Abstract
The most complex combinations of congenital cardiac malformations are found in the setting of bodily isomerism. The question remains, however, as to whether evidence of cardiac isomerism is always to be found in the setting of bodily isomerism, also known as “heterotaxy.” We have previously shown that, when assessed on the basis of the extent of the pectinate muscles relative to the atrioventricular junctions, there is always isomerism of the atrial appendages in this setting. Doubt has been remained, however, as to whether these cardiac features can accurately be recognized during life. We have now encountered two patients showing features of the left and right bodily isomerism. Examinations of these patients made using computed tomography show that all features of isomerism, no matter how complex, can now be visualized during life. The images currently presented show, furthermore, that the features of the so-called “heterotaxy” can be seen during life, not only within the heart but also in all the thoracic and abdominal organs, albeit that the isomeric features are confined to the thoracic organs. Based on the images presented, we argue that if each system of organs is analyzed and described in independent fashion; then it is possible for clinicians to exclude any suggestion of ambiguity and to provide accurate descriptions of the overall arrangement. We further discuss the appropriate terminology to describe the entity we prefer to call isomerism, along with the indications and usefulness of computed tomography in revealing the anatomic features of the congenitally malformed heart.
Keywords: Computed tomography, heterotaxy, isomerism, situs ambiguus, splenic syndrome, virtual dissection
INTRODUCTION
It is well recognized that the most complex combinations of congenital cardiac malformations are found in the setting of the so-called “visceral heterotaxy.”[1] It is now equally well recognized that the introduction of the segmental approach to diagnosis[2] revolutionized the analysis of hearts with complex congenital cardiac malformations. It is perhaps surprising, therefore, that controversy should continue regarding the optimal approach to segmental diagnosis in the setting of the so-called “heterotaxy.”[3,4] The disagreements focus on whether or not it is possible to recognize isomeric features within the malformed hearts. The presence of pulmonary and bronchial isomerism is now well accepted.[5,6] Furthermore, Van Mierop and Wiglesworth had long since pointed to the presence of isomerism of the sinus nodes in patients lacking a spleen.[7] Van Mierop et al., however, subsequently opted for description of the subsets of patients with heterotaxy in terms of asplenia and polysplenia.[8] This is now known to be less than satisfactory, since a proportion of the patients with isomeric right bronchi, in whom absence of the spleen is anticipated, are known to possess either solitary or multiple spleens.[9] This leads to the somewhat nonsensical description of “asplenia with a solitary spleen.”[10] Similar discrepancies are known to exist in patients said to have polysplenia since not all have multiple spleens.[9] For cardiologists, however, the heart is the primary focus during the diagnosis of those patients known to have the so-called “heterotaxy.” The question remains, therefore, as to whether evidence of cardiac isomerism is always to be found in this setting, and if so, where it is to be found. Distinguished authorities continue to deny the presence of such isomerism, and argue in favor of description on the basis of uncertainty, describing the arrangements in terms of “situs ambiguus.”[3] Those who do not share this opinion argue that, if isomeric features exist within the heart, then it is preferable to describe what is present, rather than opting for ambiguity.[4] The latter argument is based on morphological studies that have shown the uniform presence of isomeric atrial appendage in the hearts from patients known to have heterotaxy when assessed on the basis of the extent of the pectinate muscles relative to the atrioventricular junctions.[9,10,11] Doubt remains, nonetheless, as to whether these features can accurately be recognized during life. We have now encountered two patients in whom examinations using computed tomography show clearly that it is possible to recognize, when present, the isomeric features during life, not only within the heart, but also in all the thoracic organs. The findings also reveal the precise arrangement of the abdominal organs although these are not isomeric. Our current experience now shows that if each system of organs is analyzed and described in independent fashion, then it is possible for clinicians to exclude any suggestion of ambiguity and to provide accurate descriptions of the overall arrangement of the bodily organs.
CASE 1
Brief case description
A 63-year-old woman without any special history was admitted to our hospital because of atrial flutter, with a 2:1 ventricular response. After the spontaneous termination of the atrial flutter 2 days after the admission, her electrocardiogram showed the absence of P-waves, with escape junctional rhythm, and symptomatic sinus pauses of >5 s. We made the diagnosis of sick sinus syndrome, and implanted a pacemaker. We also initiated an electrophysiological study, hoping to achieve catheter ablation of the atrial flutter; however, the investigation was discontinued when we noted interruption of the inferior caval vein.
