled secondary antibody). (D) Expression of genes encoding IgE receptors assessed by real-time PCR analysis. Real-time PCR analysis was performed using the ΔΔCt method for relative quantification. Expression mRNA levels of the target gene were normalized to the average of the HKGs and calculated relative to the corresponding mRNA levels of Raji cells (equated to 1.0). Shown are genes encoding IgE receptors such as FCER1A (encoding the high-affinity receptor for IgE, alpha subunit) and FCER2 (also known as CD23, encoding the low-affinity receptor for IgE); additionally, CD19 is shown as the B-cell identity marker. Results are shown as mean values in one experiment ± SD and are representative of two independent experiments. *Note that FCER1A in Raji cells was classified as a low expressing gene in our analysis (30 < Ct < 35).
Discussion
The P-STS cell line was cultivated from a neuroendocrine tumor (“carcinoid”) of the terminal ileum which had already metastasized and caused flushes and bronchoconstriction in the patient28. In accordance with the characterization of the primary tumor and previous results from immunocytochemical staining showing 5-HT only in isolated cells28, only a low amount of 5-HT was detected in the cell lysate. Expression of the mRNA for TPH1, the rate-limiting enzyme for synthesis of 5-HT expressed specifically in enteroendocrine cells1, was confirmed. In addition, expression of synaptophysin and CgA, a protein important for vesicle biogenesis54, qualifies this cell line as a model for EC cells. Substance P, encoded by the TAC1 gene and secreted by less than 20% of 5-HT-producing neuroendocrine tumors55, is not expressed in P-STS cells. EC cells are found in close contact with nerve endings of submucosal neurons of the enteric reflex pathways which are largely cholinergic56, 57. ACh secreted by enteric nerves promotes intestinal secretion and motility56. In rabbit duodenal30 and guinea pig and human colonic EC cells7, cholinergic agonists induce a rise in [Ca2+]i, the main physiological trigger of vesicle exocytosis. In accordance with their EC cell phenotype, the same reaction to ACh was seen in P-STS cells and - as expected29 – could be inhibited by simultaneous addition of SST.
The neurotransmitters isoproterenol, 5-HT or GABA did not have any statistically significant influence on [Ca2+]i or the [Ca2+]i response to ACh in the screening experiments employed. This may be due to the insensitivity of the [Ca2+]i response assay caused by large differences in response between different samples of the same type.
A rise in [Ca2+]i reaching statistical significance was induced in P-STS cells by elevation of the extracellular [Ca2+] from 1 mM Ca2+ contained in the culture medium to 6 mM in the presence of 3 mM Trp indicating the presence of functional CaSRs. Immunofluorescence microscopy showed that CaSR expression in P-STS cells was comparable in intensity and pattern to expression in THP-1-derived macrophages. However, CaSR protein was lost in almost all cells in the course of further cultivation and this was reflected in a concomitant loss of the functional response. In analogy to colon cancer cells34 this loss of the CaSR might be connected to the observed gradual increase in cell proliferation. In the intestine, ACh, acidic pH as well as CaSR activation enhance bicarbonate secretion by epithelial cells which is important for mucus secretion and barrier function of the gut wall32. 5-HT has been shown to evoke bicarbonate release from mouse duodenal mucosa via a cholinergic neuronal reflex pathway and probably also via 5-HT4 receptors on intestinal epithelial cells58, suggesting that EC cells augment responses of epithelial cells to these signals.
Unexpectedly, HA significantly enhanced the [Ca2+]i response elicited by ACh in P-STS cells. A change in [Ca2+]i is an important early event in signaling cascades elicited by HA via H1R, H3R and H4R and has also been observed for H2R43, 59. However, when added alone, HA caused only a slight elevation in [Ca2+]i which did not reach statistical significance. In the human intestine mast cells and neutrophils are the main sources of HA60. Food allergic persons have increased levels of mast cells and HA secretion in the intestine61. Increased secretion of 5-HT from EC cells upon antigen ingestion might contribute to symptoms like diarrhea and is thought to be proinflammatory10. In line with this, antigen-induced mast cell degranulation in the small intestine induced mucosal hypersecretion and muscle contractions in the antigen-sensitized guinea-pig model62. Mast cell activation has also been implicated in irritable bowel syndrome and symptoms in diarrhea-predominant irritable bowel syndrome tend to be alleviated by mast cell stabilizing drugs60, 63.
An investigation into the mechanism of HA action on P-STS cells showed that HA not only enhanced cell activation by ACh but also by the Ca2+ ionophor A23187, suggesting that reaching a certain level of [Ca2+]i might be enough to augment the cellular susceptibility to a second stimulus causing a rise in [Ca2+]i. Adding a tenfold higher HA concentration did not further increase the [Ca2+]i response, while the cells strongly responded to changes in final HA concentrations between 1 and 10 µM.
