Abstract
Introduction:
Three-dimensional. (3D) printing is an innovative manufacturing process that allows computer-assisted conversion of 3D imaging data into physical “printouts” Healthcare applications are currently in evolution.
Objective:
The objective of this study was to explore the feasibility and impact of using patient-specific 3D-printed cardiac prototypes derived from high-resolution medical imaging data (cardiac magnetic resonance imaging/computed tomography [MRI/CT]) on surgical decision-making and preoperative planning in selected cases of complex congenital heart diseases (CHDs).
Materials and Methods:
Five patients with complex CHD with previously unresolved management decisions were chosen. These included two patients with complex double-outlet right ventricle, two patients with criss-cross atrioventricular connections, and one patient with congenitally corrected transposition of great arteries with pulmonary atresia. Cardiac MRI was done for all patients, cardiac CT for one; specific surgical challenges were identified. Volumetric data were used to generate patient-specific 3D models. All cases were reviewed along with their 3D models, and the impact on surgical decision-making and preoperative planning was assessed.
Results:
Accurate life-sized 3D cardiac prototypes were successfully created for all patients. The models enabled radically improved 3D understanding of anatomy, identification of specific technical challenges, and precise surgical planning. Augmentation of existing clinical and imaging data by 3D prototypes allowed successful execution of complex surgeries for all five patients, in accordance with the preoperative planning.
Conclusions:
3D-printed cardiac prototypes can radically assist decision-making, planning, and safe execution of complex congenital heart surgery by improving understanding of 3D anatomy and allowing anticipation of technical challenges.
Keywords: Cardiac magnetic resonance imaging, congenital heart disease, surgical planning, three-dimensional printing
INTRODUCTION
Congenital heart diseases (CHDs) include an extraordinary range of morphological malformations. In-depth understanding of anatomy and physiology is fundamental to diagnosis and management. Imaging modalities include echocardiography, catheter angiography, computed tomography (CT), and magnetic resonance imaging (MRI) aid diagnosis and surgical planning. While the process of image acquisition is often standardized, interpretation can be subjective, dependent upon experience, and expertise. Heart is a three-dimensional (3D), dynamic, and complex organ; challenges lie in interpreting 2D slices or arrays of imaging information and mentally reconstructing them into their 3D perspective. Once interpreted by the imager, communication of complex anatomy to surgical colleagues is still a challenge. Conventionally, this is achieved through joint review and discussion of imaging data, at times augmented by simple tools such as representative hand-drawn sketches/illustrations. While functional, there are inherent shortcomings in this approach. First, it relies on accuracy of interpretation and communication of imaging information. Second, it often does not lend itself well to 3D spatial understanding.
This becomes particularly important when trying to understand and plan interventions for complex anatomical lesions with combinations of abnormalities in viscerocardiac situs and position, complex intracardiac defects, and abnormal spatial relationships of the major vessels. Lack of understanding and communication of spatial anatomy can lead to suboptimal preoperative planning translating into surgical surprises, unexpected intraoperative technical challenges, and long surgical/cardiopulmonary bypass (CPB) time. These can potentially impact both short- and long-term surgical outcomes. In resource-limited environments like India where both access and expertise for complex heart surgery are limited,[1] excellent preoperative planning is particularly important to ensure optimal repair with optimal use of available resources.
3D printing also known as “additive manufacturing” is an innovative manufacturing process that uses computer-aided processing of 3D imaging data to create physical “printouts” of virtual objects. Specialized hardware (3D printers) can convert digital data into physical objects by adding layer upon layer of material, fused together, to create physical replicas of the virtual objects.[2] Utilization of 3D printing for healthcare needs is a relatively new concept and still in evolution.[2,3,4,5,6,7] Complex CHD offers a unique opportunity for applying 3D printing technology to address existing lacunae in anatomical detailing, communication, and surgical planning.
Objective
To explore the feasibility and impact of using patient-specific 3D cardiovascular models generated by rapid prototyping (3D printing) technology on surgical decision-making and surgical planning in selected cases of complex CHDs.
MATERIALS AND METHODS
Five patients with complex CHD with unresolved clinical management decisions were chosen for comprehensive cardiac MRI assessment and 3D prototyping of their structural heart diseases. Clinical details and anatomical diagnosis are summarized in Table 1. Three patients had previously undergone palliative surgeries. Four of the patients in this cohort had previously been evaluated at multiple institutions and been refused surgery stating high surgical risk and/or uncertain outcomes. All patients had previously undergone extensive clinical evaluation and cardiovascular imaging, followed by discussion and review in departmental surgical conferences. Specific anatomical challenges impeded surgical decision-making for definitive procedures in each of these patients.
Table 1.