Cardiac computed tomography
She subsequently underwent electrocardiographically gated coronary arterial computed tomographic angiography to evaluate her cardiac anatomy. The images were acquired using commercially available dual-source computed tomographic scanners (SOMATOM Definition Flash, Siemens Healthcare, Forchheim, Germany), employing a standard protocol of retrospective scanning, combined with a dose modulation protocol, with a contrast material of 60 mL. Images were acquired during a deep inspiratory breath-hold using the parameters of tube voltage of 100 kV, a gantry rotation of 280 ms, and a temporal resolution of 77 ms. Images were taken at mid-diastole, specifically 80% of the R-R interval. The axial image data were reconstructed using parameters of a section thickness of 0.6 mm, an interval of 0.3 mm, a field of view of 24 cm, and a matrix of 512 × 512. The effective radiation dose was 8.8 mSv. Subsequent analyses were performed using a commercially available workstation (Ziostation version 2.1.7.1, Ziosoft Inc., Tokyo, Japan).
Anatomic findings
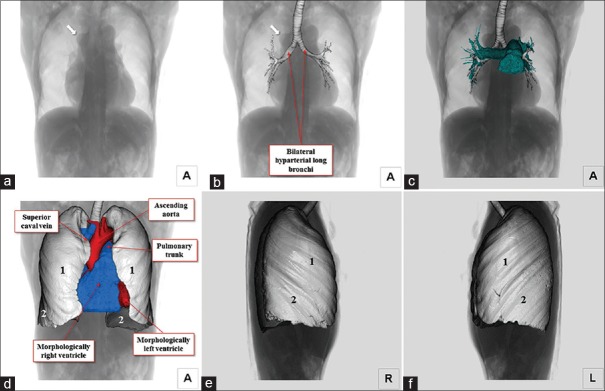
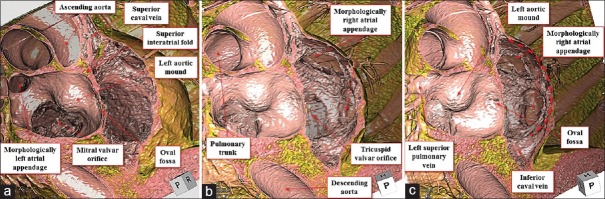
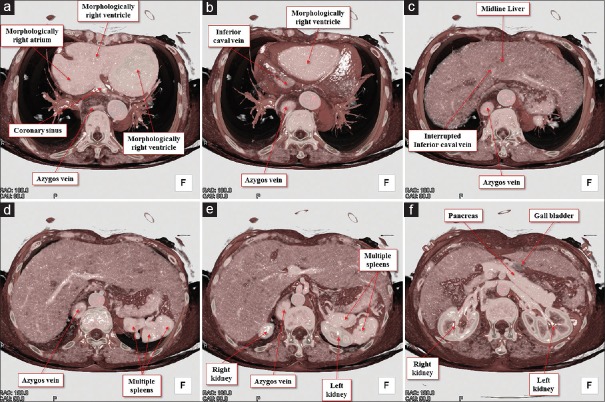
The heart was located centrally within the thorax, with its major axis directed leftward [Figure 1]. In Figures 2 and 3, we show conventional two-dimensional multiplanar reconstruction images. Assessment of the bronchial tree revealed a bilateral morphologically left arrangement [Figures 1 and 4], as evidenced by the bilateral long and hyparterial long bronchi, with a relatively sharp bronchial angulation.[12] The isomeric arrangement was further confirmed by the presence of bilaterally bilobed lungs [Figure 1]. The superior caval vein was right-sided as usual [Figures 1 and 4], however, the inferior caval vein was interrupted, with continuation through the azygos venous system to the right-sided superior caval vein [Figures 2–4]. The azygos vein itself was right-sided relative to the descending aorta [Figures 2 and 4]. The coronary sinus was identified in the left-sided atrioventricular groove and was draining into the morphologically right atrium [Figure 2]. We identified four pulmonary draining into the roof of the morphologically left atrium [Figure 4]. Despite the isomeric arrangement of the bronchi and lungs, there was usual arrangement of the atrial appendages, confirmed by noting the extent of the pectinate muscles relative to the atrioventricular junctions[9] [Figures 2, 4 and 5]. The atrioventricular connections were concordant, with separate atrioventricular junctions guarded by tricuspid and mitral valves, respectively [Figure 2]. There was right-handed ventricular topology, with the morphologically right ventricle located rightward and anterior relative to the morphologically left ventricle [Figures 2 and 4]. The ventriculoarterial connections were also concordant, with the aortic root located rightward and infero-posterior relative to the pulmonary root, the arterial trunks spiraling as they extended into the mediastinum [Figures 2 and 4]. The aortic arch was left-sided [Figures 2 and 4]. Within the abdomen, the liver occupied a midline position while multiple spleens were identified on the left side [Figure 6], along with the gall bladder and pancreas. We also observed anomalous arrangements of the abdominal arteries, with the common hepatic artery branching from the superior mesenteric artery, and extending around the head of the pancreas.
