Abstract
Background:
Anomalous origin of left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital malformation. We sought to evaluate in-hospital and mid-term outcomes of patients with a diagnosis of ALCAPA who underwent surgical repair.
Objectives:
The objective of this study is to evaluate the mid-term outcomes of surgical repair of ALCAPA at our center and to analyze the surgical techniques used.
Materials and Methods:
In a retrospective study, we analyzed early and mid-term clinical and echocardiographic data to determine the outcomes of patients who underwent surgical repair of ALCAPA in our institution between 2005 and 2015.
Results:
Twenty-one patients underwent surgical repair for ALCAPA using aortic reimplantation (n = 10, 47.6%), ostial closure (n = 8, 38.1%), or ligation (n = 3, 14.3%). The median age of patients was 24 months (range 22 days to 51 years). There were 2 (9.5%) in-hospital mortalities in infants undergoing the reimplantation technique. All patients were followed up for a median of 21 months (range 1–60 months). No patients required reoperation, and there was no mortality from discharge to mid-term follow-up. Severe early postoperative mitral regurgitation (MR) was associated with composite end-point, defined as a combination of mortality after surgery, moderate to severe MR, and moderate to severe left ventricular dysfunction at late follow-up (P = 0.019) while mitral valve repair was not (P = 0.469).
Conclusion:
The surgical management of ALCAPA can be associated with good in-hospital and mid-term outcomes regardless of the age, at which the patient has been operated.
Keywords: Anomalous origin of left coronary artery from the pulmonary artery, ligation, mitral regurgitation, mitral valve repair, ostial closure, reimplantation
INTRODUCTION
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital cardiac malformation. It can lead to ischemia induced left ventricular (LV) dysfunction and mitral regurgitation (MR).[1] The clinical spectrum of ALCAPA was described by Bland, White, and Garland in 1933, and the condition is sometimes referred to as “the Bland-White-Garland syndrome.”[2]
If left unrepaired, this anomaly can lead to mortality during infancy in approximately 90% of patients.[1,3,4] Myocardial ischemia in the first few months of life leads to typical presentation in infancy as the infant suffers from failure to thrive, profuse sweating, dyspnea, pallor, and poor feeding; however, these symptoms are rarely evident before 2 months of age. As the pulmonary vascular resistance decreases, the runoff into the pulmonary artery from the left coronary artery increases, and myocardial dysfunction becomes more apparent.[1,3] The patient may present later in life (adult presentation), and rarely malignant arrhythmia leading to sudden death may be the presenting symptom of this anomaly at an older age.[3]
Surgical correction on diagnosis is the gold standard of treatment.[5,6,7] Several surgical techniques have been described for the repair of ALCAPA.[1,8,9,10] In 1960, Sabiston et al. described the ligation of the left coronary artery at its origin from the pulmonary artery.[11] Cooley reported the first creation of a dual coronary system by performing the first ligation with saphenous vein graft (SVG) to the ALCAPA.[8] Later in 1974, Neches et al. reported the reimplantation of the left main coronary artery on to the aorta.[12] Although surgical repair results in excellent outcomes and gradual improvement in LV function, a great deal of debate still remains regarding the ideal initial management of MR. We report the mid-term outcomes of 21 consecutive ALCAPA patients who underwent surgical repair at our center during a 10-year period.
MATERIALS AND METHODS
Study protocol and population
In a retrospective study, we aimed to analyze the surgical techniques used for the repair of ALCAPA at our center, Rajaie Cardiovascular Medical and Research Center, and to evaluate the mid-term outcomes, in particular, LV function and MR. This study protocol was approved by the Local Ethics Committee in our institution.
From 2005 to 2015, 21 patients had undergone surgical repair for ALCAPA with different surgical techniques at our institution. Baseline demographics and surgical data were collected from their charts along with early and mid-term clinical and echocardiographic follow-up data.