The clinical and morphological details of the patients
Patient 1: Despite diagnosis of CHD in infancy, he had been refused cardiac surgery at multiple institutions in the past due to complex anatomy. Surgical challenges to biventricular repair were identified as: (i) Complex cardio-visceral spatial arrangement - visceroatrial situs inversus, dextrocardia, double-outlet right ventricle (DORV); (ii) difficult left ventricle (LV)-to-aorta route, requiring a long, complex patch; (iii) requirement for right ventricle (RV)-to-pulmonary artery (PA) conduit
Patient 2: Subsequent to previous palliative surgeries (Blalock–Taussig shunt at 2 months and Glenn surgery at 4 years of age), uncertainty regarding suitability for biventricular repair had prevented further surgical intervention. Anticipated surgical challenges were (i) complex spatial anatomy with abnormal viscerocardiac situs, atrioventricular (AV) discordance, aorta from RV, with pulmonary atresia; (ii) complex repair – redo sternotomy, atrial switch with difficult LV-to-aorta routing, and RV-PA conduit; (iii) predicted right ventricular volume suboptimal for biventricular repair; (iv) limited space to place RV-PA conduit
Patient 3: Despite neonatal diagnosis of complex CHD with heart failure, he had been refused surgery at multiple outside institutions due to anatomical complexity. Surgical challenges were (i) criss-cross AV relationship, complex ventricular septal anatomy, DORV from superiorly placed RV; (ii) uncertainty regarding routability of LV-to-aorta; (iii) need for aortic arch repair
Patient 4: Palliated in infancy with pulmonary artery band (PAB) for an underlying diagnosis of criss-cross heart with VSD, subsequent repairs had been deferred due to complexity of spatial anatomy
Patient 5: She had undergone multiple palliative surgeries at outside institutions, favoring single ventricle pathway due to technically difficult biventricular options. She was hemodynamically unsuitable for Fontan surgery (elevated LV end-diastolic pressure). Complex ventricular septal anatomy with multiple septal defects posed challenge to biventricular repair.
Cardiac magnetic resonance imaging
All patients underwent cardiac MRI for comprehensive anatomical and physiological assessment as well as to acquire 3D volumetric imaging data for 3D prototyping. Cases 1–3 were imaged on 1.5 Tesla, GE Signa HDxt scanner with eight-channel dedicated cardiac surface coil; cases 4–5 were imaged on 1.5 Tesla Siemens Magnetom Avanto scanner. All MRI scans were carried out and analyzed by a pediatric cardiologist and cardiac radiologist with expertise in cardiac MRI. Sequences acquired included 2D steady-state free precession (SSFP) cine sequences in orthogonal stacks, four-chamber, two-chamber, ventricular short axis, and ventricular outflow views for assessing cardiovascular anatomy in its dynamic perspective. 2D SSFP stack in the ventricular short axis planes was used to assess ventricular geometry, dimensions, and systolic functions. Phase-contrast acquisitions were used for blood flow assessments across relevant blood vessels to quantify cardiac output, Qp: Qs, and differential flows. Contrast-enhanced magnetic resonance angiography (MRA) was done in all cases after injecting 0.2 mmol/kg bodyweight of gadolinium contrast medium. Thin slice high-resolution coronal acquisitions were made when the contrast media opacified all four cardiac chambers on MR fluoroscopy. Other scan parameters include 2.6 mm slice thickness, 256 × 192 matrix, 0.75 number of excitation, parallel imaging acceleration factor of 2, and elliptic centric acquisition. Myocardial delayed enhancement sequence was acquired to look for late gadolinium enhancement. Additional 64-slice multidetector CT was done for case 3. Imaging datasets were exported in “Digital Imaging and Communications in Medicine” (DICOM) format onto GE Report Card™ or Osirix™ platforms for postprocessing, physiological assessment, multiplanar viewing, and 3D reconstruction of angiographic anatomy.
CMR data were analyzed and reviewed and discussed in combined surgical conferences to identify surgical challenges and define specific study questions for 3D printing.
Three-dimensional printing
The DICOM datasets were imported into dedicated postprocessing software (Materialise Mimics®, Materialise NV, Leuven, Belgium). Data were processed to reduce imaging noise and isolate the anatomy of interest. Cardiac chambers were segmented through semi-automated heart segmentation tool (CT heart). The region of interest was further refined with interactive editing operations. Valve leaflets and chordal apparatus were excluded from the segmented anatomy since the scans did not have sufficient spatial resolution to accurately define these structures, and since vulvar anatomy was not part of the study questions. After completing segmentation, a 3D computer model was rendered for visualization and measurements. The Materialise Mimics® software was then utilized to create a wall around the blood volume to allow visualization of intracardiac structures. In a final step, the surface of the model was smoothed and prepared to ensure suitability for 3D printing before exporting in the “standard tessellation language” (STL) format. 3D models were printed out using a 3D printer with the HeartPrint® flex technology (Materialise NV, Leuven, Belgium) or selective laser sintering (SLS) technique (polyamide).