Figure 1.
Volume-rendered images viewed from the anterior direction. Fluoroscopy-like image (a) is combined with the trachea and bronchi (b). Bilateral diaphragms are located at the same height. Bilateral long bronchi with relatively sharp bronchial bifurcation angle are observed. Note the prominent azygos arch (arrows). Bilateral pulmonary arteries override the bilateral hyparterial long bronchi (c). Fluoroscopy-like image (a) is merged with the heart and bilateral bilobed lungs (d). Pulmonary morphology of the right (e) and left (f) lung show bilateral bilobed lungs
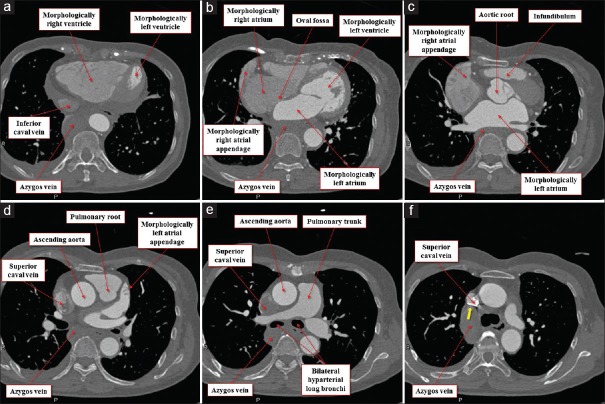
Figure 2.
Multiplanar reconstruction images viewed from the inferior direction at the level of the inferior caval vein (a), oval fossa (b), aortic root (c), left atrial appendage (d), pulmonary bifurcation (e), and azygos continuation (yellow arrow) to the superior caval vein (f). Note the wide extent of the pectinate muscles in the morphologically right appendage (b and c), whereas the pectinate muscle is confined within the left atrial appendage (d)
Figure 3.
Multiplanar reconstruction images viewed from the apical (left anterior oblique) direction at the level of the papillary muscle (a), tendinous chord (b), atrioventricular junction (c), coronary sinus (d), oval fossa (e), and azygos continuation (yellow arrows) to the superior caval vein (f). Note the wide extent of the pectinate muscles in the morphologically right appendage (d-f), whereas the pectinate muscle is confined within the left atrial appendage (b)
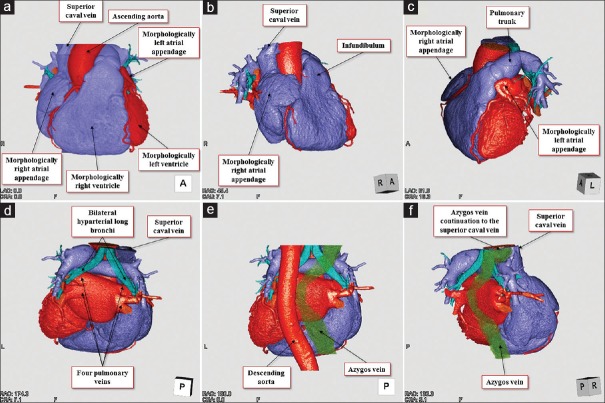
Figure 4.
