Abstract
Venoms secreted by the venom gland (VG) of parasitoid wasp help ensure successful parasitism by host immune suppression and developmental regulation. Cotesia vestalis, a larval endoparasitoid, and Diadromus collaris, a pupal endoparasitoid, parasitize the diamondback moth (DBM), Plutella xylostella. To explore and compare the venom components of two endoparasitoids, we sequenced transcriptomes of the VGs and wasp bodies without VGs (BWVGs) of the two endoparasitoids. Statistically enriched GO terms and KEGG pathways of the two VGs compared to respective whole-body background were similar and reflected active protein biosynthesis activities in the two VGs. 1,595 VG specific genes of the D. collaris VG and 1,461 VG specific genes of the C. vestalis VG were identified by comparative transcript profiling. A total of 444 and 513 genes encoding potential secretory proteins were identified and defined as putative venom genes in D. collaris VG and C. vestalis VG, respectively. The putative venom genes of the two wasps showed no significant similarity or convergence. More venom genes were predicted in D. collaris VG than C. vestalis VG, especially hydrolase-coding genes. Differences in the types and quantities of putative venom genes shed light on different venom functions.
Introduction
Hymenopteran parasitoids introduce venoms into their hosts at oviposition that facilitate development of their progeny. Venoms can cause paralysis, suppression of immune responses, modulation of the nutritional environment, and alteration of host development, either alone or in combination with other factors1. The venom components of Hymenopteran parasitoids are diverse, often consisting of a complex mixture of proteinaceous as well as nonproteinaceous biomolecules. Components can include neurotoxins, amines, small peptides, and mid- to high-molecular-weight enzymes2. The venom components of 17 parasitoid species, representing five families, have been analyzed. About 60 proteins found in parasitoid venoms share significant homology with proteins with known functions. However, no known functions exist for the vast majority of parasitoid venom proteins so their specific roles in parasitism are unknown3.
Cotesia vestalis (Braconidae), a larval endoparasitoid, and Diadromus collaris (Ichneumonidae), a pupal endoparasitoid, have been recorded in many parts of the world as two of the most important biological control agents of the diamondback moth (DBM), Plutella xylostella (Plutellidae), the most significant cosmopolitan pest of crucifer vegetable crops (Fig. 1)4, 5. These two wasps both parasitize the DBM but use different arsenal combinations. C. vestalis possess all parasitic factors, such as venom, polydnavirus (PDV) and teratocytes originating from the serosal membrane that surrounds the developing embryo of the parasitoid, whereas D. collaris uses only venom for host parasitism6–8. Therefore, it seems that one parasitic weapon in D. collaris could complete the mission undertaken by three parasitic weapons in C. vestalis. Crude venom alone from C. vestalis has a limited effect on hemocytes and probably synergizes the effect of calyx fluid or polydnavirus9 while venom combined with other parasitic factors, such as PDVs can affect host protein metabolism, suppress immune responses, and cause parasitic castration by degenerating host testes10, 11. Venom of D. collaris can impair cell and humor-mediated immune responses of the host by changing the total number, morphology, and behavior of hemocytes and inhibiting the phenoloxidase activity of the hemolymph12, 13. Therefore, venom components and functions of the two wasps should be different and compatible with their specific parasitic lifestyles. Because the two wasps parasitize the same host, it was intriguing to compare their venom components which had not previously been studied.
Figure 1.

Two wasps and their venom apparatuses. (A) and (C) C. vestalis and its venom apparatus. (B) and (D) D. collaris and its venom apparatus.
Conventional methods of combining venom protein separation with bioactivity assays are time-consuming and low throughput while high throughput proteomics methods are dependent on genomic information14. It is difficult to collect adequate pure venom for proteome research15. The venom organ of parasitoid wasps is tiny (Fig. 1), the amount of venom is extremely limited, and contamination from the venom duct or venom glands (VGs) is inevitable when venom protein samples are prepared for proteome analysis. High-throughput transcriptomic analysis has recently been applied to study the VGs of parasitic wasps, and the feasibility of this technology has been proven16–19. Therefore, we also turned to VG transcriptome analysis for studying the venom components of the two wasp species.
In this study, we sequenced transcriptomes of VGs and bodies without venom glands (BWVGs) of these two species using Illumina technology. De novo assembly identified tens of thousands of distinct sequences. Genomic features of the two VGs were analyzed. Putative genes related to venom functions were discovered by secretory protein prediction and comparative transcriptome analysis. Our results provide insight into how venom functions in host-parasitoid interactions and will facilitate identification of more Hymenopteran venom genes.
Results and Discussions
Transcriptome overview
For VG and BWVG of D. collaris, Illumina sequencing yielded 26, 777, 782 and 88, 360, 364 reads with nucleotide sizes of 2,284, 178,940 and 7,139, 171,340 bp, respectively (Table 1). For VG and BWVG of C. vestalis, Illumina sequencing yielded 26, 234, 320 and 86,756,318 reads with nucleotide sizes of 2,199,844,980 and 7,203, 796,020 bp, respectively. All high-quality reads were assembled de novo by the Trinity program. We obtained 34,063 and 63,325 transcripts from VG and BWVG of D. collaris while 26, 066 and 51,641 transcripts from VG and BWVG of C. vestalis. After further process of sequence splicing and redundancy removal with sequence clustering software, we obtained 50,763 and 43,785 ALL-transcripts with an average length of 1005 nt and 828 nt for D. collaris and C. vestalis, respectively (Table 1). Next, we analyzed the length distribution of all-transcripts sequences. Although most sequences (>50%) were between 100 to 500 bp, 7186 sequences longer than 2,000 bp were identified in D. collaris. A similar trend was observed in C. vestalis (Supplementary Fig. S1A).
Table 1.
Summary of the transcriptomes.
| DCBWVGs | DCVGs | DC | CVBWVGs | CVVGs | CV | |
|---|---|---|---|---|---|---|
| Total number of reads | 88,360,364 | 26,777,782 | — | 86,756,318 | 26,234,320 | — |
| Total base pairs (bp) | 7,139,171,340 | 2,284,178,940 | — | 7,203,796,020 | 2,199,844,980 | — |
| GC percentage | 47.53% | 45.69% | — | 41.93% | 39% | — |
| Average read length (bp) | 90 | 90 | — | 90 | 90 | — |
| Total number of contigs | 108,198 | 65,680 | — | 88,392 | 49,265 | — |
| Mean length of contigs (bp) | 409 | 305 | — | 372 | 284 | — |
| Total unique sequences | 63,325 | 34,063 | 50,763 | 51,641 | 26,066 | 43,785 |
| Number of sequences in all-transcripts | 28,394 | 48,725 | — | 41,796 | 32,775 | — |
| Sequences with E-value <10−5 | 16,121 | 23,276 | 26,753 | 22,148 | 15,723 | 26,483 |
DCVGs: D. collaris venom glands; DCBWVGs: D. collaris bodies without venom glands; CVVGs: C. vestalis venom glands; CVBWVGs: C. vestalis bodies without venom glands.