Virtual 3D models were verified through multiple interactive sessions between the clinical team and the technology team involved in segmentation and postprocessing. This was done to ensure that the segmented anatomy was true to patient anatomy as depicted by the original MRI/CT scans. The clinical team was represented by a pediatric cardiologist directly involved with the primary image acquisition and interpretation, with understanding of patient-specific anatomical issues and study questions. Multiple corrections and iterations of segmentation were required to ensure correct representation of patient anatomy, correcting for imaging artifacts, and errors in thresholding. All models were created as hollow models. Planes in which the models would be opened up to allow internal inspection were decided based on patient-specific anatomy – to provide optimal anatomic understanding, and simulating the “surgeon's view.” The final virtual 3D-segmented hollow models were interrogated in detail using 3D viewing tools before final conversion to STL format for printing.
Material used for prints were based on case-specific requirements. In 4/5 patients, we opted for hard, inflexible models; for patient 2, we opted for a semi-transparent flexible print. Case 4 was printed in multiple colors for easy anatomic understanding.
Patient review and evaluation of three-dimensional-printed heart models
All cases were rediscussed in the combined surgical conference. All available clinical and imaging data were reviewed, along with the physical inspection of the 3D-printed heart models by a team of four experienced pediatric cardiac surgeons and four pediatric cardiologists involved in the care of these patients.
RESULTS
Cardiac MRI added to anatomical and physiological understanding in all cases, helping identify specific surgical challenges that needed further elucidation. Gadolinium-enhanced MRA data were used to create the 3D prints in 4/5 cases. Suboptimal spatial resolution and image quality of MRA necessitated the use of 64-slice multidetector CT angiography for 3D printing in one case (patient 3). Each print was designed to address the patient-specific issues.
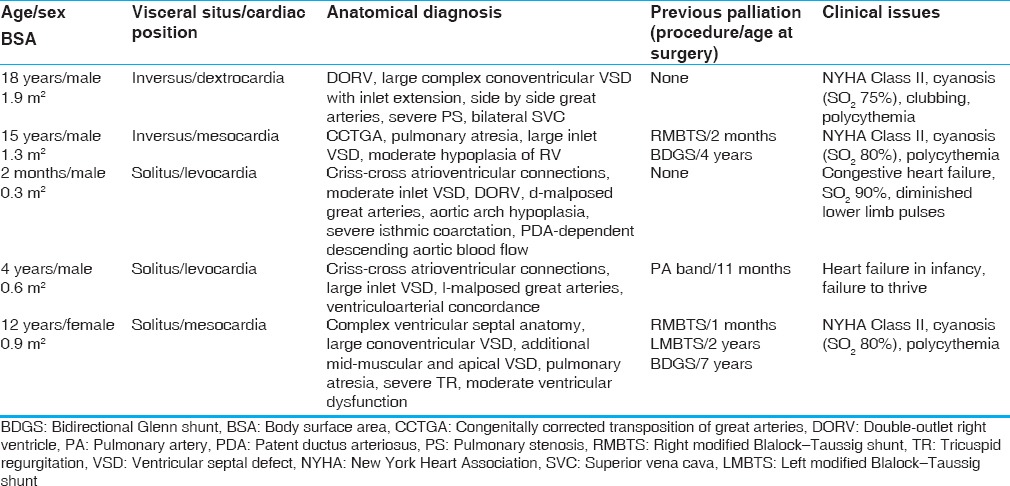
3D print for patient 1 was created with polyamide using SLS technique [Figures 1 and 2]. It was designed with posterior walls of the atria removed to allow a view through the AV valve annuli, similar to the surgeons’ perspective after atriotomy.
Figure 1.
The conversion of imaging data from patient 1 into its three-dimensional print. (a) Volume rendered gadolinium-enhanced three-dimensional magnetic resonance angiogram depicting spatial anatomy of the ventricles and the great arteries. (b) The hollow heart virtual three-dimensional model generated after postprocessing of the original imaging data. (c) The three-dimensional model printed in polyamide material using SLS technique. These images show that the three-dimensional model is identical to the original anatomy. Ao: Aorta, LV: Left ventricle, MPA: Main pulmonary artery, RV: Right ventricle, SLS: Slow laser sintering
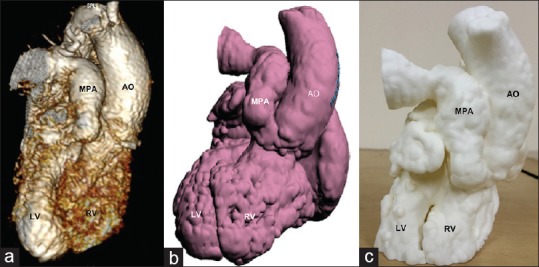
Figure 2.