Volume-rendered dye-cast images of the entire heart viewed from the frontal (a), right anterior oblique (b), left anterior oblique cranial (c), posterior (d and e), and right posterior oblique directions (f). The difference in the external aspect between the wide right atrial appendage and small left atrial appendage is recognized. Bilateral pulmonary arteries override the bilateral hyparterial long bronchi (d-f). The azygos vein ascending along the right side of the descending aorta drains into the superior caval vein (e and f)
Figure 5.
Volume-rendered virtual dissection images viewed from the base of the heart. The apex rotates leftward from the panel (a-c). Finger-like orifice of the left atrial appendage with smooth atrioventricular vestibule around the mitral valvar orifice is noted (a). On the contrary, in the right atrial appendage, the presence of pectinate muscles (red dotted curve) extending widely around the muscular atrioventricular vestibule of the tricuspid valvar orifice is noted (b and c)
Figure 6.
Volume-rendered images of the body trunk viewed from the inferior direction. The horizontal plane-cut is made from the superior to inferior direction (a-f) to reveal the arrangement of the abdominal organs. Note the prominent azygos vein with interruption of the inferior caval vein, midline liver, multiple spleens, and left-sided gall bladder
Summary of findings
The arrangement of the bronchial tree, the morphology of the lungs, the presence of an interrupted inferior caval vein with azygos continuation to the right-sided superior caval vein, and the presence of a midline liver with multiple spleens on the left side are all consistent with left isomerism. The atrial appendages, however, were normally arranged, as was the remainder of the cardiac anatomy. At present, it is usually the arrangement of the thoracic organs that permits segregation of the so-called “heterotaxy” into the right and left isomeric subsets.[13] Within the heart, however, it is only the atrial appendages, when assessed on the basis of their pectinate muscles, which are truly isomeric.[10] In our previous experience, all hearts obtained from patients with isomerism of the morphologically left bronchuses also had morphologically left atrial appendages bilaterally.[13] This patient, however, shows that bronchi and pulmonary isomerism can coexist with multiple spleens, yet the atrial appendages and the heart can be arranged in usual fashion. To the best of our knowledge, our patient is the first case whom these features have been demonstrated in unequivocal fashion during life. This shows that isomeric features are not uniform even within the thorax, even though we have previously considered segregation based on the atrial appendages to be the most constant and representative feature in the setting of isomerism.[9,13] It is a simple matter, nonetheless, to describe the usual arrangement of the atrial appendages, with the accompanying normal cardiac anatomy, in association with left bronchial and pulmonary isomerism. The abdominal organs are then as anticipated for the pulmonary rather than the cardiac anatomy. The case emphasizes that even when arrangements within different systems of organs are themselves disharmonious, and seemingly “ambiguous”, independent description of each set of organs based on sequential and segmental approaches removes any potential ambiguity.[10,12] Another unusual feature of our patient, nonetheless, was to find the azygos vein, which was conveying the abdominal venous return to the heart in the setting of interruption of the inferior caval vein, on the opposite side to the abdominal aorta. It is usually the case that the azygos vein and the aorta are on the same side of the spine in the setting of the left isomerism, with the azygos vein in posterior position. The findings in our case would permit the inference to be made of the presence on usual atrial arrangement despite the interruption of the inferior caval vein. The frequency of the arrhythmias, furthermore, is known to be greater in the setting of isomerism,[14] associated with an increased risk of developing atrial flutter.[14] Those with left isomerism are also more likely to have sick sinus syndrome and junctional rhythm.[15] It is again paradoxical, therefore, that our patient demonstrated sick sinus syndrome, with an overall constellation of findings in keeping with left isomerism, but in the setting of usual arrangement of the atrial appendages.
CASE 2
Brief case description
The patient was a 51-year-old woman, who had been diagnosed previously as having “asplenia syndrome.” The initial diagnosis had been a common atrium with an allegedly single ventricle, transposed arterial trunks, and totally anomalous pulmonary venous connection, all in the setting of a right-sided heart. She had been managed conservatively without any surgical corrections. She was admitted to our hospital because of atrial tachycardia with a 2:1 ventricular response. Two days after the admission, she underwent electrical cardioversion, which converted the atrial tachycardia successfully to normal sinus rhythm. We did not attempt to perform either an electrophysiological study or catheter ablation of the atrial tachycardia.