Diagnostic evaluation
All patients underwent chest X-ray and transthoracic echocardiography (TTE) preoperatively. In addition, computed tomographic angiography and cardiac catheterization were also performed in most patients to confirm the diagnosis. LV dysfunction was classified as none (LV ejection fraction (LVEF) ≥60%); mild (LVEF 50%–59%); moderate (LVEF 35%–49%); and severe (LVEF <35%). MR was graded as none/trivial (0), mild (1), moderate (2), or severe (3).
Surgery time and techniques
Surgical repairs were implemented as soon as the diagnosis was made. We used three main surgical techniques to repair ALCAPA, including reimplantation, ostial closure, and ligation. There was no preference to choose the surgery type in an individual, except for three patients who underwent ligation technique without arresting the heart since they had severe myocardial dysfunction. Moreover, mitral valve (MV) repair was performed in four infants/children and in all three adult patients.
At aortic reimplantation used in ten patients (47.6%), the left coronary artery was mobilized, and a large coronary ostial button was harvested from the pulmonary artery, which was then anastomosed to an opening in the left posterolateral aspect of the aorta. Furthermore, MV repair was concomitantly performed by posterior annuloplasty in some patients.
The ostial closure with or without concomitant coronary artery bypass graft (CABG) surgery was performed in 8 patients (38.1%) during which, direct closure of orifice of the anomalous coronary artery from the pulmonary artery was performed. In four of these eight patients, the orifice of the anomalous coronary artery was closed by direct suturing from inside the pulmonary artery with or without cardiac arrest. MV repair by ring annuloplasty was concomitantly performed in one of them. In the other four patients, ostial closure with concomitant CABG surgery was performed along with MV repair using ring annuloplasty in all of them.
The third technique included ligation of the anomalous left coronary artery after its origin from the pulmonary artery in three patients. In these patients, the anomalous connection of the left coronary artery was dissected and ligated close to the pulmonary trunk wall with a single suture and metal clip. This was done without cardiopulmonary bypass (CPB) in two patients and with CPB and mild hypothermia (34°C–32°C) in another patient, but without aortic cross-clamp.
Statistical analysis
Continuous variables were reported as median (interquartile range) analyzed using Kruskal–Wallis test. Categorical variables were reported as number (percentage), and Chi-square test or Fisher exact tests were used. Two-sided P values were calculated. All statistical analyses were performed by the SPSS software, version 21.0 (IBM Inc., Somers, NY, USA).
Follow-up
Both in-hospital and mid-term follow-up data were collected. All patients were evaluated after surgery by physical examination and TTE, and then they were followed up in clinic at regular intervals.
RESULTS
Baseline characteristics
Between 2005 and 2015, 21 consecutive patients underwent the surgical repair of ALCAPA with or without concomitant MV repair. The median age of patients at surgery was 24 months (range 22 days to 51 years). There were 12 males (57%) and nine females (43%).
The presenting symptoms in infants were poor feeding, pallor, and crying indicating congestive heart failure (n = 9); however, a 22-day-old patient presented with cardiogenic shock due to massive MI. Among children, symptoms at presentation were dyspnea (n = 4), typical chest pain (n = 1), and palpitation (n = 1); however, two patients were asymptomatic diagnosed incidentally during routine echocardiographic examination. In adult patients, the presentation included one patient who survived a cardiac arrest, one patient with dyspnea who had history of surgery in childhood for ASD closure (i.e., the diagnosis of ALCAPA had been missed), and one patient who presented with typical chest pain.
The preoperative electrocardiogram (ECG) in infants showed signs of the myocardial ischemia in nine patients (Q waves in leads I and aVL in three patients; ST-T segment changes in the lateral anterior leads V4–V6 and high lateral leads I and aVL in six patients) and was normal in one patient. Among children, there were signs of myocardial ischemia in seven patients (Q waves in leads I and aVL in four patients; ST-T segment changes in the lateral anterior leads V4–V6 and high lateral leads I and aVL in three patients), and only one child had a normal ECG. Among adults, one patient had ST-T segment changes in leads I and aVL, and two patients had normal ECG.