Our studies with HA agonists and antagonists/inverse agonists indicate the presence of functional H3Rs and H4Rs on P-STS cells, but gave no indication for the presence of H1Rs or H2Rs. Their expression might be too low for detection with inverse agonists48. The inhibitory effect of the selective H3R agonist methimepip on the [Ca2+]i response upon simultaneous addition of ACh or A23187 is in line with data from synaptic nerve endings where [Ca2+]i and neurotransmitter release are reduced by agonists at H3R43, 64.
The significant enhancing effect of the H4R agonist 4-methylhistamine on the [Ca2+]i response to ACh in our experiments indicates the presence of H4Rs on P-STS cells. Elevation of [Ca2+]i as well as degranulation and release of pro-inflammatory cytokines in response to 4-methylhistamine has previously been shown in human mast cells65. The apparent inhibitory effect of the H4R inverse agonist/antagonist JNJ-7777120 on the response of P-STS cells to ACh even suggests the enhancement of the cellular response to ACh by constitutive H4R activity. Constitutive activity of H4Rs has been observed in transformed cells, but up to date little if any evidence for constitutive activity in native systems has been obtained43. As we were not able to detect release of 5-HT or CgA from P-STS cells, we can only conclude from their [Ca2+]i response that 4-methylhistamine activates the cells less efficiently than HA when added at the same concentration, as has also been observed for eosinophil shape change66. Considering this, our data suggest that the enhancing effect of HA on the [Ca2+]i response to ACh might be mediated via H4R. In accordance with these putative results, H4Rs have been implicated in visceral hypersensitivity in rats67.
The antipsychotic betahistine68, and the tricyclic antidepressant imipramine are known HA receptor ligands. Betahistine is a H1R agonist42, while imipramine has antihistaminic as well as antimuscarinic activity51. Their effects on the [Ca2+]i response of P-STS cells to ACh were studied. In accordance with its lack of gastrointestinal side effects in clinical studies68 betahistine had no effect on the [Ca2+]i response to ACh, while imipramine inhibited this response. This is in line with the clinical observation that tricyclic antidepressants cause constipation69.
The facts that (i) pseudoallergic symptoms like bronchoconstriction and anaphylaxis may be associated with neuroendocrine tumors, and (ii) that the allergic mediator HA exerted a stimulatory effect on P-STS cells, prompted us to look for the presence of IgE receptors on these cells. IgE receptors on neuroendocrine cells in the gut might further contribute to an imbalance in motility and secretion in food patients with food allergy by releasing 5-HT. Crosslinking of high- as well as low-affinity IgE receptors has been implicated in raising [Ca2+]i 52, 70 and might therefore cause granule exocytosis from EC cells if present at the cell surface. Neither the IgE-binding α subunit of the high-affinity IgE receptor (FcεRIα) nor the low-affinity IgE receptor CD23 were detected at mRNA or protein level in P-STS cells, however. Inflammatory bowel disease patients tend to have high levels of IgG antibodies specific for luminal antigens71 and – although hotly debated - cancelling of foods with high serum IgG has been suggested to reduce stool frequency72. Not only IgE-antigen complexes, but also crosslinked IgG can induce mast cell degranulation63 and via the mediator HA may cause 5-HT release from EC cells. However, no indication of a direct interaction of heat-aggregated IgG with P-STS cells was found.
In this work we confirmed that the P-STS tumor cell line is a valuable model for human EC cells with expression of neuroendocrine markers, albeit low 5-HT content. Importantly, the cell line shows the rise in [Ca2+]i in response to ACh expected for EC cells. This response is significantly enhanced by simultaneous addition of HA, suggesting a participation of EC cells in symptoms of intestinal disorders associated with increased mast cell numbers like food allergy or irritable bowel syndrome. Studies with specific HA receptor ligands indicate the presence of H4Rs and of H3Rs with functional analogy to nerve cell H3Rs in this ileal EC cell line.
Methods
Cell culture
P-STS midgut neuroendocrine tumor cells (semi-adherent, originally isolated from a WHO III neuroendocrine tumor of the terminal ileum) were established and provided by R. Pfragner28. They were free of mycoplasma as judged from immunofluorescence microscopy after staining of fixed and permeabilized cells with Hoechst dye 33342. P-STS were grown in a 1:1 mixture of medium M199 and Ham’s F12 nutrient mixture supplemented with 10% heat-inactivated fetal calf serum, 100 U ml−1 penicillin G and 100 µg ml−1 streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. Raji Burkitt’s lymphoma B cells (ATCC Catalog No. CCL-86), JW8/5/13 plasmacytoma cells (ECACC No. 87080706) secreting mouse/human chimeric IgE specific for the (4-hydroxy-3-iodo-5-nitrophenyl)acetyl (NIP) group73 and THP-1 monocytes (ATCC Catalog No. tib-202) were grown in RPMI 1640 medium containing the same supplements. RBL SX-38 cells74 (kindly provided by Jean-Pierre Kinet) were grown in DMEM medium in the presence of 250 µg ml−1 G418 sulfate. Treatment of THP-1 or P-STS cells with IL-4 was for 3 d at a concentration of 10 ng ml−1 where indicated.