(a) The transatrial visualization of the intracardiac anatomy using the virtual hollow heart model of patient 1. (b) Similar perspective using the three-dimensional print. VSD is labeled with *. Ao: Aorta, LV: Left ventricle, RV: Right ventricle, VSD: Ventricular septal defect
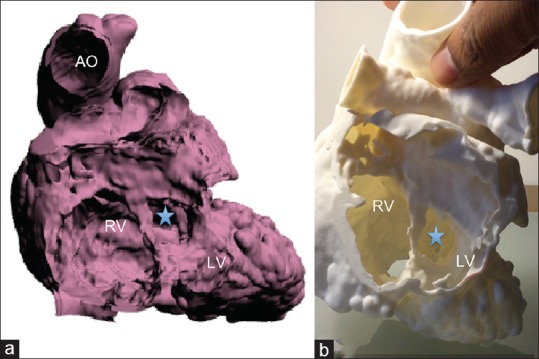
The model for patient 2 was printed in HeartPrint® Flex material, a semitransparent, flexible material [Figure 3]. The model was designed with the posterior atrial walls removed allowing transatrial viewing of both the ventricles.
Figure 3.
(a and c) The computer-generated virtual three-dimensional models of patient 2. (b and d) The three-dimensional printed model (HeartPrint® flex) viewed from similar perspectives. Ao: Aorta, LV: Left ventricle, RV: Right ventricle, VSD is marked by arrow in (d)
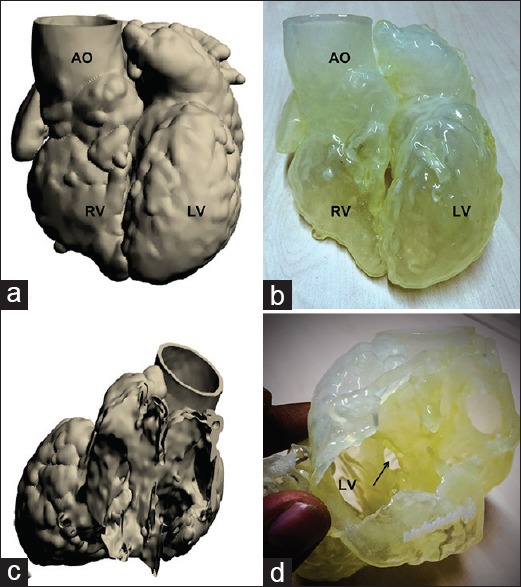
The prototype for patient 3 was built using powder-based “sandstone” material, producing a hard hollow model of the heart. Two cuts were prescribed during the design stage – a posterior parasagital cut allowing removal of the atria, and a mid-septal level ventricular short axis cut. Thus, the final model had three parts which when put together simulated the full heart; detachment of the atrial part allowed a view into the ventricles through the two AV valve annuli with easy visualization of the ventricular septal anatomy and the inlet ventricular septal defect [Figure 4]. Detachment of the ventricular apical slice allowed a view into the ventricular cavities and the origins of aorta and PA from the superiorly placed RV.
Figure 4.
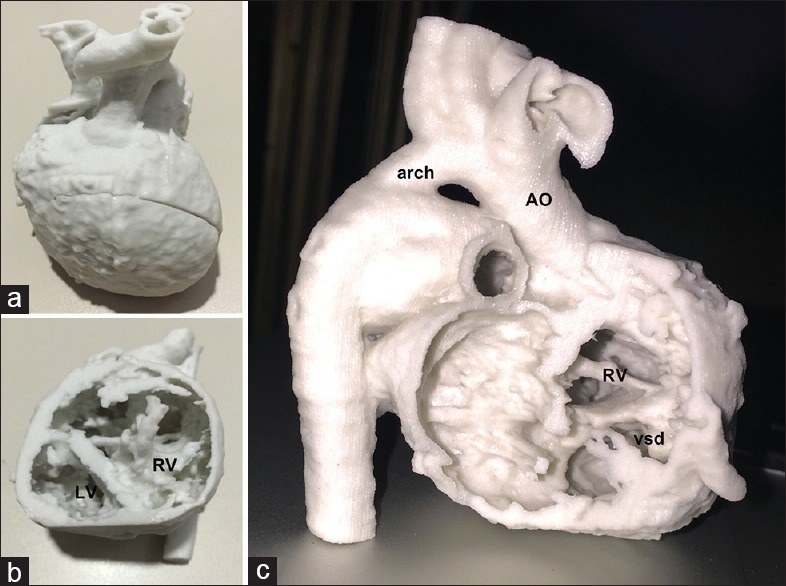
(a-c) The three-dimensional print of patient 3 (criss-cross heart, double-outlet right ventricle), printed using “sandstone” material. (a) The “whole heart” perspective. (b) The view into the heart after removing the apical part of the model. (c) The transatrial perspective. Ao: Aorta, arch: Aortic arch, RV: Right ventricle, vsd: Ventricular septal defect
The model for patient 4 was printed in semiflexible resin with color-coding of chambers for easy understanding of “criss-cross heart” anatomy [Figure 5a]. The model for patient 5 was printed in transparent, hard material allowing easy visualization of the intracardiac anatomy [Figure 5b].