Cardiac computed tomography
She underwent electrocardiographically gated coronary arterial computed tomographic angiography to evaluate her complicated cardiac anatomy. Acquisitions were performed using commercially available dual-source computed tomographic scanners (SOMATOM Force, Siemens Healthcare, Forchheim, Germany), employing a low-dose protocol of acquisition mode of high-pitch dual spiral scan, tube voltage of 70 kVp, rotation time of 250 ms, temporal resolution of 66 ms, and effective radiation dose of 1.9 mSv, with a contrast agent volume of only 20 mL. Images were acquired during a deep inspiratory breath hold. Images were taken at mid-diastole, specifically 70% of the R-R interval. The axial image data were reconstructed using parameters of a section thickness of 0.6 mm, an interval of 0.3 mm, a field of view of 24 cm, and a matrix of 512 × 512. Subsequent analyses were performed using a commercially available workstation (Ziostation version 2.1.7.1, Ziosoft Inc., Tokyo, Japan).
Anatomic findings
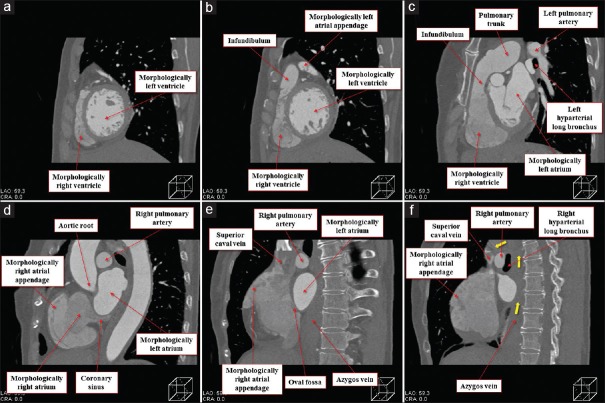
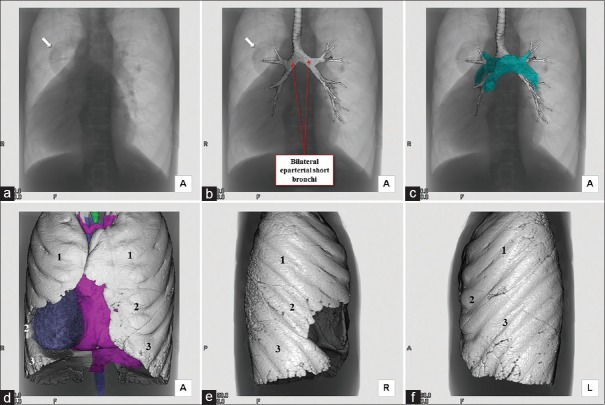
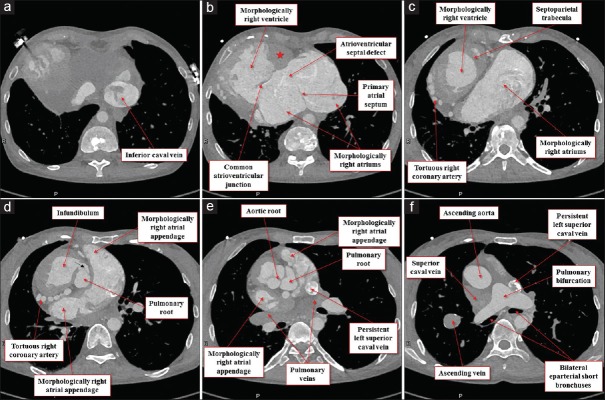
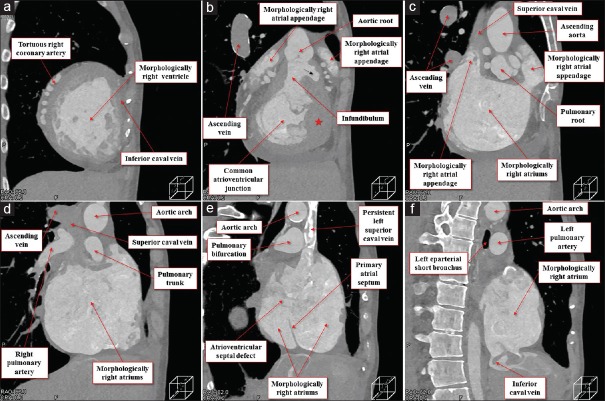
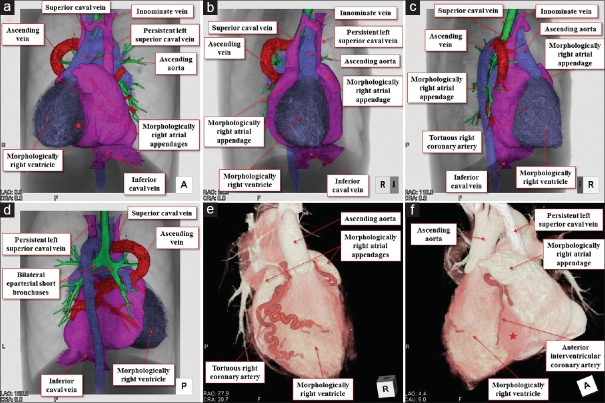
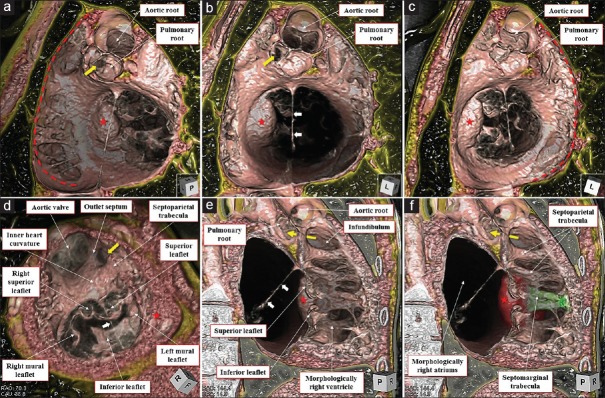
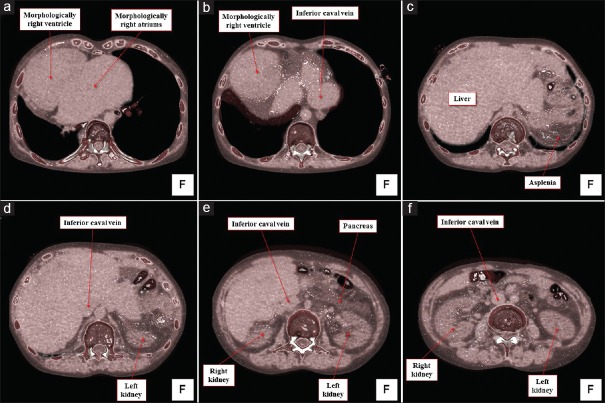
The heart was located in the right hemithorax, with its major axis directed rightward [Figure 7]. In Figures 8 and 9, we show conventional two-dimensional multiplanar reconstruction images from the computed tomographic dataset. There was an isomeric arrangement of the bronchial tree [Figure 7], with both bronchi being eparterial and short, having a relatively wide angle of bifurcation.