Antibodies and reagents
Mouse anti-CaSR [5C10] and donkey anti-goat Cy3-IgG were from Abcam, all other secondary fluorescent antibodies as well as FITC-conjugated goat anti-IgE and Fluo-4AM were from Life Technologies, FITC-conjugated goat anti-IgG was from Millipore, rabbit anti-synaptophysin (GTX100865) was from GeneTex, goat anti-CgA and mouse anti-CD23[BU38] were from Santa Cruz, the 5-HT ELISA kit (ADI-900-175) was from Enzo Life Sciences and the human CgA ELISA kit (ab196271) was from Abcam. A23187 Ca2+ ionophore, phorbol 12-myristate 13-acetate (PMA), LPS from Escherichia coli 055:B5, bovine serum albumin (BSA, heat shock fraction), isoproterenol, neomycin trisulfate, ACh chloride, SST, arginine monohydrochloride (Arg), HA dihydrochloride, γ-aminobutyric acid (GABA), imipramine hydrochlorid and lyophilized human IgG were from Sigma Aldrich, AC-265347 was from Santa Cruz and NPS-2143 was from Selleckchem. All HA receptor ligands (4-methylhistamine dihydrochloride, JNJ 7777120 dihydrobromide, methimepip dihydrobromide, ranitidine hydrochloride, BF2.649 (pitolisant) hydrochloride and mepyramine (pyrilamine) maleate) were from Tocris. 4-hydroxy-3-nitrophenylacetyl coupled in a ratio of 15:1 to bovine serum albumin (NIP-BSA) was from Biosearch Technologies and recombinant human IL-4 was from R&D Systems. Human IgG was heat-aggregated by incubation at 63 °C for 60 min at a concentration of 2 mg ml−1 in 0.9% sodium chloride and before use of the supernatant in experiments was centrifuged at 1.000× g for 2 min.
EBV detection
Nucleic acid extraction was performed with 200 µl suspension of ~105 cells by use of the automated NucliSens EasyMag extractor, according to the manufacturer’s instructions (Biomerieux, Marcy l’Etoile, France). EBV-specific real-time TaqMan PCR was performed with primers and probe located in the BNRF1 p143 gene (nonglycosylated membrane protein), as described by Niesters et al.75. The detection limit of real-time PCR was 200 copies ml−1, as determined by a commercially available, particle-counted, EBV standard (Advanced Biotechnologies Inc., Columbia, Md., USA).
Real-time PCR analysis
Total RNA was isolated using Absolutely RNA Miniprep Kit (Stratagene by Agilent) according to the manufacturer’s protocol. 1 µg RNA was used for cDNA generation using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems by Thermo Fisher Scientific) according to the instructions of the manufacturer. Real-time PCR analysis was performed using Power SYBR Green PCR Master Mix (Applied Biosystems by Life Technologies) on ABI 7900HT instrument equipped with SDS 2.3 software (Applied Biosystems by Thermo Fisher Scientific). All primers were self-designed using the Primer Express 3.0 software (Applied Biosystems by Thermo Fisher Scientific) and validated using the Human Total RNA Master Panel (Takara, Clontech Laboratories Inc.) as described76, 77. Primer sequences are summarized in Table 1. For relative quantification, data were analyzed by ΔΔCT or ΔCT methods as indicated. Expression levels of target genes were normalized to the average of the selected housekeeping genes (HKGs) EEF1A1 and UBC. Ct values of genes expressed at the limit of detection were set to 40.
Table 1.
Real-time PCR primers. Gene symbol, Gene ID and sequences of forward and reverse primers are indicated.