For annotation, all-transcripts sequences were searched by BLASTx against the non-redundant (nr) NCBI database using a cut-off E-value of 10−5. 26,753 (53%) and 26,483 (60%) sequences returned an above cut-off BLAST result for D. collaris and C. vestalis. The proportion of sequences with matches in nr databases was greater among the longer assembled sequences (Supplementary Fig. S1B). The E-value distribution of best hits against the nr database showed that about 59% of the mapped sequences have strong homology (smaller than 1.0E−50), whereas 41% of the homolog sequences range from 1.0E−5 to 1.0E−50 in D. collaris (Fig. 2). All-transcripts sequences of C. vestalis has nearly the same E-value distribution pattern. Similarity distribution analysis shows that over 60% matches are more than 60% similar in D. collaris and C. vestalis. As to the species distribution, the two wasp transcriptomes were very similar. The highest percentage of unigenes of the two wasps matched the genes of the alfalfa leafcutter bee Megachile rotundata, followed by the jewel wasp Nasonia vitripennis, and the Jerdon’s jumping ant Harpegnathos saltator (Fig. 2).
Figure 2.
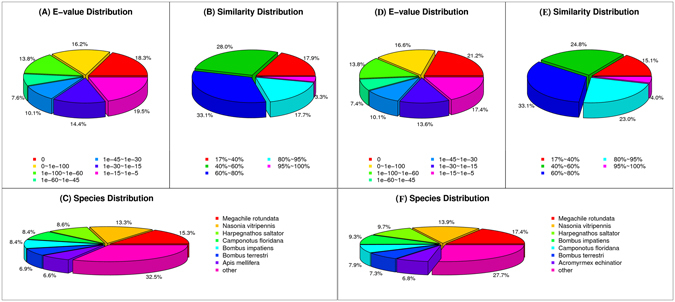
Characteristics of homology search of Illumina sequences against the nr database. (A) and (D): E-value distribution of BLAST hits for D. collaris all-transcripts and C. vestalis all-transcripts with a cut-off E-value of 1.0E−5. (B) and (E): Similarity distribution of the top BLAST hits for D. collaris all-transcripts and C. vestalis all-transcripts. (C) and (F): Species distribution is shown as a percentage of the total homologous sequences with an E-value of at least 1.0E−5 in D. collaris all- transcripts and C. vestalis all-transcripts. We used the first hit of each sequence for analysis.
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of venom gland genes
Among the all-transcripts, the transcripts having reads in VG or BWVG represented VG transcriptomes or BWVG transcriptomes, respectively. D. collaris and C. vestalis VG transcriptomes consisted of 32775 and 28394 transcripts, respectively (Table 1).
The GO classification system allows descriptions of gene products in terms of their associated biological processes, cellular components, and molecular functions. Overall, 7698 genes of D. collaris VG and 7189 genes of C. vestalis VG were assigned to GO terms. In both VGs, sequences to which GO categories were assigned had the greatest representation in ‘Cellular process’. For both VGs, in the three main divisions (cellular component, molecular function, and biological process) of the GO classification, the categories ‘Cell’, ‘Binding’, and ‘Cellular process’ were dominant, respectively (Fig. 3).
Figure 3.
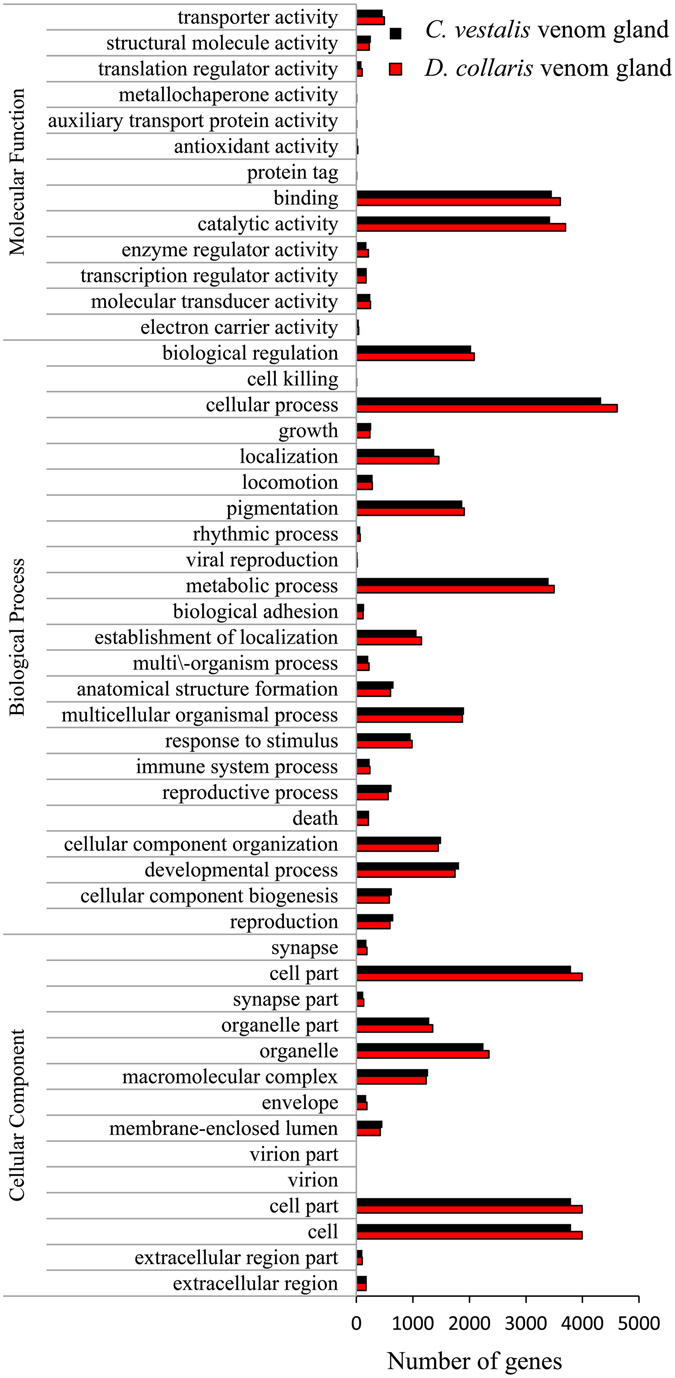
GO term distribution of venom gland genes at level two.
The distribution pattern of GO terms in the two VGs showed great similarity at levels 2, 3, 4, and 5 across GO categories with respect to the number of transcripts linked to each GO term. Pearson’s correlation coefficients were all significant (>0.99 with P-value ≪ 0.01 at three levels) either for the whole GO terms or each main division (Supplementary Table S1), indicating that the two VG transcriptomes had significantly similar function profiles or patterns. The semantic similarity of two GO term sets measured by G-SESAME was 0.75056 (Maximum value = 1), indicating relatively higher similarity.
To study the physiological characters of the VGs, statistically enriched GO terms were identified and analyzed compared to the whole-body transcriptome background. In the category of ‘Molecular Function’, ‘Catalytic activity’ was enriched in the two VGs at level two while ‘Binding’ was only enriched in D. collaris VG. Analysis of ‘Cell Component’ category enrichment indicated that ‘Organelle’, ‘Cell’, ‘Organelle part’, ‘Membrane-enclosed lumen’, and ‘Macromolecular complex’ were significantly enriched at level two in both VGs. For the category of ‘Biological Process’, ‘Metabolic process’ was enriched at level two in both VGs while ‘Cellular process’ was only enriched in C. vestalis VG at level two in the ‘Biological Process’ category (Supplementary Table S2).