Cardiomegaly (cardiothoracic ratio > 0.55) was detected in 18 patients (85%). The diagnosis of ALCAPA was made by a combination of imaging modalities as follows: Computed tomography (CT) angiography with cardiac catheterization and TTE (n = 3); CT angiography with TTE (n = 8); cardiac catheterization with TTE (n = 8); and just by TTE (n = 2).
The anomalous left coronary artery originated from the posterior facing sinus of the pulmonary trunk in the majority of patients (n = 18), taking the normal course, and distribution of the left main coronary artery while the origin was from the anterior nonfacing sinus in two children, and coursing to the left around the pulmonary artery until the atrioventricular groove. In one patient, the anomalous left coronary artery arose from the right pulmonary artery and then took an intramural course in the posterior wall of the main pulmonary artery (n = 1; infant). Other baseline characteristics are summarized in Table 1.
Table 1.
Demographics, intraoperative characteristics, and outcomes according to age groups
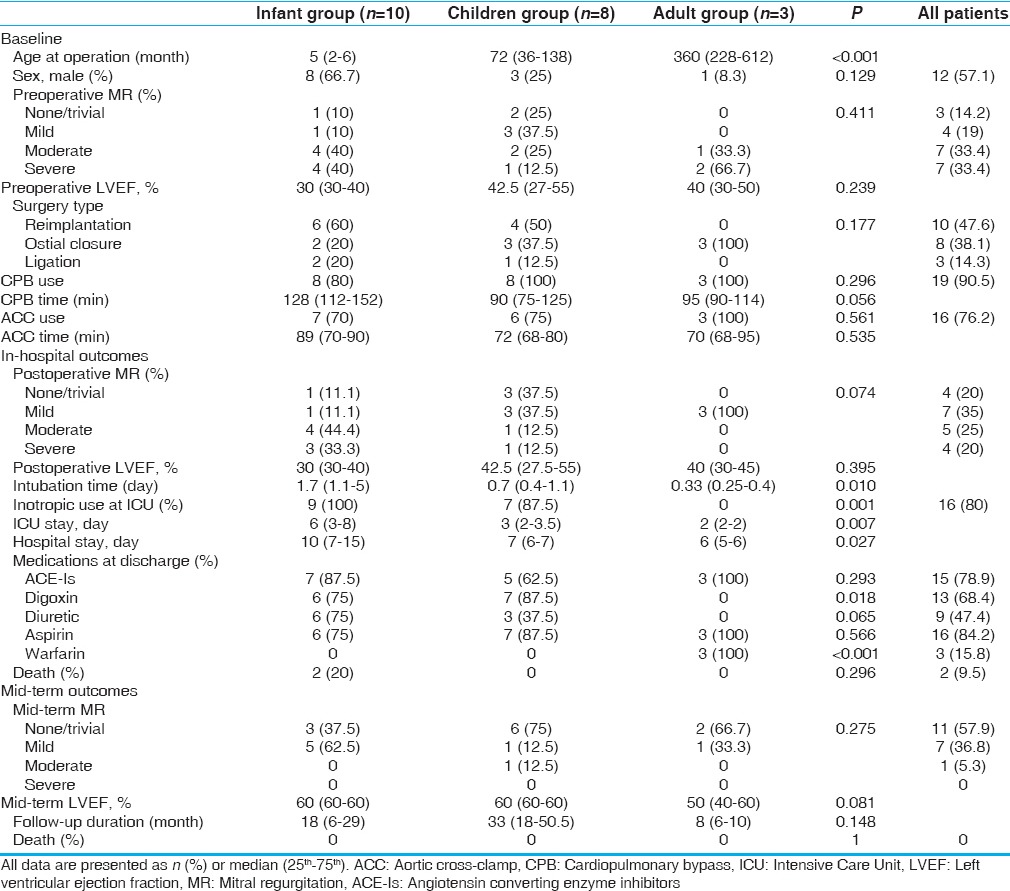
In-hospital outcomes
Five patients (four infants and one child) underwent delayed sternal closure (24–96 h) due to either borderline hemodynamics or generalized oozing from the mediastinum. Another infant with preoperative, LVEF of 25% was unable to wean from CPB and required LV assist device for a duration of 3 days; consequently, he was discharged in good condition. One patient had mediastinal bleeding postoperatively and required re-exploration.