| Gene symbol | Gene ID | Forward Primer | Reverse Primer |
|---|---|---|---|
| CHGA′) | 1113 | CTGCGCCGGGCAAGT | CATCACCTCGGTATCCCCTTT |
| SYP | 6855 | ACACATGCAAGGAGCTGAGAGA | CAGGTTCAGGAAGCCGAACA |
| TPH1 | 7166 | TTCTATACCCCAGAGCCAGATACC | TGGGAGAATTGGGCAAAACT |
| TAC1 | 6863 | GCGACCAGATCAAGGAGGAA | GGTCTCCGGGCGATTCTC |
| FCER1A | 2205 | CATGGAATCCCCTACTCTACTGTGT | CCTTAGGTTTCTGAGGGACTGCTA |
| FCER2 | 2208 | AGGTGTCCAGCGGCTTTGT | AGCACTTCCGTTGGAAATTGA |
| CD19 | 930 | TGACCCCACCAGGAGATTCTT | CACGTTCCCGTACTGGTTCTG |
| EEF1A1 | 1915 | ATTACAGGGACATCTCAGGCTGAC | CATTCTTGGAGATACCAGCTTCAA |
| UBC | 7316 | ATTTGGGTCGCAGTTCTTG | TGCCTTGACATTCTCGATGGT |
′)Gene names: chromogranin A (CHGA), synaptophysin (SYP), tryptophan hydroxylase 1 (TPH1), tachykinin precursor 1 (TAC1), Fc fragment of IgE receptor Ia (FCER1A), Fc fragment of IgE receptor II (CD19), eukaryotic translation elongation factor 1 alpha 1 (EEF1A1), ubiquitin C (UBC).
Fluorescence microscopy
Cells grown on cover slips were fixed for 20 min with 4% paraformaldehyde in phosphate buffered saline without Ca2+ or Mg2+ (PBS), and - if not indicated otherwise - the cells were permeabilized for 5 min with 0.2% v/v Triton X-100 in PBS. Blocking and incubation with antibodies was in 5% goat serum in PBS at room temperature. Nuclei were stained with Hoechst dye 33342 in PBS. Cells were mounted in a 9:1 (v/v) mixture of glycerol and 1 M Tris-HCl (pH 8.6) containing 25% diazabicyclo(2,2,2)octane (Merck) and were viewed under a Zeiss Axioplan 2 fluorescence microscope using Axiovision software.
[Ca2+]i imaging
P-STS cells were plated in 24-well plates at a density of 102 to 103 per well. For the experiment the medium was removed and the cells were incubated for 45 min with 3 µM Fluo-4 AM (Life Technologies, stock solution 0.4 mg ml−1 in DMSO) in 200 µl serum-free medium per well. Then this solution was carefully exchanged for the same volume of pure medium and the cells in 24-well plates were placed on the inverted microscope Axio Observer Z1 equipped with a high resolution AxioCam MRc 5 camera (Carl Zeiss, Jena/Göttingen, Germany) and incubated there for 30 min avoiding agitation. After focusing on a suitable group of cells, pictures were taken every 10 s (program Axiovision). Between the second and third picture the test substance was carefully added in 200 µl medium. For quantification the mean change in fluorescence intensity was calculated from at least 20 cells with the program Image J. The increase in fluorescence before addition of the test substance was extrapolated for calculation of the baseline fluorescence increase. The fluorescence increase above baseline caused by addition of the test substance was calculated from the third picture taken, e.g. from the time point 10 s after starting the addition of the test substance.
Statistical analysis of [Ca2+]i responses
It was obvious that increases in fluorescence calculated from the [Ca2+]i imaging experiments did not display a normal distribution, especially when using strong agonists (an example is given in Fig. 5A right panel). Additionally, the strength of reaction to the same agonist varied between experiments, possibly due to differences in cell density influencing mutual inhibition or activation between adjacent cells78. Statistical significance of the differences of mean values between 2 samples of interest was calculated by the non-parametric Mann-Whitney U-test using IBM SPSS Statistics for Windows version 24 (IBM Corporation, USA). The frequency distributions obtained with strong agonists appeared strongly right skewed or bimodal and as very high values are not adequately considered by rank tests, the Mann-Whitney U-test might for these cases underestimate statistical significance. For some of the data statistical significance of the difference of mean values was also calculated from ranked data with the unpaired two-tailed t-test assuming unequal variances79. The slightly lower p-values obtained with this procedure are given in the figure legends. All samples were considered independent. Experiments were initially conducted as screening experiments with different sample numbers per group. To increase statistical reliability and strength, in all further experiments all treatments were conducted in parallel resulting in equal sample numbers in each treatment group.
5-HT quantification in cell lysate and supernatant
P-STS cells were plated at a density of 106 cells per well in a 24-well plate. The cells were washed with PBS, detached with trypsin/EDTA (removed immediately) at 37 °C and resuspended in 5% w/v BSA in serum-free medium. An aliquot was lysed by three times freezing and thawing followed by 3 min of treatment in a sonication bath and the 5-HT content of the supernatant after high-speed centrifugation was determined by ELISA following the instructions in the kit. Per well of the 96-well ELISA plate 90 µl of sample (containing 5% BSA) were added to 10 µl of assay buffer. 5-HT standards were added to the same matrix.
Acknowledgements
We would like to thank Prof. Bruno Niederle for scientific discussion and Martin Svoboda for designing primers. This project was supported by the Austrian Science Fund FWF grant SFB F4606-B28 to EJJ and projects P23228-B19 and P22441-B13 to DM.