Figure 5.
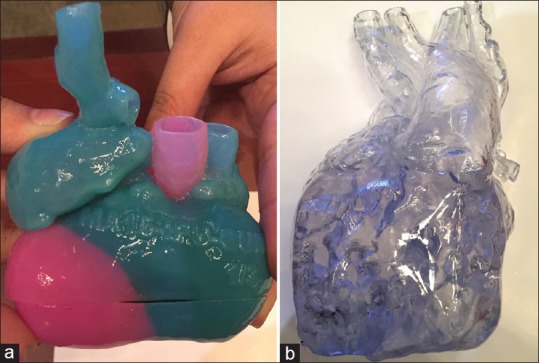
(a) The three-dimensional-printed heart model of patient 4 (criss-cross heart) printed in multiple colors. (b) The heart model of patient 5, printed in transparent, hard material
All 3D prints had excellent correlation with the anatomy on the original DICOM datasets. Measured dimensions of valve annuli, septal defects, and vessels on the physical models were nearly identical to those on respective multiplanar reconstructions of raw data and the virtual 3D models. Spatial anatomy was true to MR/CT angiographic reconstructed anatomy.
Exposure of the internal anatomy by predesigned splits in the models was satisfactory in all cases, allowing optimal visualization of intracardiac anatomy. Specific regions of interest for each case were well depicted, especially the ventricular septum, ventricular outflow tracts, and great artery relationships.
Impact on surgical decision-making
There was universal agreement among team members that the models provided a radical and novel 3D perspective into patient-specific cardiac anatomy. Tactile experience of handling the prototypes and viewing the defects provided valuable information similar to that obtained during direct intraoperative inspection. It allowed greater ease of understanding specific technical challenges and enabled detailed surgical planning for each patient.
Patient 1
The model helped visualize the location, size, and shape of VSD, relationship with outflow tracts, and the specific technical challenges involved in routing the LV to the aorta. It was apparent that intraventricular tunneling would require RV ventriculotomy in addition to atriotomy. Surgeons were able to anticipate and precisely plan the location of the ventriculotomy incision, length/shape, and lie of the VSD patch and site for placement of RV-to-PA conduit. External inspection provided clear understanding of surface anatomy and spatial relationships of the cardiac chambers and great arteries. Previous opinion to defer surgery was overturned in favor of proceeding with biventricular repair (Rastelli operation).
Patient 2
Inspection of the 3D model allowed clearer understanding of the technical challenges and approach to creating the LV-aorta pathway and placing RV-PA conduit. It was clear that the final volume of the morphological RV after intraventricular tunneling and RV-PA conduit placement would be insufficient for biventricular repair; therefore, the team opted for one and a half ventricle approach retaining the Glenn shunt (atrial switch with Rastelli operation).
Patient 3
The model revealed technical improbability of achieving biventricular repair since the aorta was far removed from the VSD, and the location of the tricuspid valve apparatus was completely in the potential LV-aorta pathway. Previous plan of direct surgical inspection of intracardiac anatomy under CPB toward attempting biventricular repair was therefore abandoned in favor of left thoracotomy, aortic arch repair, ligation of patent ductus arteriosus, and PAB.
Patient 4
The model allowed clear understanding of spatial relationships, ventricular septal morphology, and size and location of VSD. Aorta ran an inferior course to connect to the posterior aspect of morphological LV. Brief inspection of the 3D model was sufficient to decide on the relatively straightforward surgical plan for total correction – VSD patch closure through atriotomy.
Patient 5
The transparent model allowed excellent visualization of the complex ventricular septal morphology and enabled surgical planning of the intraventricular tunneling, and patch closure of the mid-muscular defect. Decision was taken for biventricular correction (Rastelli operation with tricuspid valve repair).
Intraoperative anatomy and surgical correlation
Intraoperative cardiac anatomy was found to be identical to the respective 3D models for all cases. All surgeries were accomplished in accordance with the preoperative plans; CPB time was minimized.
Surgical outcomes
All patients had optimal postoperative recovery with uncomplicated course. They continue to be clinically well on 1-year follow-up. Patient 3 underwent second-stage palliation (Glenn shunt) at the age of 6 months.
DISCUSSION
3D printing or rapid prototyping technology has been in existence for over two decades and has been used for engineering and industrial applications. Its use in solving healthcare problems is a relatively recent development. It enables creation of a physical to scale replica of any virtual 3D object through a computer-controlled process. The virtual object (digital image data) may be one created entirely using computer-aided design (CAD) or it may be derived from 3D image scan of an actual object. Thus, 3D reconstructions of biological structures derived from volumetric medical imaging are also amenable to 3D prototyping.[8,9,10] This opens up the exciting possibility of viewing, handling, and interacting with medical imaging data in a completely novel way.