The KEGG orthology (KO) is a classification system that provides an alternative functional annotation of genes by their associated biological pathways. A total of 7604 D. collaris and 7338 C. vestalis VG genes were assigned to KOs based on sequence homologies. D. collaris and C. vestalis VG genes were mapped to 252 and 256 KEGG pathways, respectively (Supplementary Table S3). A total of 252 pathways were shared by the two transcriptomes. Pathways ‘Asthma’, ‘Retinol metabolism’, ‘Porphyrin and chlorophyll metabolism’, and ‘Terpenoid backbone biosynthesis’ were only found in D. collaris VG. The pathways with the most representation in both VGs were ‘Metabolic pathways’, ‘spliceosome’, and ‘RNA transport’. In almost all pathways, the two transcriptomes had similar representations in the number of distinct annotations within each pathway. For the 252 shared pathways, Pearson’s correlation coefficient in percentages of transcript representations, indicated significant similarity in percentages of transcript representations (r > 0.97, p < 3.82 E-177) (Supplementary Table S3).
Enrichment analysis was also performed to identify the over-expressed pathways with the whole-body transcript distribution as background. Totally, 44 and 36 enriched pathways (P ≤ 5.0E−3) were identified in D. collaris VG and C. vestalis VG (Supplementary Table S4). Among them, 24 pathways were enriched in both VGs. At level four, ‘Spliceosome’, ‘Ubiquitin mediated proteolysis’, ‘Tuberculosis’, and ‘RNA degradation’ were the top four enriched pathways in D. collaris VG. ‘Spliceosome’, ‘Protein processing in endoplasmic reticulum’, and ‘Proteasome’, were the top 3 enriched pathways in C. vestalis VG. At level two, ‘Genetic information processing’ and ‘Metabolism’ were dominant in both VGs (Supplementary Table S4).
VGs are specific organs for production of venom macromolecules and secretions, and these organs have a high level of metabolic activity. Previous research demonstrated that the ultrastructure of the secretory units of the gland tubules in D. collaris was consistent with the model of a type III gland cell, which was quite similar to the C. vestalis VG and VGs described in other parasitoids20–24. The apparatuses of the two VG cells were abundant and included Golgi apparatus, rough endoplasmic reticulum, and mitochondria. The abundance of these components was consistent with intense protein synthesis and suggests vigorous activities in the VG cells6, 20. Indeed, many over-expressed GO terms and KEGG pathways reflected these features. Most enriched GO terms in ‘Cell Component’ such as ‘Organelle’, ‘Organelle part’, and ‘Membrane-enclosed lumen’ were consistent with the observation of abundant apparatuses in gland cells. In the meantime, the enrichment of the pathways of ‘Genetic information processing’ and ‘Metabolism’ and the over-expressed GO terms of ‘Metabolic process’ and ‘Macromolecular complex’ were also consistent with the active processes of macromolecule biosynthesis and catabolism in gland cells. Interestingly, ‘Catalytic activity’ and ‘Binding’ enriched in ‘Molecular Function’ were also the most represented functional categories assigned to the VG ESTs from the saw-scaled viper, Echis ocellatus 25, the solitary hunting wasp species, Orancistrocerus drewseni 26, the endoparasitic wasp, Chelonus inanitus 27, and the ant, Tetramorium bicarinatum 19. Vincent et al.27 suggested that catalytic activity and binding categories thus may constitute a hallmark of the VG transcriptomes analyzed to date27.
In conclusion, the obvious similarity in distribution profiles and enrichment results of GO terms and KEGG pathways between the two wasp VGs might reflect the similar secretory structure and function of two VGs at the genetic level. These data will help draw a general pattern for the biosynthesis and secretion of venom proteins of two VGs.
Differently expressed genes in VG compared to BWVG
Differences in reads frequencies in the VG and BWVG libraries were used to estimate differences in gene expression level between two libraries. We identified 22,543 and 23,040 genes that were expressed at significantly different levels between VG and BWVG in D. collaris and C. vestalis (Fig. 4 and Supplementary Table S5). Of these, 4396 were up-regulated and 18147 were down-regulated in the D. collaris VG while 3831 genes were up-regulated and 19,209 genes were down-regulated in the C. vestalis VG (Fig. 4 and Supplementary Table S5).
Figure 4.
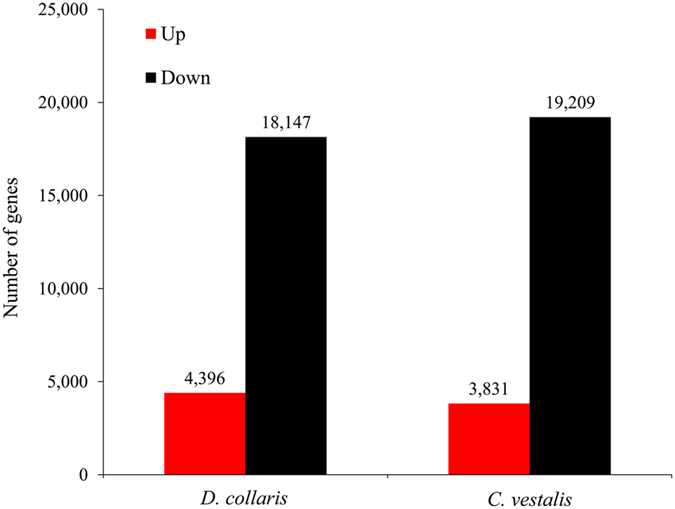
Changes in gene expression profiles between venom glands (VGs) and bodies without venom glands (BWVGs).
We also identified 1,595 and 1,461 VG specific genes in up-regulated genes that were only expressed in D. collaris and C. vestalis VGs. Of these, 422 D. collaris (26%) and 581 C. vestalis (40%) genes could be annotated based on alignments to the nr database (Supplementary Tables S6 and S7). Many were highly expressed. FPKMs (Fragments Per kb per Million fragments) of 38 D. collaris VG and 6 C. vestalis VG specific genes were >1000. Only 19 D. collaris VG and 1 C. vestalis VG highly expressed genes were annotated while the rest showed no similarity to any known proteins. CL3939. Contig3_All of D. collaris VG that matched a protein inhibitor and Unigene30513_All of C. vestalis that was similar to a venom protein were the two most highly expressed annotated genes. CL2424. Contig1_All of D. collaris VG with a 91787.8632 FPKM and Unigene32174_All of C. vestalis VG with a 62339.5501 FPKM, with no annotation and the most highly expressed, should be completely new proteins. Interestingly, the annotation rate was obviously lower in gland specific genes than other VG genes. Perhaps these genes, probably the most possible VG function related, evolved and diverged more rapidly. Next, these VG specific genes were classified through GO and KEGG annotation. At level 2, ‘Catalytic activity’ and ‘Binding’ were dominant in ‘Molecular Function’, providing the same result as in the two VG transcriptomes mentioned above. KEGG annotations of VG specific genes of the two wasps differed considerably at level 3 (Supplementary Tables S6 and S7). ‘Metabolic pathways’ contained the most VG specific genes of D. collaris VG while ‘Ribosome’ was the highest in occurrence in the VG specific genes of the C. vestalis VG. This is consistent with the function of active protein synthesis and metabolic activities in the VG.