Author Contributions
B.P. and E.J.J. designed the study concept; B.P. performed most experiments and wrote the article; E.J.J. contributed valuable experimental ideas and to results’ interpretation; A.M. and D.M. performed and interpreted the real-time PCR; S.A. performed the EBV analysis; R.P. helped with practical work; R.P. and E.J.J. revised the article.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Beatrix Pfanzagl, Email: beatrix.pfanzagl@meduniwien.ac.at.
Erika Jensen-Jarolim, Email: erika.jensen-jarolim@meduniwien.ac.at.
References
- 1.Bertrand PP, Bertrand RL. Serotonin release and uptake in the gastrointestinal tract. Autonomic neuroscience: basic & clinical. 2010;153:47–57. doi: 10.1016/j.autneu.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Hansen MB, Witte AB. The role of serotonin in intestinal luminal sensing and secretion. Acta physiologica. 2008;193:311–323. doi: 10.1111/j.1748-1716.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 3.Shajib MS, et al. Interleukin 13 and serotonin: linking the immune and endocrine systems in murine models of intestinal inflammation. PloS one. 2013;8:e72774. doi: 10.1371/journal.pone.0072774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gershon MD. Review article: serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Alimentary pharmacology & therapeutics. 2004;20(Suppl 7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 5.Xue J, Askwith C, Javed NH, Cooke HJ. Autonomic nervous system and secretion across the intestinal mucosal surface. Autonomic neuroscience: basic & clinical. 2007;133:55–63. doi: 10.1016/j.autneu.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. Journal of clinical gastroenterology. 2005;39:S184–193. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- 7.Raghupathi R, et al. Identification of unique release kinetics of serotonin from guinea-pig and human enterochromaffin cells. The Journal of physiology. 2013;591:5959–5975. doi: 10.1113/jphysiol.2013.259796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kendig DM, Grider JR. Serotonin and colonic motility. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2015;27:899–905. doi: 10.1111/nmo.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turvill JL, Farthing MJ. Role of the neuroendocrine system in pathogenesis of gastroenteritis. Current opinion in infectious diseases. 2000;13:523–529. doi: 10.1097/00001432-200010000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Khan WI, Ghia JE. Gut hormones: emerging role in immune activation and inflammation. Clinical and experimental immunology. 2010;161:19–27. doi: 10.1111/j.1365-2249.2010.04150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Current opinion in endocrinology, diabetes, and obesity. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocrine-related cancer. 2010;17:909–918. doi: 10.1677/ERC-10-0152. [DOI] [PubMed] [Google Scholar]
- 13.Sjolund K, Sanden G, Hakanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed] [Google Scholar]
- 14.Schimmack S, Svejda B, Lawrence B, Kidd M, Modlin IM. The diversity and commonalities of gastroenteropancreatic neuroendocrine tumors. Langenbeck’s archives of surgery/Deutsche Gesellschaft fur Chirurgie. 2011;396:273–298. doi: 10.1007/s00423-011-0739-1. [DOI] [PubMed] [Google Scholar]
- 15.Moyana TN, Zhang D, Xiang J. Single jejunoileal and right colonic carcinoids as midgut tumors. A study collating immunophenotypes and histogenesis. Annals of clinical and laboratory science. 1995;25:504–512. [PubMed] [Google Scholar]
- 16.Yantiss RK, Odze RD, Farraye FA, Rosenberg AE. Solitary versus multiple carcinoid tumors of the ileum: a clinical and pathologic review of 68 cases. The American journal of surgical pathology. 2003;27:811–817. doi: 10.1097/00000478-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Gregersen T, et al. Rectal Mechano-sensory Function in Patients with Carcinoid Diarrhea. Journal of neurogastroenterology and motility. 2015;21:423–32. doi: 10.5056/jnm15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blatman KH, Ditto AM. Chapter 25: Idiopathic anaphylaxis. Allergy Asthma Proc. 2012;33(Suppl 1):S84–87. doi: 10.2500/aap.2012.33.3558. [DOI] [PubMed] [Google Scholar]
- 19.Metcalfe DD. Differential diagnosis of the patient with unexplained flushing/anaphylaxis. Allergy Asthma Proc. 2000;21:21–24. doi: 10.2500/108854100778249006. [DOI] [PubMed] [Google Scholar]
- 20.Paulson AS, Bergsland EK. Systemic therapy for advanced carcinoid tumors: where do we go from here? Journal of the National Comprehensive Cancer Network: JNCCN. 2012;10:785–793. doi: 10.6004/jnccn.2012.0078. [DOI] [PubMed] [Google Scholar]