There were two in-hospital mortalities (9.5%). The first was a 22-day-old patient who presented with extensive myocardial infarction and severe MR who underwent coronary reimplantation. The patient was not able to wean from CPB and died of acute heart failure. The second was a 2-month-old with severe LV dysfunction who also underwent coronary reimplantation. He was weaned from CPB with high-dose inotropes and ultimately died due to multiorgan failure after prolonged ventilation.
The median intubation times in infants, children, and adults were 1.7, 0.7, and 0.33 days, respectively (P = 0.01). Moreover, hospital and intensive care unit stays were higher in infants compared to children and adults [Table 1]. A 6-month-old infant who had severe MR preoperatively and was treated using reimplantation without MV repair needed reoperation for MV repair because of failure to wean from the ventilator after 16 days. Following MV repair, the patient was extubated after 2 days and discharged in stable condition.
Mid-term outcomes
Patients were followed up for a median of 21 months (range 1–60 months). There was no mortality or re-operation during the follow-up period. The majority of patients had none/trivial MR (57.9%) at late follow-up, and no patients had severe MR. There was no difference in the severity of MR among the age groups [Table 1].
The status of mitral regurgitation and left ventricular function
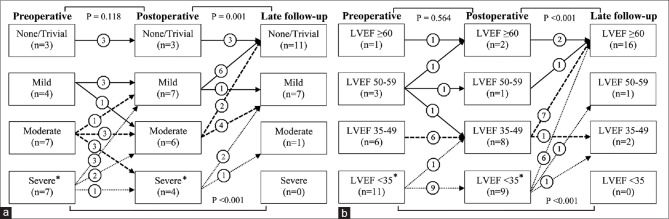
There were no significant differences between preoperative and early postoperative severity of MR and LV function (P = 0.118 and P = 0.564, respectively). However, the rate of none/trivial MR was significantly higher at mid-term follow-up compared with preoperative and early postoperative findings (P < 0.001 and P = 0.001, respectively). Moreover, the majority of patients (16 patients, 84.2%) at mid-term follow-up had LVEF of ≥60% [Figure 1].
Figure 1.
Fate of mitral valve regurgitation (a) and left ventricular function (b) during follow-up period. *Two cases of intra- and post-operative mortality
Subgroup analysis by the mitral valve repair
Of patients undergoing MV repair, five patients had severe MR, and two had moderate MR preoperatively. The severity of MR at mid-term follow-up compared to preoperative status was not significantly different between patients with or without MV repair (P = 0.324). Other characteristics were comparable between patients with or without concomitant MV repair. We also excluded MV repair for adults and performed another analysis including infants and children, in which there was no significant difference regarding preoperative and late outcomes for MR severity between patients with or without concomitant MV repair (P = 0.196).
Predictors of end-point
We defined a composite end-point as a combination of mortality after surgery plus moderate to severe MR and moderate to severe LV dysfunction at mid-term follow-up. On univariate analysis, severe early postoperative MR was the only variable associated with composite end-point (P = 0.019). Moreover, patients with preoperative moderate to severe MR were shown to have a trend toward composite end-point compared to those without (P = 0.07). We did not find any relationship between preoperative LVEF and composite end-point (P = 0.349).
DISCUSSION
In this cohort of ALCAPA patients, based on a single-institution report, we found two in-hospital mortalities (9.5% of patients), and both were infants who underwent reimplantation of the anomalous ALCAPA. Most patients had higher rates of none/trivial MR or LVEF ≥60% at mid-term follow-up compared to preoperative or early postoperative assessments. There were no significant differences regarding preoperative and late MR severity between patients with or without concomitant MV repair. In addition, severe early postoperative MR was associated with composite end-point as a combination of mortality after surgery plus moderate to severe MR and moderate to severe LV dysfunction at mid-term follow-up, while MV repair was not.