To validate the gene expression data obtained through statistical comparison of FPKM value, we compared the gene expression profiles of VG and BWVG using quantitative PCR (qPCR). A good correlation was obtained for the results of RNA-Seq and qPCR analysis in 10 genes from each wasp (Supplementary Tables S8 and S9).
Secreted protein prediction and function analysis
Venoms were secreted by VG cells from the parasitoid wasps. Therefore, venom proteins are expected with signal peptides in their amino acid sequences. A total of 532 and 457 potential secretory proteins were identified in the D. collaris VG and the C. vestalis VG while 499 and 419 had annotations in the nr database (Supplementary Table S10). These genes, encoding potential secretory proteins, were defined as putative venom genes of the two wasps and further analyzed.
A total of 116 D. collaris VG and 70 C. vestalis VG secretory proteins were homologs of known Hymenopteran venom proteins. However, most putative secretory proteins showed no significant similarity to known venom proteins. There exists a great possibility that these secretory proteins represent novel venom proteins for each wasp. Among all VG genes, significantly more homologous sequences of known Hymenopteran venom proteins were in the putative secreted proteins (21.8% in D. collaris VG and 15.3% in C. vestalis VG) than those in non-secretory sequences (3.5% in D. collaris VG and 1.42% in C. vestalis VG), especially in the up-regulated subgroups of secretory proteins (27% in D. collaris VG and 21.4% in C. vestalis VG) (Supplementary Table S10).
Immunological similarities exist across venoms of many Hymenopteran species. Antibodies raised against Chelonus nr. curvimaculatus (Braconidae) venom reacted with venom proteins from the Formicidae, Vespidae, and Apidae. Venom proteins from mostly primitive parasitic wasps and ants showed much higher cross-reactivity than aculeate wasp and bee venom28. Conversely, four venom proteins from the ectoparasitoid wasp Eupelmus orientalis were recognized by polyclonal antibodies raised against venom proteins from Apis mellifera 1, 29. Recently, antibodies against P. puparum calreticulin, GOBP-like venom protein, venom protein U, serine protease 22, and serine protease homolog 29 were found to cross detect the venom proteins in N. vitripennis 16. Comparative analysis indicates that venoms of social Hymenoptera species are qualitatively similar with venoms produced by parasitic aculeates30–32. Many types of venom proteins in parasitic wasps are also present in social and solitary wasps or bees as allergens such as antigen 5 and acid phosphatase33. These immunological similarities, due to similar amino acid identity or similar post-translational modifications, suggest evolutionary conservation of venom composition and perhaps functionality in spite of their apparently different functions34. Therefore, the continuity of venom protein evolution could be the reason for this great similarity with other Hymenopteran venom proteins in putative secreted proteins and provides further evidence that our approach has led to the successful identification of venom proteins.
D. collaris VG has 116 putative secretory proteins sharing homology with venom components detected in other wasps, including pupal endoparasitoids (Supplementary Table S10). Pupal endoparasitoids like D. collaris are restricted primarily to a few subfamilies of the Ichneumonidae30, 35, 36. The best-studied species is Pimpla hypochondriaca, a solitary pupal endoparasitoid, which injects a venom that paralyzes and immunosuppresses its Lepidopteran host30, 37. Neither embryos nor feeding-stage larvae of P. hypochondriaca appear to play a role in altering host development or immune defenses30. A number of venom genes have been identified by random sequencing of the venom gland cDNA library and classical bottom-up proteomic approaches2, 37, 38. D. collaris as a solitary pupal endoparasitoid lives a parasitic life similar to P. hypochondriaca and thus its progeny face the similar challenges. Because there are no additional parasitoid-associated factors, such as PDVs and teratocytes, in D. collaris, venom may play a role similar to that of P. hypochondriaca venom in host immune suppression and host regulation12, 13. A similarity search of putative secreted proteins against P. hypochondriaca venom proteins returned matches to trehalase, metalloprotease, cysteine-rich venom protein 6, cysteine-rich venom protein 2, and two other proteins. This indicates venom components with potentially similar functions in D. collaris venom.
C. vestalis VG has 70 putative secretory proteins significantly matched to venom genes of other wasp species, including PDV-producing parasitoids (Supplementary Table S10). For many PDV-producing parasitoids like C. vestalis, venom proteins are required for PDV function or to provide synergistic effects. This ranges from complete independence of some ichneumonid PDVs (ichnoviruses) to variable dependency of braconid PDVs (bracoviruses) on venom2. Yu et al.9 found that C. vestalis venom alone may be insufficient to suppress the host immune system, but it might synergize the effects of calyx fluid or polydnavirus as in other insect-host systems9. A number of putative secreted proteins in the C. vestalis VG transcriptome showed evident similarity to venom proteins from PDV-producing parasitoids such as Cotesia rubecula. For example, the Unigene32176_All coded for a protein most similar to “Venom protein Vn4.6” which seemed to interfere with the activation of host hemolymph prophenoloxidase39. Intriguingly, Unigene32176_All, with 32,734 FPKM, was also the most frequently sequenced transcript with annotation in either C. vestalis VG or genes coding for secretory proteins. CL1620. Contig8_All matched to venom protein Vn50 and Unigene8653_All had a 0 e-value against calreticulin in C. rubecula venom. This might indicate similar venom functions present in C. vestalis venom.
Many venom proteins have been discovered to suppress host immunity including humoral and cellular immunity, and dominant in quantity among all the venom proteins3. Host immune suppression by the two wasp venoms has been primarily studied as mentioned above in the introduction part. Putative venom proteins of the two parasitoids contain homologs of immune-suppressing proteins (Supplementary Table S10). Proteins similar to immune-suppressing proteins, including Vn5040, calreticulin41, and super oxide dismutase42, were found in putative venom proteins of two wasps. Proteins with significant similarity to Vn4.639 and serpin43 also existed in C. vestalis potential secretory venom proteins. These proteins might help the parasitoids escape the host immunity responses and will be the focus of our study in the future. For the rest of putative venom-coding genes, either venom gene homologies or novel venom genes, their potential roles in interactions with the host should also be taken into consideration during the studies in the future.
All genes encoding secretory proteins consist of 111 up-regulated and 287 down-regulated genes in D. collaris VG, while 56 up-regulated and 312 down-regulated genes in C. vestalis VG (Supplementary Table S10). Previous studies of venom genes have demonstrated that most venom coding genes were either up-regulated or even venom tissue specific39, 41–47. Therefore, these secreted proteins encoded by up-regulated transcripts are likely to be real venom proteins. Among the up - regulated genes, 34 and 11 genes were only expressed in D. collaris VG and C. vestalis VG including some highly-expressed transcripts (FPKM > 1000). These included CL2038. Contig3_All, Unigene35108_All, and CL3939. Contig1_All in D. collaris VG and Unigene32174_All and Unigene30513_All in C. vestalis VG. Although several VG specific genes still have no function annotations, these genes should be the most probable venom genes (Supplementary Table S10).