Utility of this concept lies in the fact that anatomical challenges are integral to evaluation and management of a large variety of medical conditions including CHD. Volumetric scans using CT, MRI, and lately ultrasound allow rapid acquisition of 3D image datasets in form of multiple contiguous slices of 2D imaging data that can be aggregated to allow computerized 3D visualizations and multiplanar reconstructions. In specific clinical situations, this greatly enhances anatomical understanding and treatment planning. However, most such 3D visualizations are confined to the 2D screens of imaging workstations, limiting their access and utility. 3D printing technology allows the possibility of converting such imaging datasets into physical models, providing unique, easily accessible, tactile tools to understand anatomy, and plan management.
There are two components to the process of 3D printing. The first is creation of a virtual 3D model using specialized software; the second is the process of actual physical “printing out.” When derived from medical imaging data, software is used to select and segment the region of interest, excluding all “noise” (unnecessary data), followed by creation of a “virtual 3D model.” The virtual model is a computerized rendering of the entire stack of contiguous slices of the imaging data layered one on top of the other to create the “whole” object. This virtual model is then edited, using CAD tools and finally converted into specific digital formats (STL, OBJ, etc.) that can be interpreted and processed by the hardware (3D printer). The printer proceeds to “printout” the object layer by layer, stacking them up, and fusing them together to create the complete physical model.
3D printing found its first and most common application in healthcare in dental, craniomaxillofacial, and orthopedic surgeries, all of which involve imaging and reconstructing dense bony structures.[2,5,11,12,13,14] Cardiovascular applications have been limited and relatively more recent.[15,16,17,18,19,20,21,22,23,24,25] Being dynamically mobile soft-tissue structure, the cardiac anatomy is more difficult to accurately image and reconstruct. Furthermore, unlike bony structures, the heart is a hollow organ; the standard 3D rendered visualizations derived from contrast-enhanced MR or CT angiography are essentially voluminograms of the cardiac cavities filled with contrast. Viewing of intracardiac anatomic details requires further postprocessing to generate a “hollow heart” model. This is done by computationally building a cast around the voluminogram and then subtracting the blood pool contrast. Thus, when printed out, the surface or myocardial thickness is not anatomically true (it is computationally designed), but the internal anatomy is precise.
Precise diagnosis and surgical repair of complex CHD are challenging even in high-resource, high-expertise environments. In limited-resource environments, complex lesions with potential for biventricular corrections are often relegated to single ventricle palliations due to resource limitations and uncertainty regarding technical feasibility of complex corrective surgeries.[26] Now, with increasing expertise, there is a conscious effort to attempt total correction even in technically difficult lesions, but efficient use of resources is important.
In all five cases described here, there were definite anatomical complexities, which would be deemed challenging by most pediatric cardiac experts. Despite multiple collective reviews of all available clinical and imaging data in a high-expertise setting, there were concerns regarding feasibility of safe surgeries – leading to indecision and postponement of intervention. 3D prints had dramatic direct impact on decision-making in all these cases by allowing the team members to interact with patient anatomy in a radically different manner as compared to merely reviewing images on a computer screen. They provided a direct, tactile, and extremely flexible means of viewing and understanding patient anatomy. It became possible to look inside the hollow models, identify intracardiac defects, and precisely determine the surgical challenges. Where hours of cumulated discussion and reviews had failed, minutes with the model were enough to convince the team regarding the surgical options. Meticulous surgical planning was possible in each case, including planning of the surgical approach, site(s) of cardiotomy, steps in intraventrular tunneling, length/shape of VSD patches, and site for placement of conduits. The team was convinced regarding the correlation of the models with the anatomy depicted on MRI/CT scans. Models were reviewed by surgeons again just before going to operating room in preference to reviewing the conventional raw images. There was strong subjective perception among the surgeons that the 3D-printed models aided them in decision-making, perioperative planning, anticipation of anatomical challenges, and safe execution of complex surgeries. Surgical time, CPB times were minimized and postoperative recovery was optimal and uncomplicated. These cases are proof of concept that 3D printing can be a valuable tool for aiding complex congenital heart surgery even in a resource-limited environment. Ability to optimally utilize available resources to ensure safe and optimal surgery while minimizing surgical morbidities can potentially offset the costs involved in volumetric imaging and 3D printing.
Even after having served their purpose of assisting surgery, the prints continue to be used as morphology models to help fellows’ in-training understand these examples of complex CHD. In the absence of easy access to actual anatomic specimens, 3D-printed anatomical specimens can prove to be a valuable tool for teaching, training, and simulation. They can also serve as excellent tools for communication with patients and their families and for preoperative counseling.