There is no consensus on the best surgical technique for the treatment of ALCAPA; however, according to the preceding studies, the creation of dual coronary system with direct aortic reimplantation has been considered for the management of ALCAPA anomaly.[1,13,14] Two cases of in-hospital mortalities developed in whom reimplantation surgical technique was implemented. We think that the ligation technique could lead to better outcomes compared with reimplantation technique in patients presenting with cardiogenic shock in the background of ischemic heart. It may be explained by this issue that the ligation technique obviates the need to arrest the heart in contrast by reimplantation technique. All patients who have a single coronary supply need special follow-up as they may be at risk of developing ventricular dysfunction or arrhythmias and are especially prone to sudden death. Furthermore, it has been recommended by some studies that these patients may benefit from an elective establishment of a dual coronary system.[15,16,17] Further large-scale studies are required to examine this issue. In addition, in one patient, the left anomalous left coronary artery was arising from the right pulmonary artery at its origin from the main pulmonary artery, then it coursed posteriorly and had intramural course, and this anatomy was not suitable for reimplantation technique, therefore, ostial closure technique was used. It has been reported by preceding studies that venous grafts for ALCAPA are at greater risk of developing late obliterative changes compared to arterial ones,[18] therefore, we recommend that for the patients who received SVG, a strict follow-up is needed as the longevity of these grafts is not as good as the left internal mammary artery grafts or the native reimplanted coronary artery.
We found a significant improvement in the postoperative LV function at late echocardiographic examination compared to the preoperative and early postoperative examinations. Kudumula et al. in a retrospective cohort of infants and children with ALCAPA have found 88% of patients with normal LV function at late follow-up.[19] In addition, other reports have demonstrated significant improvement in LV function at long-term follow-up.[1,13,14,20,21,22] The ALCAPA syndrome is characterized by chronic low perfusion of the myocardium and potential revivability in the territory of anomalous artery. These are the basis for functional recovery and good clinical outcomes after revascularization in the ALCAPA syndrome. Based on this hypothesis, it has been postulated that the LV recovery is attributable to the recovery of hibernating myocardium following the revascularization of anomalous artery territory.[23]
One of the main factors influencing the outcomes of ALCAPA repair is the severity of MR. The majority of MR is functional with the potential for improvement after ALCAPA repair.[14,19,24] We found that moderate to severe preoperative MR had worse outcomes, although it was not statistically significant. In addition, our results have shown significant improvement in the severity of MR, whether concomitant MV repair was performed or not. Among infants and children, most centers prefer not to repair MV at the initial management of ALCAPA anomaly, but there is controversy with regard to the management of MR.[1] We did not find a significant difference in the late MR severity in infants and children between patients who underwent MV repair and those who did not. Therefore, we suggest that preoperative MR in these patients should be corrected when they cannot be weaned from pump or postoperative ventilator support. In adult patients, however, structural MV abnormalities are more probable to develop due to chronic MR, and MV repair is recommended at the initial operation as the LV function recovery alone may not correct the preoperative MR.
Limitations
We should keep in mind some limitations when considering our results. First, this study was retrospective in nature with a small sample size of at a single institution. Second, we used three different surgical approaches in our institution. Although there was no preference to choose the surgical technique, we are unable to definitely conclude which approach was superior to the others because of the small sample size. Third, although there is no consensus regarding MV repair in patients with ALCAPA, we had no predefined indications for MV repair; therefore, our results cannot be generalized, and further studies are required to examine whether MV repair can improve outcomes.