A total of 182 and 177 distinct domains were identified in the putative secreted proteins of D. collaris VG and C. vestalis VG, respectively (Supplementary Table S10). Of these, 94 domains were shared. All of these putative secretory proteins of two wasp species appeared to fall into seven different broad functional categories by combining domain and nr annotation data according to Poirie et al.3. These categories included (1) enzymes; (2) protease inhibitors; (3) immune related proteins; (4) recognition/binding proteins; (5) neurotoxin-like/paralytic factors; (6) chaperones, and (7) others (Fig. 5). Apparently more secretory proteins were classified into the enzyme category in D. collaris VG (170) than C. vestalis VG (125). The enzyme category was further classified into subcategories and compared based on their specific functions (Supplementary Table S10). Significantly more hydrolases were in D. collaris VG secretory proteins (120) than C. vestalis VG secretory proteins (88), such as peptidase (17 in D. collaris VG and six in C. vestalis VG), esterase (16 in D. collaris VG and six in C. vestalis VG) and trehalase (four in D. collaris VG and 0 in C. vestalis VG) (Fig. 5 and Supplementary Table S10).
Figure 5.
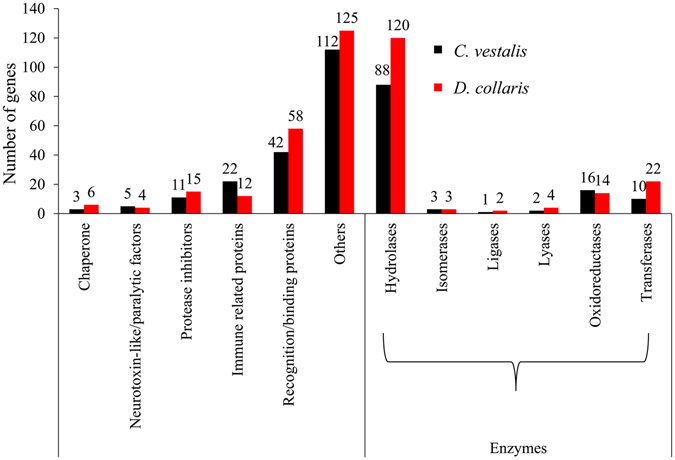
Function classification of putative secretory proteins.
Sequence similarities of secreted proteins and non-secreted body proteins between the two VGs were compared using blastp48. Results showed that the e-value distribution between secreted proteins of the two VGs resembled that between non-secreted proteins (Pearson coefficient = 0.93, p < 0.01) (Fig. 6).
Figure 6.
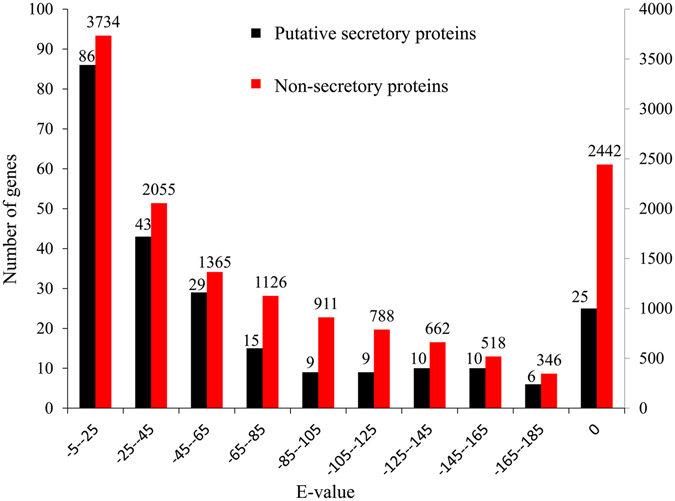
Sequence similarities of secreted proteins and non-secreted proteins between two venom glands.
Most putative venom genes were different between two wasp species and no significant similarity was discovered between putative venom genes of the two wasps (Supplementary Table S10). Convergent evolution apparently did not happen in the two venoms even though both venoms are used to suppress immune reactions of the same host9, 12. The parasitization of different development phases by two wasps, wasp phylogeny, and how the venom interacts with host physiology might have had greater influence on the venom components. Recent studies on the wasp Leptopilina species (Figitidae) indicated that venom composition could mainly differ even between closely-related species parasitizing the same host49, 50.
More putative venom genes, especially genes coding for hydrolases, were predicted in the D. collaris VG transcriptome than in the C. vestalis VG transcriptome. Many factors such as the limited number of known venom genes or incomplete N-terminal unigene regions could have affected the prediction results. However, considering that C. vestalis possesses three parasitic factors while D. collaris has only venom, venom from D. collaris ought to have more functions to accomplish successful parasitism. Li et al.12 reported that venom of D. collaris might be able to destroy the structure of the host fat body and adipocytes to release nutrition for progeny development. Hydrolases should play a role in this process12. However, no proteins with any similar function were discovered in C. vestalis venom. Therefore, the advantage of D. collaris VG in putative venom genes, especially hydrolase related genes, may not be a mere coincidence.
Conclusion
We sequenced the transcriptomes of two endoparasitoids of P. xylostella using Illumina sequencing technology. A great number of unique transcripts were assembled and annotated. An evident similarity between the two VGs was discovered in the distribution profiles of GO terms and KEGG pathways in the two VG transcriptomes. Enriched GO terms and pathways of the two VGs and VG specific genes were consistent with active activities of protein biosynthesis in the VGs. Putative venom genes of the two wasps showed no obvious similarity or convergence although the wasps parasitize different stages of the same host. More venom genes were predicted in D. collaris VG than C. vestalis VG, especially hydrolase-coding genes. The differences in the types and quantities of putative venom genes between the two wasp species shed some light on divergent venom functions of the two endoparasitoids. We speculated that the divergence of two venom gland transcriptomes might suggest that the evolution of two venom glands has led to the diversity of venom components and functions adapting to specific parasitic lifestyles while their similarities in distribution profiles and enrichment results of GO terms and KEGG pathways may reflect their origin from the common ancestor and retain a potential conserved transcriptome profile for venom production. Taken together, our results provide an invaluable resource for the identification of additional Hymenopteran venom genes and will contribute to the understanding of how venom functions in host-parasitoid interactions.
Methods
Insect rearing and sample preparation
Parasitoids and the host P. xylostella were maintained as previously described5, 6. Briefly, an abundance of hosts at proper stages were exposed to each parasitoid for parasitization. Larvae parasitized by C. vestalis were reared on cabbage while pupae parasitized by D. collaris were maintained in a container until emergence of adult wasps. Adult wasps were fed 20% honey water. Both parasitoid species and their host were maintained at 25 ± 1 °C, 65% relative humidity under a 14 h light:10 h dark photoperiod. VGs were dissected from 0–7 day old mated female wasps, and then both VGs and BWVGs were collected in 1.5-ml microtubes containing Trizol reagent (Invitrogen Life technologies, CA, USA), respectively. After homogenization, samples were stored in a −70 °C refrigerator. The total RNA was extracted using Trizol reagent according to the manufacture’s protocol.
RNA isolation and library preparation
Total RNA was extracted from VG and BWVG using TRIZOL reagent according to the manufacturer’s protocol. RNA integrity was confirmed using the 2100 Bioanalyzer (Agilent Technologies) with a minimum RNA integrated number value of 8. The samples for transcriptome analysis were prepared using Illumina’s kit following manufacturer’s instructions. Briefly, poly(A) mRNA was purified from 20 μg of total RNA using oligo(dT) magnetic beads and fragmented into short sequence by fragmentation buffer. The cleaved poly(A) RNA fragments were used for first strand cDNA synthesis using random hexamer-primer followed by second strand cDNA synthesis using RNaseH and DNA polymerase I. After the end repair and ligation of adaptors, the products were purified and enriched with PCR to create a cDNA library.