The potential for healthcare applications of 3D printing is tremendous and includes direct clinical applications across medical specialties, creation of patient-specific implants, prosthesis and surgical guides, development and testing of medical devices, creation of morphology specimens for teaching and training, development of surgical skill/simulation laboratories, tools for communication and even bioprinting of human tissues and organs. Currently, 3D printing technology is expensive and the process is logistically challenging. Costs depend on the size of model, material used, and the in-built costs of specialized software, printers, and skilled personnel. Engineering expertise for designing and printing mostly rests with specialized 3D printing service providers outside of medical institutions. Turnaround times from imaging to receiving the 3D print can range from a few days to weeks. However, recognizing the tremendous potential for the technology in medicine, selected healthcare institutions (including ours) are beginning to invest in developing the infrastructure and capability in-house. With advancements in technology and in material sciences, it is likely to become less expensive and more accessible in the future. Ability to optimally utilize available resources to ensure safe and optimal surgery while minimizing surgical morbidities can potentially offset the costs involved in volumetric imaging and 3D printing.
While the idea of “printing out” medical scans may seem simplistic and attractive to a physician, it is important to understand that it is a complex process. High-quality imaging is imperative; choice of imaging modality should be based on the specific study questions as well as patient safety. Accurate interpretation of imaging data by a physician with expertise in imaging and familiarity with the patient's clinical context is important for identifying indications and specific study questions for 3D printing. Next, postprocessing of images and extraction of the virtual model, which is often done by members from the technology team, needs to be assisted by strong input from the clinical team to ensure accuracy and eliminate artifacts. Design details such as type of model (solid versus hollow) and designing of splits to enable internal viewing need to be determined based on case-specific requirements. Finally, the finish and quality of the final print will depend on the type of printer and the material used, which again may be dictated by the specific indications for printing. Thus, 3D printing for CHD is not “plug and play.” It requires involvement and integration of skills in imaging, image interpretation, and technology, with close collaboration between cardiologists, radiologists, CAD software experts, and 3D printer hardware specialists.
CONCLUSIONS
3D-printed heart models can radically assist surgical decision-making and surgical planning in selected cases of complex CHD, allowing safe execution of complex repairs.
Limitations
Our experience is among the first instances of the use of 3D printing to aid congenital heart surgery in India. While it highlights the feasibility and utility of the modality, the number of patients and the spectrum of lesions represented in this study are small. Future studies need to be designed to measurably assess the impact of 3D print assisted CHD surgery on CPB time, operative outcomes, and treatment costs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Antoine Hollander from Materialise NV, Leuven, Belgium, for technological collaboration in postprocessing imaging data and creating 3D prints for patients 1, 2, 4, 5. We would also like to thank Firoza Kothari, Anatomiz 3D, Mumbai, India, for technological assistance with the 3D print for patient 3.
REFERENCES
- 1.Kumar RK. Universal heart coverage for children with heart disease in India. Ann Pediatr Cardiol. 2015;8:177–83. doi: 10.4103/0974-2069.164674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU, et al. 3D printing based on imaging data: Review of medical applications. Int J Comput Assist Radiol Surg. 2010;5:335–41. doi: 10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 3.Kim MS, Hansgen AR, Wink O, Quaife RA, Carroll JD. Rapid prototyping: A new tool in understanding and treating structural heart disease. Circulation. 2008;117:2388–94. doi: 10.1161/CIRCULATIONAHA.107.740977. [DOI] [PubMed] [Google Scholar]
- 4.Webb PA. A review of rapid prototyping (RP) techniques in the medical and biomedical sector. J Med Eng Technol. 2000;24:149–53. doi: 10.1080/03091900050163427. [DOI] [PubMed] [Google Scholar]