CONCLUSIONS
In conclusion, surgical management of ALCAPA has good mid-term outcomes regardless of the patient age at repair. Preoperative moderate to severe MR and severe early postoperative MR were associated with worse outcomes, but MV repair was not. On the other hand, MV repair at the initial management of ALCAPA, in infants and children, showed acceptable mid-term outcomes, although there are no clear recommendations for identifying patients who would benefit from such an intervention.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mongé MC, Eltayeb O, Costello JM, Sarwark AE, Carr MR, Backer CL. Aortic implantation of anomalous origin of the left coronary artery from the pulmonary artery: Long-term outcomes. Ann Thorac Surg. 2015;100:154–60. doi: 10.1016/j.athoracsur.2015.02.096. [DOI] [PubMed] [Google Scholar]
- 2.Lange R, Vogt M, Hörer J, Cleuziou J, Menzel A, Holper K, et al. Long-term results of repair of anomalous origin of the left coronary artery from the pulmonary artery. Ann Thorac Surg. 2007;83:1463–71. doi: 10.1016/j.athoracsur.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Dodge-Khatami A, Mavroudis C, Backer CL. Anomalous origin of the left coronary artery from the pulmonary artery: Collective review of surgical therapy. Ann Thorac Surg. 2002;74:946–55. doi: 10.1016/s0003-4975(02)03633-0. [DOI] [PubMed] [Google Scholar]
- 4.Wesselhoeft H, Fawcett JS, Johnson AL. Anomalous origin of the left coronary artery from the pulmonary trunk. Its clinical spectrum, pathology, and pathophysiology, based on a review of 140 cases with seven further cases. Circulation. 1968;38:403–25. doi: 10.1161/01.cir.38.2.403. [DOI] [PubMed] [Google Scholar]
- 5.del Nido PJ, Duncan BW, Mayer JE, Jr, Wessel DL, LaPierre RA, Jonas RA. Left ventricular assist device improves survival in children with left ventricular dysfunction after repair of anomalous origin of the left coronary artery from the pulmonary artery. Ann Thorac Surg. 1999;67:169–72. doi: 10.1016/s0003-4975(98)01309-5. [DOI] [PubMed] [Google Scholar]
- 6.Laks H, Ardehali A, Grant PW, Allada V. Aortic implantation of anomalous left coronary artery. An improved surgical approach. J Thorac Cardiovasc Surg. 1995;109:519–23. doi: 10.1016/S0022-5223(95)70283-0. [DOI] [PubMed] [Google Scholar]
- 7.Muzaffar T, Ahmad Ganie F, Gpoal Swamy S, Wani NU. The surgical outcome of anomalous origin of the left coronary artery from the pulmonary artery. Int Cardiovasc Res J. 2014;8:57–60. [PMC free article] [PubMed] [Google Scholar]
- 8.Cooley DA, Hallman GL, Bloodwell RD. Definitive surgical treatment of anomalous origin of left coronary artery from pulmonary artery: Indications and results. J Thorac Cardiovasc Surg. 1966;52:798–808. [PubMed] [Google Scholar]
- 9.Meyer BW, Stefanik G, Stiles QR, Lindesmith GG, Jones JC. A method of definitive surgical treatment of anomalous origin of left coronary artery. A case report. J Thorac Cardiovasc Surg. 1968;56:104–7. [PubMed] [Google Scholar]
- 10.Takeuchi S, Imamura H, Katsumoto K, Hayashi I, Katohgi T, Yozu R, et al. New surgical method for repair of anomalous left coronary artery from pulmonary artery. J Thorac Cardiovasc Surg. 1979;78:7–11. [PubMed] [Google Scholar]
- 11.Sabiston DC, Jr, Neill CA, Taussig HB. The direction of blood flow in anomalous left coronary artery arising from the pulmonary artery. Circulation. 1960;22:591–7. doi: 10.1161/01.cir.22.4.591. [DOI] [PubMed] [Google Scholar]