[12] In keeping with this finding, both lungs [Figure 7] were trilobed, indicating the presence of right isomerism. There were bilateral superior caval veins, each draining directly to both sides of the atrial roof, with a bridging brachiocephalic vein [Figures 8 and 10]. The inferior caval vein, which was left-sided and anterior to the descending aorta, was draining into the left-sided atrium [Figures 8 and 10]. The coronary sinus was absent. There was supracardiac totally anomalous pulmonary venous return, with four pulmonary veins draining into the right-sided superior caval vein through an ascending vein [Figures 8–10]. As identified on the basis of the extent of the pectinate muscles relative to the atrioventricular junctions,[9] both atrial appendages were of right morphology [Figures 8–11]. There was a common atrioventricular junction, guarded by a common atrioventricular valve [Figures 8 and 11] which drained exclusively to a dominant morphologically right ventricle [Figures 8–11]. Since the common atrioventricular valve was exclusively committed to the morphological right ventricle, the superior and inferior leaflets were no longer bridging [Figure 11]. The site of an incomplete and solidified left ventricle was identified at the inferior and left side of the dominant ventricle [Figures 8–11]. The site of the incomplete left ventricle was delimited from the morphologically right ventricle by the anterior interventricular coronary artery [Figure 10]. When judged relative to the overall ventricular mass, the small ventricle remained in the “hip pocket” although it was positioned anteriorly relative to the heart itself. The ventriculoarterial connection was double-outlet from the dominant right ventricle [Figures 10 and 11], with both arterial roots supported by completely muscular infundibulums [Figure 11], albeit with subpulmonary infundibular stenosis [Figures 8, 9 and 11]. The aortic root was located rightward and supero-anteriorly relative to the pulmonary root [Figures 8 and 10]. The aortic arch was left-sided [Figures 8 and 10]. The spleen was absent [Figure 12], with the liver and gallbladder on the right side, and the stomach and pancreas on the left side [Figure 12].