- 21.Modlin IM, et al. Gastrointestinal neuroendocrine (carcinoid) tumours: current diagnosis and management. The Medical journal of Australia. 2010;193:46–52. doi: 10.5694/j.1326-5377.2010.tb03742.x. [DOI] [PubMed] [Google Scholar]
- 22.Izikson L, English JC, 3rd, Zirwas MJ. The flushing patient: differential diagnosis, workup, and treatment. Journal of the American Academy of Dermatology. 2006;55:193–208. doi: 10.1016/j.jaad.2005.07.057. [DOI] [PubMed] [Google Scholar]
- 23.Khasraw M, Gill A, Harrington T, Pavlakis N, Modlin I. Management of advanced neuroendocrine tumors with hepatic metastasis. Journal of clinical gastroenterology. 2009;43:838–847. doi: 10.1097/MCG.0b013e3181b152a1. [DOI] [PubMed] [Google Scholar]
- 24.Grozinsky-Glasberg S, Shimon I, Rubinfeld H. The role of cell lines in the study of neuroendocrine tumors. Neuroendocrinology. 2012;96:173–187. doi: 10.1159/000338793. [DOI] [PubMed] [Google Scholar]
- 25.Ellis LM, Samuel S, Sceusi E. Varying opinions on the authenticity of a human midgut carcinoid cell line–letter. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:5365–5366. doi: 10.1158/1078-0432.CCR-10-2550. [DOI] [PubMed] [Google Scholar]
- 26.Rinner B, et al. Molecular evidence for the bi-clonal origin of neuroendocrine tumor derived metastases. BMC genomics. 2012;13:594. doi: 10.1186/1471-2164-13-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drexler HG, Matsuo Y. Malignant hematopoietic cell lines: in vitro models for the study of multiple myeloma and plasma cell leukemia. Leukemia research. 2000;24:681–703. doi: 10.1016/S0145-2126(99)00195-2. [DOI] [PubMed] [Google Scholar]
- 28.Pfragner R, et al. Establishment and characterization of three novel cell lines - P-STS, L-STS, H-STS - derived from a human metastatic midgut carcinoid. Anticancer research. 2009;29:1951–1961. [PubMed] [Google Scholar]
- 29.Forsberg EJ, Miller RJ. Regulation of serotonin release from rabbit intestinal enterochromaffin cells. The Journal of pharmacology and experimental therapeutics. 1983;227:755–766. [PubMed] [Google Scholar]
- 30.Forsberg EJ, Miller RJ. Cholinergic agonists induce vectorial release of serotonin from duodenal enterochromaffin cells. Science. 1982;217:355–356. doi: 10.1126/science.7089569. [DOI] [PubMed] [Google Scholar]
- 31.Glassmeier G, et al. Inhibition of L-type calcium channels by octreotide in isolated human neuroendocrine tumor cells of the gut. Biochemical and biophysical research communications. 1998;250:511–515. doi: 10.1006/bbrc.1998.9344. [DOI] [PubMed] [Google Scholar]
- 32.Tang L, et al. Calcium-sensing receptor stimulates Cl(−)- and SCFA-dependent but inhibits cAMP-dependent HCO3(−) secretion in colon. American journal of physiology. Gastrointestinal and liver physiology. 2015;308:G874–883. doi: 10.1152/ajpgi.00341.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macleod RJ. CaSR function in the intestine: Hormone secretion, electrolyte absorption and secretion, paracrine non-canonical Wnt signaling and colonic crypt cell proliferation. Best practice & research. Clinical endocrinology & metabolism. 2013;27:385–402. doi: 10.1016/j.beem.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Tennakoon, S., Aggarwal, A. & Kallay, E. The calcium-sensing receptor and the hallmarks of cancer. Biochimica et biophysica acta, doi:10.1016/j.bbamcr.2015.11.017 (2015). [DOI] [PubMed]
- 35.Wiedenmann B, Huttner WB. Synaptophysin and chromogranins/secretogranins–widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Archiv. B, Cell pathology including molecular pathology. 1989;58:95–121. doi: 10.1007/BF02890062. [DOI] [PubMed] [Google Scholar]
- 36.Modlin IM, Kidd M, Pfragner R, Eick GN, Champaneria MC. The functional characterization of normal and neoplastic human enterochromaffin cells. The Journal of clinical endocrinology and metabolism. 2006;91:2340–2348. doi: 10.1210/jc.2006-0110. [DOI] [PubMed] [Google Scholar]
- 37.Breunig E, et al. Histamine excites neurones in the human submucous plexus through activation of H1, H2, H3 and H4 receptors. The Journal of physiology. 2007;583:731–742. doi: 10.1113/jphysiol.2007.139352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook AE, et al. Biased allosteric modulation at the CaS receptor engendered by structurally diverse calcimimetics. British journal of pharmacology. 2015;172:185–200. doi: 10.1111/bph.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward DT, Riccardi D. New concepts in calcium-sensing receptor pharmacology and signalling. British journal of pharmacology. 2012;165:35–48. doi: 10.1111/j.1476-5381.2011.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clemmensen C, Smajilovic S, Wellendorph P, Brauner-Osborne H. The GPCR, class C, group 6, subtype A (GPRC6A) receptor: from cloning to physiological function. British journal of pharmacology. 2014;171:1129–1141. doi: 10.1111/bph.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xi YH, et al. The functional expression of calcium-sensing receptor in the differentiated THP-1 cells. Molecular and cellular biochemistry. 2010;342:233–240. doi: 10.1007/s11010-010-0489-3. [DOI] [PubMed] [Google Scholar]
