Abstract
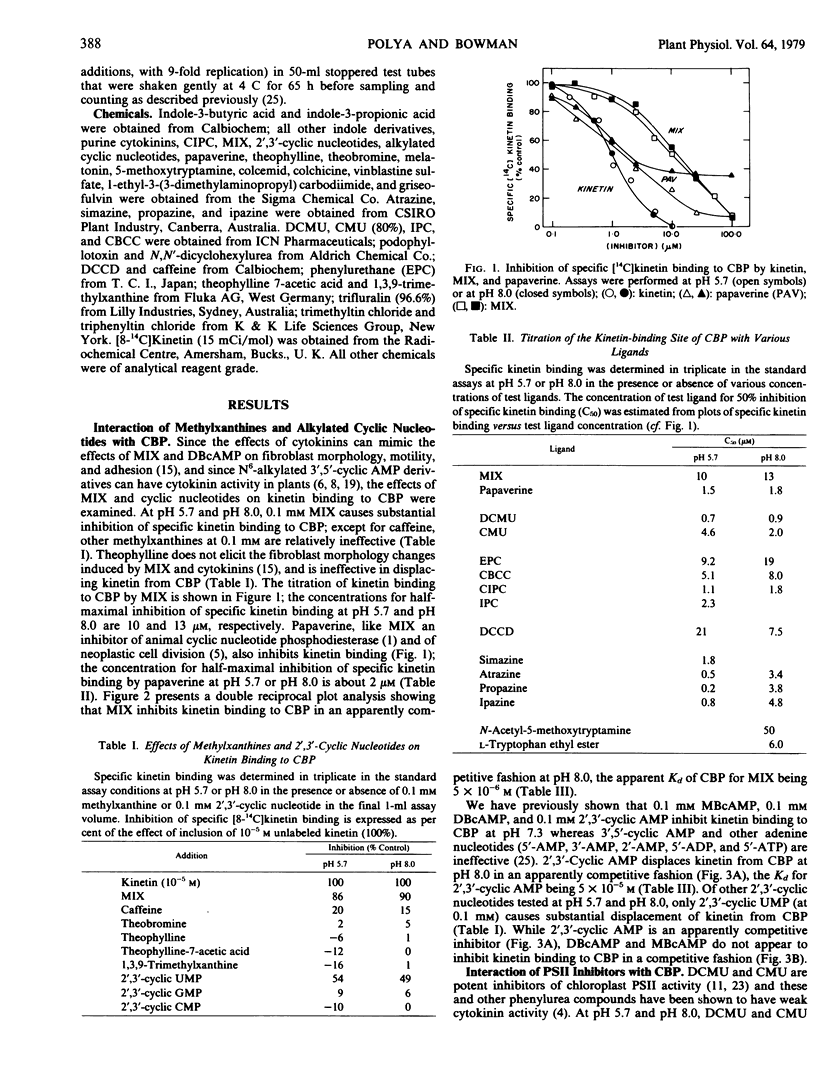
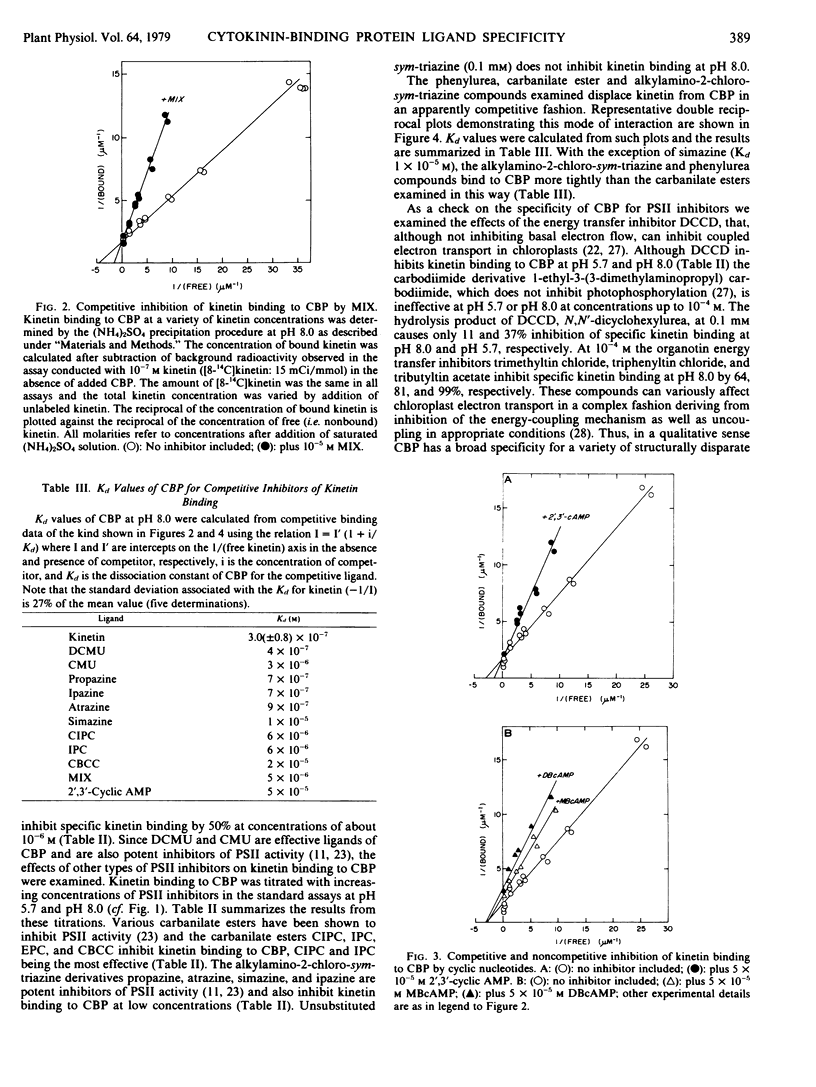
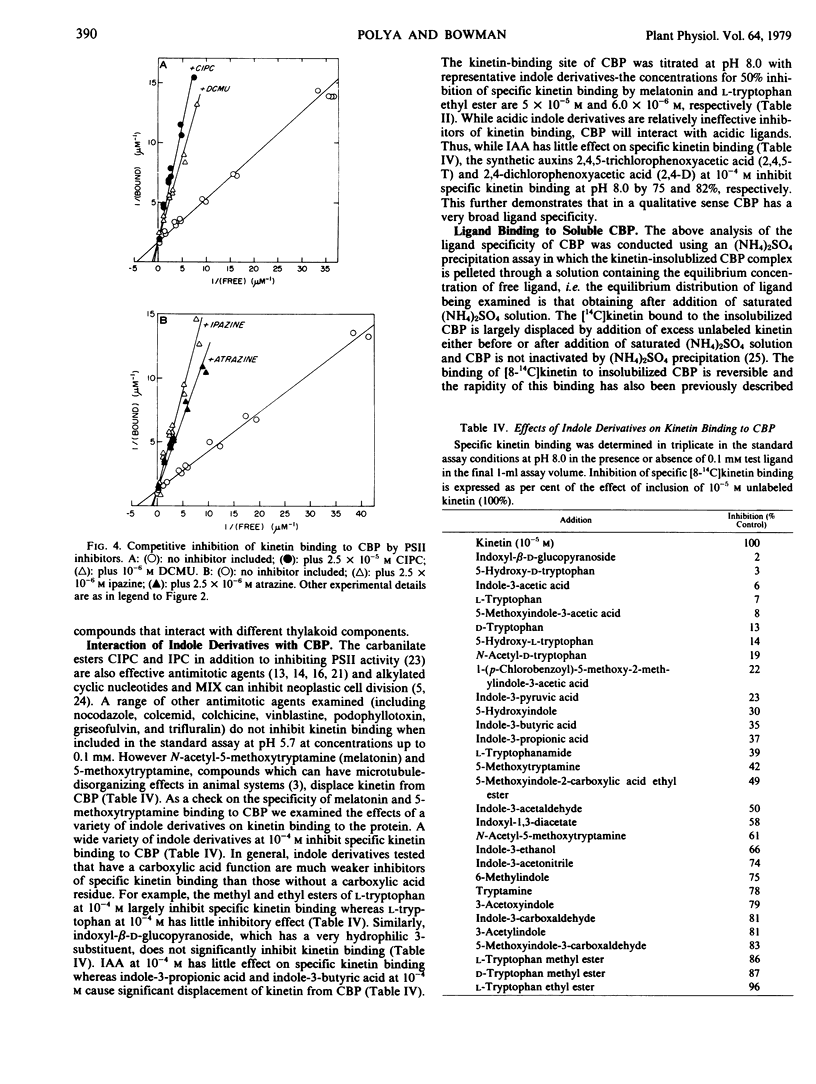
A soluble cytokinin-binding protein from wheat germ that has a high affinity for a range of purine cytokinins also interacts with a variety of nonpurine compounds that can affect cytokinin-modified processes in animal or plant cells or which bind to proteins known to interact with certain cytokinins. A variety of structurally disparate compounds which inhibit chloroplast photosystem II activity (including phenylurea, carbanilate, and alkylamino-2-chloro-sym-triazine compounds) displace kinetin from the protein in an apparently competitive fashion. However, various energy transfer inhibitors (including organotin compounds and N,N′-dicy-clohexylcarbodiimide) also inhibit kinetin binding to the protein. N6,2-0′-Dibutyryl-3′,5′-cyclic AMP and 1-methyl-3-isobutylxanthine (the effects of which on fibroblast morphology and motility can be mimicked by cytokinins) are inhibitors of kinetin binding to the protein. A variety of compounds that can have antimitotic effects (including 1-methyl-3-isobutylxanthine and certain alkylated cyclic nucleotide, carbanilate, and tryptamine compounds) displace kinetin from the protein. However, a variety of indole derivatives also displace kinetin from the cytokinin-binding protein, which in a qualitative sense has a broad ligand specificity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton A. R., Polya G. M. The specific interaction of cibacron and related dyes with cyclic nucleotide phosphodiesterase and lactate dehydrogenase. Biochem J. 1978 Nov 1;175(2):501–506. doi: 10.1042/bj1750501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M. I., Zwar J. A. Cytokinin activity of some substituted ureas and thioureas. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):245–265. doi: 10.1098/rspb.1966.0067. [DOI] [PubMed] [Google Scholar]