Transcriptome assembly and annotation
Four cDNA libraries were sequenced at the Beijing Genome Institute (Shenzhen, China) on the Illumina HiSeq™ 2000 platform. Transcriptome de novo assembly was accomplished with assembling program - Trinity51. After removal of adaptor sequences, empty reads and low quality sequences, sequences from the two libraries were assembled into contigs. Then the reads were mapped back to contigs. Contigs from the same transcript were detected and further assembled with paired-end reads. For transcripts from each of the two libraries, TGIC52 and Phrap53 were used to assemble to non-redundant (nr) all-transcripts by gene clustering.
Transcript sequences were first aligned by blastx (http://blast.ncbi.nlm.nih.gov/Blast.cgi) against protein databases like nr, Swiss-Prot, and KEGG, retrieving proteins with the highest similarity with the given transcripts along with their protein functional annotations. With nr annotation, GO annotation, and functional classification for transcripts were analyzed using Blast2GO54, 55 and WEGO software56. Orientation and coding sequence (CDS) of sequences which had no hits in blast were predicted using ESTScan57. Transcript expression level was calculated using the FPKM method (Fragments Per kb per Million reads).
Pearson’s correlation coefficient was used to evaluate the correlation values with respect to the percentages of transcript representations linked to each GO term or KEGG biological pathway between two transcriptomes.
G-SESAME (http://bioinformatics.clemson.edu/G-SESAME/index.php) was used to measure the semantic similarity of GO term sets58, 59.
Secretory protein prediction and protein domain identification
The BLAST results were used to extract CDSs from transcripts. The CDS of the transcripts that has no significant hit in BLAST search were predicted by ESTScan57. Prediction of signal peptides was carried out using the SignalP 3.0 Server (http://www.cbs.dtu.dk/services/SignalP-3.0/)60. To remove sequences that also contained a transmembrane domain in addition to the signal peptide, we used TMHMM Server (http://www.cbs.dtu.dk/services/TMHMM/) to predict transmembrane region. The putative protein that has a signal peptide and with no or one transmembrane domain would be considered as a potential secreted protein61, 62.
Protein domains were identified by searching the Pfam database (http://pfam.xfam.org) using the HMMER web server (http://www.ebi.ac.uk/Tools/hmmer/)63, 64.
Identification of statistically enriched ontologies and pathways
The hypergeometric test was used to measure significantly enriched GO terms in the target gene groups in comparison with the background62, 65. The calculating formula used was , where N is the number of all genes with GO annotation; n is the number of differentially expressed genes (DEGs) in N; M is the number of genes that are annotated to a certain GO terms; and m is the number of DEGs in M. The GO terms with the p-value cut-off of 5.0E−3 were deemed to be enriched. In addition, to identify the enriched pathways, the hypergeometric test was used similarly to measure the relative coverage of the annotated KEGG orthologous groups of a pathway in the background, and pathways with a p-value cut-off of 5.0E−3 were considered as enriched66.
In this paper, the wasp transcriptome means the all-transcripts. And the transcripts having reads in VG or BWVG represented VG transcriptome or BWVG transcriptome, respectively.
Identification of differentially expressed genes
The expression differences between two samples were calculated with the FDR (false discovery rate) method. The FDR was applied to determine the threshold of the P-value in multiple tests and analyses67. An FDR < 0.001 and an absolute value of log2Ratio ≥ 1 were used as the threshold to judge the significance of gene expression differences.
Quantitative real-time PCR (qRT-PCR) analysis
To confirm the results of the FPKM comparison, the expression profiles of 10 selected genes were measured using qPCR. Total RNAs of VG and BWVG were extracted using the SV Total RNA Isolation System (Promega, Fitchburg, USA). One microgram of RNAwas reverse transcribed for first-strand cDNA synthesis with the ReverTra Ace qPCR RT Kit (TOYOBO, Osaka, Japan). qRT-PCR was performed in ABI7500 Real-Time System (Applied Biosystems, Foster City, CA, USA) using SYBR Premix Ex Taq TM II (Takara, Shiga, Japan). The cycling parameters were 95 °C for 60 seconds followed by 40 cycles of 95 °C for 15 s and 60 °C for 35 s. For each gene, three biological replicates were analyzed and the average threshold cycle (Ct) was calculated. The results were normalized to the expression level of the C. vestalis 18S rRNA gene (GenBank accession number: JX399880) and D. collaris 18S rRNA gene (GenBank accession number: KX912696). Finally, the relative expression level was calculated using the 2−△△Ct method68.
Data deposition
The four data sets of Illumina sequencing are available at the NCBI Short Read Archive (SRA) with the accession number: SRR1022346 (D. collaris VG), SRR4294717 (D. collaris BWVG), SRR1032213 (C. vestalis VG) and SRR3948414 (C. vestalis BWVG). The assembled sequences have been deposited in the NCBI’s TSA database: GEZZ00000000 (D. collaris all-transcripts) and GFAF00000000 (C. vestalis all-transcripts).
Electronic supplementary material
Acknowledgements
This research was supported by the 973 Program (2013CB127600), and the State Key Program of National Natural Science Foundation of China (31630060).