- 42.Seifert R, et al. Molecular and cellular analysis of human histamine receptor subtypes. Trends Pharmacol Sci. 2013;34:33–58. doi: 10.1016/j.tips.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panula P, et al. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol Rev. 2015;67:601–655. doi: 10.1124/pr.114.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ligneau X, et al. BF2.649 [1-{3-[3-(4-Chlorophenyl)propoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: Preclinical pharmacology. The Journal of pharmacology and experimental therapeutics. 2007;320:365–375. doi: 10.1124/jpet.106.111039. [DOI] [PubMed] [Google Scholar]
- 45.Thurmond RL, et al. A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. The Journal of pharmacology and experimental therapeutics. 2004;309:404–413. doi: 10.1124/jpet.103.061754. [DOI] [PubMed] [Google Scholar]
- 46.Arrang JM, Morisset S, Gbahou F. Constitutive activity of the histamine H3 receptor. Trends Pharmacol Sci. 2007;28:350–357. doi: 10.1016/j.tips.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Seifert R, et al. Paradoxical stimulatory effects of the “standard” histamine H4-receptor antagonist JNJ7777120: the H4 receptor joins the club of 7 transmembrane domain receptors exhibiting functional selectivity. Molecular pharmacology. 2011;79:631–638. doi: 10.1124/mol.111.071266. [DOI] [PubMed] [Google Scholar]
- 48.Bakker RA, et al. In vitro pharmacology of clinically used central nervous system-active drugs as inverse H(1) receptor agonists. The Journal of pharmacology and experimental therapeutics. 2007;322:172–179. doi: 10.1124/jpet.106.118869. [DOI] [PubMed] [Google Scholar]
- 49.Kitbunnadaj R, et al. N-substituted piperidinyl alkyl imidazoles: discovery of methimepip as a potent and selective histamine H3 receptor agonist. J Med Chem. 2005;48:2100–2107. doi: 10.1021/jm049475h. [DOI] [PubMed] [Google Scholar]
- 50.Reher TM, Neumann D, Buschauer A, Seifert R. Incomplete activation of human eosinophils via the histamine H4-receptor: evidence for ligand-specific receptor conformations. Biochem Pharmacol. 2012;84:192–203. doi: 10.1016/j.bcp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Salvi V, Mencacci C, Barone-Adesi F. H1-histamine receptor affinity predicts weight gain with antidepressants. Eur Neuropsychopharmacol. 2016;26:1673–1677. doi: 10.1016/j.euroneuro.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Acharya M, et al. CD23/FcepsilonRII: molecular multi-tasking. Clinical and experimental immunology. 2010;162:12–23. doi: 10.1111/j.1365-2249.2010.04210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia YC, et al. Functional expression of IgG-Fc receptors in human airway smooth muscle cells. American journal of respiratory cell and molecular biology. 2011;44:665–672. doi: 10.1165/rcmb.2009-0371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Chromogranin A, an “on/off” switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/S0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- 55.Kidd M, Modlin IM, Drozdov I. Gene network-based analysis identifies two potential subtypes of small intestinal neuroendocrine tumors. BMC genomics. 2014;15:595. doi: 10.1186/1471-2164-15-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harrington AM, Hutson JM, Southwell BR. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Progress in histochemistry and cytochemistry. 2010;44:173–202. doi: 10.1016/j.proghi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Lomax AE, Linden DR, Mawe GM, Sharkey KA. Effects of gastrointestinal inflammation on enteroendocrine cells and enteric neural reflex circuits. Autonomic neuroscience: basic & clinical. 2006;126–127:250–257. doi: 10.1016/j.autneu.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 58.Smith AJ, Chappell AE, Buret AG, Barrett KE, Dong H. 5-Hydroxytryptamine contributes significantly to a reflex pathway by which the duodenal mucosa protects itself from gastric acid injury. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20:2486–2495. doi: 10.1096/fj.06-6391com. [DOI] [PubMed] [Google Scholar]