- Doskeland S. O., Ueland P. M., Haga H. J. Factors affecting the binding of [3H]adenosine 3':5'-cyclic monophosphate to protein kinase from bovine adrenal cortex. Biochem J. 1977 Mar 1;161(3):653–665. doi: 10.1042/bj1610653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D. C., Murray A. W. Evidence against an involvement of cyclic nucleotides in the induction of betacyanin synthesis by cytokinins. Biochem J. 1975 Feb;146(2):333–337. doi: 10.1042/bj1460333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E., Erion J. L. A cytokinin binding protein from higher plant ribosomes. Biochem Biophys Res Commun. 1975 May 19;64(2):694–700. doi: 10.1016/0006-291x(75)90376-9. [DOI] [PubMed] [Google Scholar]
- Good N. E. Inhibitors of the Hill reaction. Plant Physiol. 1961 Nov;36(6):788–803. doi: 10.1104/pp.36.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S. M., Faulkner R. D., Hawrelak S. D. Competitive inhibition of beef heart cyclic AMP phosphodiesterase by cytokinins and related compounds. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4670–4674. doi: 10.1073/pnas.71.12.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler P. K., Jackson W. T. Isopropyl N-phenylcarbamate affects spindle microtubule orientation in dividing endosperm cells of Haemanthus katherinae Baker. J Cell Sci. 1969 Nov;5(3):727–743. doi: 10.1242/jcs.5.3.727. [DOI] [PubMed] [Google Scholar]
- Jackson W. T. Regulation of mitosis. II. Interaction of isopropyl N-phenylcarbamate and melatonin. J Cell Sci. 1969 Nov;5(3):745–755. doi: 10.1242/jcs.5.3.745. [DOI] [PubMed] [Google Scholar]
- Johnson G. S., D'armiento M., Carchman R. A. N6-substituted adenines induce cell elongation irrespective of the intracellular cyclic AMP levels. Exp Cell Res. 1974 Mar 30;85(1):47–56. doi: 10.1016/0014-4827(74)90211-0. [DOI] [PubMed] [Google Scholar]