Author Contributions
W.Z. and X.X.C. conceived and designed the experiments; W.Z., M.S., and Y.X.Q. performed the experiments and analyzed the data; W.Z., F.L., X.W.W. and X.X.C. wrote and revised the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01383-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moreau SJ, Guillot S. Advances and prospects on biosynthesis, structures and functions of venom proteins from parasitic wasps. Insect Biochem. Mol. Biol. 2005;35:1209–1223. doi: 10.1016/j.ibmb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Asgari S, Rivers DB. Venom proteins from endoparasitoid wasps and their role in host-parasite interactions. Annu. Rev. Entomol. 2010;56:313–335. doi: 10.1146/annurev-ento-120709-144849. [DOI] [PubMed] [Google Scholar]
- 3.Poirie M, Colinet D, Gatti JL. Insights into function and evolution of parasitoid wasp venoms. Curr. Opin. Insect Sci. 2014;6:52–60. doi: 10.1016/j.cois.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Liu SS, Wang XG, Shi ZH, Gebremeskel FB. The biology of Diadromus collaris (Hymenoptera: Ichneumonidae), a pupal parasitoid of Plutella xylostella (Lepidoptera: Plutellidae), and its interactions with Oomyzus sokolowskii (Hymenoptera: Eulophidae) B. Entomol. Res. 2001;91:461–469. [PubMed] [Google Scholar]
- 5.Yu RX, Shi M, Huang F, Chen XX. Immature development of Cotesia vestalis (Hymenoptera: Braconidae), an endoparasitoid of Plutella xylostella (Lepidoptera: Plutellidae) Ann. Entomol. Soc. Am. 2008;101:189–196. doi: 10.1603/0013-8746(2008)101[189:IDOCVH]2.0.CO;2. [DOI] [Google Scholar]
- 6.Li WD, Yu RX, Chen XX, He JH. Venom gland of the ichneumonid Diadromus collaris: morphology, ultrastructure and age-related changes. Fruits. 2006;13:137–143. [Google Scholar]
- 7.Bai SF, Li X, Chen XX, Cheng JA, He JH. Interspecific competition between two endopatasitoids Cotesia vestalis (Hymenoptera: Braconidae) and Oomyzus sokolowskii (Hymenoptera: Eulophidae) Arch. Insect. Biochem. Physiol. 2011;76:156–167. doi: 10.1002/arch.20399. [DOI] [PubMed] [Google Scholar]
- 8.Shi M, Huang F, Chen YF, Meng XF, Chen XX. Characterization of midgut trypsinogen-like cDNA and enzymatic activity in Plutella xylostella parasitized by Cotesia vestalis or Diadegma semiclausum. Arch. Insect. Biochem. Physiol. 2009;70:3–17. doi: 10.1002/arch.20249. [DOI] [PubMed] [Google Scholar]
- 9.Yu RX, et al. Effects of venom/calyx fluid from the endoparasitic wasp Cotesia vestalis on the hemocytes of its host Plutella xylostella in vitro. J. Insect Physiol. 2007;53:22–29. doi: 10.1016/j.jinsphys.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Bai SF, Cai DZ, Li X, Chen XX. Parasitic castration of Plutella xylostella larvae induced by polydnaviruses and venom of Cotesia vestalis and Diadegma semiclausum. Arch. Insect. Biochem. Physiol. 2009;70:30–43. doi: 10.1002/arch.20279. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim AMA, Kim Y. Parasitism by Cotesia vestalis alters the hemocyte population and immunological function of the diamondback moth, Plutella xylostella. J. Insect Physiol. 2006;52:943–950. doi: 10.1016/j.jinsphys.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Li WD, Shi M, Chen XX. Effects of parasitism by Diadromus collaris (Hymenoptera: Ichneumonidae) on morphology and ultrastructure of fat body and adipocytes of host Plutella xylostella (Lepidoptera: Plutellidae) pupae. Acta Ecol. Sin. 2007;50:662–666. [Google Scholar]
- 13.Li WD, Huang F, Chen YF, Chen XX. Immunosuppression effects of venom of pupal endoparasitoid wasp, Diadromus collaris (Gravenhorst) on its host, Plutella xylostella pupae. Acta Ecol. Sin. 2006;49:206–212. [Google Scholar]
- 14.de Graaf DC, Aerts M, Danneels E, Devreese B. Bee, wasp and ant venomics pave the way for a component-resolved diagnosis of sting allergy. J. Proteomics. 2009;72:145–154. doi: 10.1016/j.jprot.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Colinet D, Mathe-Hubert H, Allemand R, Gatti JL, Poirie M. Variability of venom components in immune suppressive parasitoid wasps: from a phylogenetic to a population approach. J. Insect Physiol. 2013;59:205–212. doi: 10.1016/j.jinsphys.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Yan Z, et al. Insights into the venom composition and evolution of an endoparasitoid wasp by combining proteomic and transcriptomic analyses. Sci. Rep. 2016;6:19604. doi: 10.1038/srep19604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke GR, Strand MR. Systematic analysis of a wasp parasitism arsenal. Mol. Ecol. 2014;23:890–901. doi: 10.1111/mec.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park D, et al. Uncovering the novel characteristics of Asian honey bee, Apis cerana, by whole genome sequencing. BMC Genomics. 2015;16:1. doi: 10.1186/1471-2164-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouzid W, et al. De Novo sequencing and transcriptome analysis for Tetramorium bicarinatum: a comprehensive venom gland transcriptome analysis from an ant species. Bmc Genomics. 2014;15:987. doi: 10.1186/1471-2164-15-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan J, Chen Xx. A comparative ultrastructure of the venom apparatus from two species of parasitic wasps (Hymenoptera) of Plutella xylostella (Lepidoptera) Belg. J. Zool. 2003;22:79–81. [Google Scholar]
- 21.Noirot C, Quennede A. Fine-structure of insect epidermal glands. Annu. Rev. Entomol. 1974;19:61–80. doi: 10.1146/annurev.en.19.010174.000425. [DOI] [Google Scholar]
- 22.Gnatzy W, Volknandt W. Venom gland of the digger wasp Liris niger: morphology, ultrastructure, age-related changes and biochemical aspects. Cell Tissue Res. 2000;302:271–284. doi: 10.1007/s004410000282. [DOI] [PubMed] [Google Scholar]
- 23.Blass S, Ruthmann AA. Fine-structure of the accessory-glands of the female genital-tract of the ichneumonid Pimpla turionellae (Hymenoptera) Zoomorphology. 1989;108:367–377. doi: 10.1007/BF00312277. [DOI] [Google Scholar]
- 24.Vanmarle J. Structure and histochemistry of venom glands of wasps Microbracon hebetor Say and Philanthus triangulum F. Toxicon. 1977;15:529–539. doi: 10.1016/0041-0101(77)90104-0. [DOI] [PubMed] [Google Scholar]
- 25.Wagstaff SC, Harrison RA. Venom gland EST analysis of the saw-scaled viper, Echis ocellatus, reveals novel alpha9beta1 integrin-binding motifs in venom metalloproteinases and a new group of putative toxins, renin-like aspartic proteases. Gene. 2006;377:21–32. doi: 10.1016/j.gene.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Baek JH, et al. Differential gene expression profiles in the venom gland/sac of Orancistrocerus drewseni (Hymenoptera: Eumenidae) Arch. Insect Biochem. Physiol. 2009;71:205–222. doi: 10.1002/arch.20316. [DOI] [PubMed] [Google Scholar]
- 27.Vincent, B. et al. The venom composition of the parasitic wasp Chelonus inanitus resolved by combined expressed sequence tags analysis and proteomic approach. BMC Genomics11, 693, doi:10.1186/1471-2164-11-693 (2010). [DOI] [PMC free article] [PubMed]
- 28.Leluk J, Schmidt J, Jones D. Comparative studies of the protein composition of Hymenopteran venom reservoirs. Toxicon. 1989;27:105–114. doi: 10.1016/0041-0101(89)90410-8. [DOI] [PubMed] [Google Scholar]
- 29.Doury G, Bigot Y, Periquet G. Physiological and biochemical analysis of factors in the female venom gland and larval salivary secretions of the ectoparasitoid wasp Eupelmus orientalis. J. Insect Physiol. 1997;43:69–81. doi: 10.1016/S0022-1910(96)00053-4. [DOI] [PubMed] [Google Scholar]
- 30.Pennacchio F, Strand MR. Evolution of developmental strategies in parasitic Hymenoptera. Annu. Rev. Entomol. 2006;51:233–258. doi: 10.1146/annurev.ento.51.110104.151029. [DOI] [PubMed] [Google Scholar]
- 31.Piek T. Neurotoxins from venoms of the Hymenoptera-25 years of research in Amsterdam. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 1990;96:223–233. doi: 10.1016/0742-8413(90)90001-P. [DOI] [PubMed] [Google Scholar]
- 32.Brochetto-Braga MR, Lima PRde. Hymenoptera venom review focusing on Apis mellifera. Cr. Acad. Sci. Urss. 2003;9:149–162. [Google Scholar]
- 33.Hoffman DR. Hymenoptera venom allergens. Clin. Rev. Allergy Immunol. 2006;30:109–128. doi: 10.1385/CRIAI:30:2:109. [DOI] [PubMed] [Google Scholar]
- 34.Quicke, D. L. Parasitic Wasps, (Chapman and Hall, 1997).