- 59.Kwan HY, et al. Stimulation of histamine H2 receptors activates TRPC3 channels through both phospholipase C and phospholipase D. European journal of pharmacology. 2009;602:181–187. doi: 10.1016/j.ejphar.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 60.Wood JD. Nonruminant Nutrition Symposium: Neurogastroenterology and food allergies. Journal of animal science. 2012;90:1213–1223. doi: 10.2527/jas.2011-4787. [DOI] [PubMed] [Google Scholar]
- 61.Smolinska S, Jutel M, Crameri R, O’Mahony L. Histamine and gut mucosal immune regulation. Allergy. 2014;69:273–281. doi: 10.1111/all.12330. [DOI] [PubMed] [Google Scholar]
- 62.Buhner S, Schemann M. Mast cell-nerve axis with a focus on the human gut. Biochimica et biophysica acta. 2012;1822:85–92. doi: 10.1016/j.bbadis.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Bischoff SC. Physiological and pathophysiological functions of intestinal mast cells. Seminars in immunopathology. 2009;31:185–205. doi: 10.1007/s00281-009-0165-4. [DOI] [PubMed] [Google Scholar]
- 64.Imamura M, Poli E, Omoniyi AT, Levi R. Unmasking of activated histamine H3-receptors in myocardial ischemia: their role as regulators of exocytotic norepinephrine release. The Journal of pharmacology and experimental therapeutics. 1994;271:1259–1266. [PubMed] [Google Scholar]
- 65.Jemima EA, Prema A, Thangam EB. Functional characterization of histamine H4 receptor on human mast cells. Mol Immunol. 2014;62:19–28. doi: 10.1016/j.molimm.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Lim HD, et al. Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist. The Journal of pharmacology and experimental therapeutics. 2005;314:1310–1321. doi: 10.1124/jpet.105.087965. [DOI] [PubMed] [Google Scholar]
- 67.Deiteren A, De Man JG, Pelckmans PA, De Winter BY. Histamine H(4) receptors in the gastrointestinal tract. British journal of pharmacology. 2015;172:1165–1178. doi: 10.1111/bph.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Provensi G, Blandina P, Passani MB. The histaminergic system as a target for the prevention of obesity and metabolic syndrome. Neuropharmacology. 2016;106:3–12. doi: 10.1016/j.neuropharm.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 69.Lucchelli A, Santagostino-Barbone MG, Barbieri A, Candura SM, Tonini M. The interaction of antidepressant drugs with central and peripheral (enteric) 5-HT3 and 5-HT4 receptors. British journal of pharmacology. 1995;114:1017–1025. doi: 10.1111/j.1476-5381.1995.tb13307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacGlashan D., Jr. IgE receptor and signal transduction in mast cells and basophils. Current opinion in immunology. 2008;20:717–723. doi: 10.1016/j.coi.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 71.Frehn L, et al. Distinct patterns of IgG and IgA against food and microbial antigens in serum and feces of patients with inflammatory bowel diseases. PloS one. 2014;9:e106750. doi: 10.1371/journal.pone.0106750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bentz S, et al. Clinical relevance of IgG antibodies against food antigens in Crohn’s disease: a double-blind cross-over diet intervention study. Digestion. 2010;81:252–264. doi: 10.1159/000264649. [DOI] [PubMed] [Google Scholar]
- 73.Bruggemann M, et al. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. The Journal of experimental medicine. 1987;166:1351–1361. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wiegand TW, et al. High-affinity oligonucleotide ligands to human IgE inhibit binding to Fc epsilon receptor I. Journal of immunology. 1996;157:221–230. [PubMed] [Google Scholar]
- 75.Niesters HG, et al. Development of a real-time quantitative assay for detection of Epstein-Barr virus. Journal of clinical microbiology. 2000;38:712–715. doi: 10.1128/jcm.38.2.712-715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mechtcheriakova D, et al. Activation-induced cytidine deaminase (AID)-associated multigene signature to assess impact of AID in etiology of diseases with inflammatory component. PloS one. 2011;6:e25611. doi: 10.1371/journal.pone.0025611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meshcheryakova A, et al. Exploring the role of sphingolipid machinery during the epithelial to mesenchymal transition program using an integrative approach. Oncotarget. 2016;7:22295–323. doi: 10.18632/oncotarget.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hraha TH, Bernard AB, Nguyen LM, Anseth KS, Benninger RK. Dimensionality and size scaling of coordinated Ca(2+) dynamics in MIN6 beta-cell clusters. Biophys J. 2014;106:299–309. doi: 10.1016/j.bpj.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zimmerman DW, Zumbo BN. Rank transformations and the power of the Student t test and Welch t’ test for non-normal populations with unequal variances. Can J Exp Psychol. 1993;47:523–539. doi: 10.1037/h0078850. [DOI] [Google Scholar]