- 12.Neches WH, Mathews RA, Park SC, Lenox CC, Zuberbuhler JR, Siewers RD, et al. Anomalous origin of the left coronary artery from the pulmonary artery. A new method of surgical repair. Circulation. 1974;50:582–7. doi: 10.1161/01.cir.50.3.582. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz ML, Jonas RA, Colan SD. Anomalous origin of left coronary artery from pulmonary artery: Recovery of left ventricular function after dual coronary repair. J Am Coll Cardiol. 1997;30:547–53. doi: 10.1016/s0735-1097(97)00175-7. [DOI] [PubMed] [Google Scholar]
- 14.Yoon YS, Park JJ, Yun TJ, Kim YH, Ko JK, Park IS, et al. Early result of surgical management of the anomalous origin of the left coronary artery from the pulmonary artery. Korean J Thorac Cardiovasc Surg. 2006;39:18–27. [Google Scholar]
- 15.Backer CL, Stout MJ, Zales VR, Muster AJ, Weigel TJ, Idriss FS, et al. Anomalous origin of the left coronary artery. A twenty-year review of surgical management. J Thorac Cardiovasc Surg. 1992;103:1049–57. [PubMed] [Google Scholar]
- 16.Kececioglu D, Voth E, Morguet A, Munz DL, Vogt J. Myocardial ischemia and left-ventricular function after ligation of left coronary artery (Bland-White-Garland syndrome): A long-term follow-up. Thorac Cardiovasc Surg. 1992;40:283–7. doi: 10.1055/s-2007-1020165. [DOI] [PubMed] [Google Scholar]
- 17.Alexi-Meskishvili V, Berger F, Weng Y, Lange PE, Hetzer R. Anomalous origin of the left coronary artery from the pulmonary artery in adults. J Card Surg. 1995;10(4 Pt 1):309–15. doi: 10.1111/j.1540-8191.1995.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 18.el-Said GM, Ruzyllo W, Williams RL, Mullins CE, Hallman GL, Cooley DA, et al. Early and late result of saphenous vein graft for anomalous origin of left coronary artery from pulmonary artery. Circulation. 1973;48(1 Suppl):III2–6. doi: 10.1161/01.cir.48.1s3.iii-2. [DOI] [PubMed] [Google Scholar]
- 19.Kudumula V, Mehta C, Stumper O, Desai T, Chikermane A, Miller P, et al. Twenty-year outcome of anomalous origin of left coronary artery from pulmonary artery: Management of mitral regurgitation. Ann Thorac Surg. 2014;97:938–44. doi: 10.1016/j.athoracsur.2013.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Alexi-Meskishvili V, Nasseri BA, Nordmeyer S, Schmitt B, Weng YG, Böttcher W, et al. Repair of anomalous origin of the left coronary artery from the pulmonary artery in infants and children. J Thorac Cardiovasc Surg. 2011;142:868–74. doi: 10.1016/j.jtcvs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Ben Ali W, Metton O, Roubertie F, Pouard P, Sidi D, Raisky O, et al. Anomalous origin of the left coronary artery from the pulmonary artery: Late results with special attention to the mitral valve. Eur J Cardiothorac Surg. 2009;36:244–8. doi: 10.1016/j.ejcts.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Cochrane AD, Coleman DM, Davis AM, Brizard CP, Wolfe R, Karl TR. Excellent long-term functional outcome after an operation for anomalous left coronary artery from the pulmonary artery. J Thorac Cardiovasc Surg. 1999;117:332–42. doi: 10.1016/s0022-5223(99)70431-9. [DOI] [PubMed] [Google Scholar]
- 23.Shivalkar B, Borgers M, Daenen W, Gewillig M, Flameng W. ALCAPA syndrome: An example of chronic myocardial hypoperfusion? J Am Coll Cardiol. 1994;23:772–8. doi: 10.1016/0735-1097(94)90767-6. [DOI] [PubMed] [Google Scholar]
- 24.Brown JW, Ruzmetov M, Parent JJ, Rodefeld MD, Turrentine MW. Does the degree of preoperative mitral regurgitation predict survival or the need for mitral valve repair or replacement in patients with anomalous origin of the left coronary artery from the pulmonary artery? J Thorac Cardiovasc Surg. 2008;136:743–8. doi: 10.1016/j.jtcvs.2007.12.065. [DOI] [PubMed] [Google Scholar]