- 35.Gauld ID. Evolutionary patterns of host utilization by Ichneumodidae parasitoids (Hymenoptera, Ichneumodidae and Braconidae) Biol. J. Linn. Soc. Lond. 1988;35:351–377. doi: 10.1111/j.1095-8312.1988.tb00476.x. [DOI] [Google Scholar]
- 36.Whitfield JB. Phylogeny and evolution of host-parasitoid interactions in hymenoptera. Annu. Rev. Entomol. 1998;43:129–151. doi: 10.1146/annurev.ento.43.1.129. [DOI] [PubMed] [Google Scholar]
- 37.Dani MP, Richards EH. Identification, cloning and expression of a second gene (vpr1) from the venom of the endoparasitic wasp, Pimpla hypochondriaca that displays immunosuppressive activity. J. Insect Physiol. 2010;56:195–203. doi: 10.1016/j.jinsphys.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Richards EH, Dani MP. A recombinant immunosuppressive protein from Pimpla hypochondriaca (rVPr1) increases the susceptibility of Lacanobia oleracea and Mamestra brassicae larvae to Bacillus thuringiensis. J. Invertebr. Pathol. 2010;104:51–57. doi: 10.1016/j.jip.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Asgari S, Zareie R, Zhang GM, Schmidt O. Isolation and characterization of a novel venom protein from an endoparasitoid, Cotesia rubecula (Hymenoptera: Braconidae) Arch. Insect. Biochem. Physiol. 2003;53:92–100. doi: 10.1002/arch.10088. [DOI] [PubMed] [Google Scholar]
- 40.Asgari S, Zhang G, Zareie R, Schmidt O. A serine proteinase homolog venom protein from an endoparasitoid wasp inhibits melanization of the host hemolymph. Insect Biochem. Mol. Biol. 2003;33:1017–1024. doi: 10.1016/S0965-1748(03)00116-4. [DOI] [PubMed] [Google Scholar]
- 41.Zhang G, Schmidt O, Asgari S. A calreticulin-like protein from endoparasitoid venom fluid is involved in host hemocyte inactivation. Dev. Comp. Immunol. 2006;30:756–764. doi: 10.1016/j.dci.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Colinet D, Cazes D, Belghazi M, Gatti JL, Poirie M. Extracellular superoxide dismutase in insects: characterization, function, and interspecific variation in parasitoid wasp venom. J. Biol. Chem. 2011;286:40110–40121. doi: 10.1074/jbc.M111.288845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colinet D, et al. A serpin from the parasitoid wasp Leptopilina boulardi targets the Drosophila phenoloxidase cascade. Dev. Comp. Immunol. 2009;33:681–689. doi: 10.1016/j.dci.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Price DRG, et al. A venom metalloproteinase from the parasitic wasp Eulophus pennicornis is toxic towards its host, tomato moth (Lacanobia oleracae) Insect Mol. Biol. 2009;18:195–202. doi: 10.1111/j.1365-2583.2009.00864.x. [DOI] [PubMed] [Google Scholar]
- 45.Labrosse C, et al. A RhoGAP protein as a main immune suppressive factor in the Leptopilina boulardi (Hymenoptera, Figitidae) - Drosophila melanogaster interaction. Insect Biochem. Mol. Biol. 2005;35:93–103. doi: 10.1016/j.ibmb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Doremus T, et al. Venom gland extract is not required for successful parasitism in the polydnavirus-associated endoparasitoid Hyposoter didymator (Hym. Ichneumonidae) despite the presence of numerous novel and conserved venom proteins. Insect Biochem. Mol. Biol. 2013;43:292–307. doi: 10.1016/j.ibmb.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y, et al. A venom gland extracellular chitin-binding-like protein from pupal endoparasitoid wasps, Pteromalus Puparum, selectively binds chitin. Toxins. 2015;7:5098–5113. doi: 10.3390/toxins7124867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Camacho C, et al. BLAST+: architecture and applications. BMC bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colinet D, et al. Extensive inter- and intraspecific venom variation in closely related parasites targeting the same host: the case of Leptopilina parasitoids of Drosophila. Insect Biochem. Mol. Biol, Insect biochemistry and molecular biology. 2013;43:601–611. doi: 10.1016/j.ibmb.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Goecks J, et al. Integrative approach reveals composition of endoparasitoid wasp venoms. PLoS One. 2013;8:e64125. doi: 10.1371/journal.pone.0064125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grabherr MG, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pertea G, et al. TIGR Gene Indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics. 2003;19:651–652. doi: 10.1093/bioinformatics/btg034. [DOI] [PubMed] [Google Scholar]
- 53.Nickerson DA, Tobe VO, Taylor SL. PolyPhred: Automating the detection and genotyping of single nucleotide substitutions using fluorescence-based resequencing. Nucleic Acids Res. 1997;25:2745–2751. doi: 10.1093/nar/25.14.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conesa A, et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 55.Conesa A, Gotz S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int. J. Plant Genomics. 2008;2008:1–13. doi: 10.1155/2008/619832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye J, et al. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 2006;34:293–297. doi: 10.1093/nar/gkl031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iseli C, Jongeneel CV, Bucher P. ESTScan: a program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1999;99:138–148. [PubMed] [Google Scholar]
- 58.Wang JZ, Du Z, Payattakool R, Yu PS, Chen CF. A new method to measure the semantic similarity of GO terms. Bioinformatics. 2007;23:1274–1281. doi: 10.1093/bioinformatics/btm087. [DOI] [PubMed] [Google Scholar]
- 59.Du Z, Li L, Chen CF, Yu PS, Wang JZ. G-SESAME: web tools for GO-term-based gene similarity analysis and knowledge discovery. Nucleic Acids Res. 2009;37:345–349. doi: 10.1093/nar/gkp463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 61.Bos JI, et al. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid) PLoS Genet. 2010;6:e1001216. doi: 10.1371/journal.pgen.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su YL, et al. Transcriptomic analysis of the salivary glands of an invasive whitefly. PLoS One. 2012;7:e39303. doi: 10.1371/journal.pone.0039303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finn RD, et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 2016;44:279–285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finn RD, et al. HMMER web server: 2015 update. Nucleic Acids Res. 2015;43:30–38. doi: 10.1093/nar/gkv397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin Y, et al. Intestinal transcriptomes of nematodes: comparison of the parasites Ascaris suum and Haemonchus contortus with the free-living Caenorhabditis elegans. PloS Negl. Trop. Dis. 2008;2:e269. doi: 10.1371/journal.pntd.0000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li XY, et al. De novo sequencing and comparative analysis of the blueberry transcriptome to discover putative genes related to antioxidants. Gene. 2012;511:54–61. doi: 10.1016/j.gene.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 67.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001;29:1165–1188. doi: 10.1214/aos/1013699998. [DOI] [Google Scholar]
- 68.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