- 5.Torabi K, Farjood E, Hamedani S. Rapid prototyping technologies and their applications in prosthodontics, a review of literature. J Dent (Shiraz) 2015;16:1–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Malik HH, Darwood AR, Shaunak S, Kulatilake P, El-Hilly AA, Mulki O, et al. Three-dimensional printing in surgery: A review of current surgical applications. J Surg Res. 2015;199:512–22. doi: 10.1016/j.jss.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 7.Martelli N, Serrano C, van den Brink H, Pineau J, Prognon P, Borget I, et al. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery. 2016;159:1485–500. doi: 10.1016/j.surg.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Sodian R, Weber S, Markert M, Rassoulian D, Kaczmarek I, Lueth TC, et al. Stereolithographic models for surgical planning in congenital heart surgery. Ann Thorac Surg. 2007;83:1854–7. doi: 10.1016/j.athoracsur.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Binder TM, Moertl D, Mundigler G, Rehak G, Franke M, Delle-Karth G, et al. Stereolithographic biomodeling to create tangible hard copies of cardiac structures from echocardiographic data: In vitro and in vivo validation. J Am Coll Cardiol. 2000;35:230–7. doi: 10.1016/s0735-1097(99)00498-2. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs S, Grunert R, Mohr FW, Falk V. 3D-imaging of cardiac structures using 3D heart models for planning in heart surgery: A preliminary study. Interact Cardiovasc Thorac Surg. 2008;7:6–9. doi: 10.1510/icvts.2007.156588. [DOI] [PubMed] [Google Scholar]
- 11.Winder J, Bibb R. Medical rapid prototyping technologies: State of the art and current limitations for application in oral and maxillofacial surgery. J Oral Maxillofac Surg. 2005;63:1006–15. doi: 10.1016/j.joms.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Wong TY, Fang JJ, Chung CH, Huang JS, Lee JW. Comparison of 2 methods of making surgical models for correction of facial asymmetry. J Oral Maxillofac Surg. 2005;63:200–8. doi: 10.1016/j.joms.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 13.Fukui N, Ueno T, Fukuda A, Nakamura K. The use of stereolithography for an unusual patellofemoral disorder. Clin Orthop Relat Res. 2003;409:169–74. doi: 10.1097/01.blo.0000057990.41099.0e. [DOI] [PubMed] [Google Scholar]
- 14.Poukens J, Haex J, Riediger D. The use of rapid prototyping in the preoperative planning of distraction osteogenesis of the cranio-maxillofacial skeleton. Comput Aided Surg. 2003;8:146–54. doi: 10.3109/10929080309146049. [DOI] [PubMed] [Google Scholar]
- 15.Cantinotti M, Valverde I, Kutty S. Three-dimensional printed models in congenital heart disease. Int J Cardiovasc Imaging. 2017;33:137–44. doi: 10.1007/s10554-016-0981-2. [DOI] [PubMed] [Google Scholar]
- 16.Hibino N. Three dimensional printing: Applications in surgery for congenital heart disease. World J Pediatr Congenit Heart Surg. 2016;7:351–2. doi: 10.1177/2150135116644886. [DOI] [PubMed] [Google Scholar]
- 17.Garekar S, Bharati A, Chokhandre M, Mali S, Trivedi B, Changela VP, et al. Clinical application and multidisciplinary assessment of three dimensional printing in double outlet right ventricle with remote ventricular septal defect. World J Pediatr Congenit Heart Surg. 2016;7:344–50. doi: 10.1177/2150135116645604. [DOI] [PubMed] [Google Scholar]
- 18.Gosnell J, Pietila T, Samuel BP, Kurup HK, Haw MP, Vettukattil JJ. Integration of computed tomography and three-dimensional echocardiography for hybrid three-dimensional printing in congenital heart disease. J Digit Imaging. 2016;29:665–9. doi: 10.1007/s10278-016-9879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farooqi KM, Gonzalez-Lengua C, Shenoy R, Sanz J, Nguyen K. Use of a three dimensional printed cardiac model to assess suitability for biventricular repair. World J Pediatr Congenit Heart Surg. 2016;7:414–6. doi: 10.1177/2150135115610285. [DOI] [PubMed] [Google Scholar]
- 20.Kurup HK, Samuel BP, Vettukattil JJ. Hybrid 3D printing: A game-changer in personalized cardiac medicine? Expert Rev Cardiovasc Ther. 2015;13:1281–4. doi: 10.1586/14779072.2015.1100076. [DOI] [PubMed] [Google Scholar]
- 21.Farooqi KM, Uppu SC, Nguyen K, Srivastava S, Ko HH, Choueiter N, et al. Application of virtual three-dimensional models for simultaneous visualization of intracardiac anatomic relationships in double outlet right ventricle. Pediatr Cardiol. 2016;37:90–8. doi: 10.1007/s00246-015-1244-z. [DOI] [PubMed] [Google Scholar]
- 22.Biglino G, Capelli C, Wray J, Schievano S, Leaver LK, Khambadkone S, et al. 3D-manufactured patient-specific models of congenital heart defects for communication in clinical practice: Feasibility and acceptability. BMJ Open. 2015;5:e007165. doi: 10.1136/bmjopen-2014-007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivieri LJ, Krieger A, Loke YH, Nath DS, Kim PC, Sable CA. Three-dimensional printing of intracardiac defects from three-dimensional echocardiographic images: Feasibility and relative accuracy. J Am Soc Echocardiogr. 2015;28:392–7. doi: 10.1016/j.echo.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Samuel BP, Pinto C, Pietila T, Vettukattil JJ. Ultrasound-derived three-dimensional printing in congenital heart disease. J Digit Imaging. 2015;28:459–61. doi: 10.1007/s10278-014-9761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valverde I, Gomez G, Gonzalez A, Suarez-Mejias C, Adsuar A, Coserria JF, et al. Three-dimensional patient-specific cardiac model for surgical planning in Nikaidoh procedure. Cardiol Young. 2015;25:698–704. doi: 10.1017/S1047951114000742. [DOI] [PubMed] [Google Scholar]
- 26.Kottayil BP, Sunil GS, Kappanayil M, Mohanty SH, Francis E, Vaidyanathan B, et al. Two-ventricle repair for complex congenital heart defects palliated towards single-ventricle repair. Interact Cardiovasc Thorac Surg. 2014;18:266–71. doi: 10.1093/icvts/ivt495. [DOI] [PMC free article] [PubMed] [Google Scholar]