- LeJohn H. B., Cameron L. E. Cytokinins regulate calcium binding to a glycoprotein from fungal cells. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1053–1060. doi: 10.1016/0006-291x(73)90800-0. [DOI] [PubMed] [Google Scholar]
- Léjohn H. B. A rapid and sensitive auxin binding system for detecting N6-substituted adenines, and some urea and thiourea derivatives, that show cytokinin activity in cell division tests. Can J Biochem. 1975 Jul;53(7):768–778. doi: 10.1139/o75-104. [DOI] [PubMed] [Google Scholar]
- Mann J. D., Cota-Robles E., Yung K. H., Pu M., Haid H. Phenylurethane herbicides: inhibitors of changes in metabolic state. I. Botanical aspects. Biochim Biophys Acta. 1967 Mar 29;138(1):133–139. doi: 10.1016/0005-2787(67)90593-x. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Samir Amer M., Kreighbaum W. E. Cyclic nucleotide phosphodiesterases: properties, activators, inhibitors, structure--activity relationships, and possible role in drug development. J Pharm Sci. 1975 Jan;64(1):1–37. doi: 10.1002/jps.2600640106. [DOI] [PubMed] [Google Scholar]
- Uribe E. G. The interaction of N,N'-dicyclohexylcarbodiimide with the energy conservation systems of the spinach chloroplast. Biochemistry. 1972 Nov 7;11(23):4228–4235. doi: 10.1021/bi00773a006. [DOI] [PubMed] [Google Scholar]
- Watling-Payne A. S., Selwyn M. J. Inhibition and uncoupling of photophosphorylation in isolated chloroplasts by organotin, organomercury and diphenyleneiodonium compounds. Biochem J. 1974 Jul;142(1):65–74. doi: 10.1042/bj1420065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Takegami T. Isolation of cytokinin binding protein from tobacco leaves by bioaffinity chromatography and its partial characterization. J Biochem. 1977 Mar;81(3):791–799. doi: 10.1093/oxfordjournals.jbchem.a131517. [DOI] [PubMed] [Google Scholar]