Figure 7.
Volume-rendered fluoroscopy-like image (a) is merged with the trachea and bronchi (b). Bilateral short bronchi with relatively wide bronchial bifurcation angle are observed. Note the abnormal tubular shadow (arrows), corresponding to the ascending vein of total anomalous pulmonary venous return. Bilateral pulmonary arteries do not override the bilateral eparterial short bronchi (c). Fluoroscopy-like image (a) is merged with the heart and bilateral trilobed lungs (d). Pulmonary morphology of the right (e) and left (f) lung show bilateral trilobed lungs
Figure 8.
Multiplanar reconstruction images viewed from the inferior direction at the level of the inferior caval vein (a), ostium premium defect (b), superior margin of the common atrioventricular valve (c), bilateral infundibulums (d), arterial roots (e), and pulmonary bifurcation (f). Red star (b) remarks the solidified incomplete left ventricle located basal inferoanterior of the morphologically right ventricle. Black arrow (d) denotes the stenotic channel toward the subpulmonary infundibulum
Figure 9.
Multiplanar reconstruction images viewed from the apical (right anterior oblique) direction at the level of the mid-ventricle (a), atrioventricular junction (b), bilateral morphologically right atrial appendage (c), mid-morphologically right atria (d), pulmonary bifurcation (e), and posterior morphologically right atria (f). Red star (b) remarks the solidified rudimentary chamber located basal infero-anterior of the morphologically right ventricle. Black arrow (b) denotes the stenotic channel toward the subpulmonary infundibulum
Figure 10.
Volume-rendered dye-cast images viewed from the anterior (a), right anterior (b), right posterior (c), and posterior directions (d). Symmetrical external aspect of the bilateral morphologically right atrial appendages sandwiching the arterial roots (b) and the aspect of the total pulmonary venous return with ascending vein draining into the superior caval vein (d) are well recognized. Note the tortuous right coronary artery (e) and anterior interventricular coronary artery (f) delimiting the morphologically right ventricle from the solidified incomplete left ventricle (red stars)
Figure 11.
Volume-rendered virtual dissection images viewed from the basal (a-c), apical (d), and right posterior oblique directions (e and f). The bilateral pectinate muscles (red dotted curves) extend widely around the common atrioventricular vestibule. The coarse trabeculation and double-outlet right ventricle are shown (e and f) with the septomarginal trabecula colored in green and solidified rudimentary chamber (red stars) colored in red (f). White arrows denote the string-like remnant of the primary atrial septum. Yellow arrows remark the stenotic channel of the subpulmonary infundibulum
Figure 12.
Volume-rendered images of the body trunk viewed from the inferior direction. The horizontal plane-cut is made from the superior to inferior direction (a-f) to reveal the arrangement of the abdominal organs. Inferior caval vein, draining into the left-sided morphologically right atrium, is located left anterior to the descending aorta. Note the asplenia
Summary of findings
The heart in our patient was in the situation often described as “dextroposition” and “dextrocardia” although these terms mean no more than the heart is in the right chest, and that the cardiac apex points rightward. It conveys no information regarding chamber organization and internal cardiac anatomy.[16] As demonstrated in the present images, the identification and categorization of the extremely varied chamber arrangement that might be anticipated in right-sided hearts is greatly facilitated by the use of a descriptive system based on sequential and segmental analyses, accounting separately for connections, relations, and morphology.[16]
In our patient, all the findings are consistent with the arrangement best described as right isomerism. These patients are likely to have atrial tachycardias,[15] as was the case in our patient. In general, patients with right isomerism have more complex cardiovascular malformations than those with left isomerism,[12] again as shown in the present case. Accordingly, when taken alongside the extracardiac abnormalities, such as splenic dysfunctions, prognosis tends to be poor compared with those with left isomerism although even those with multiple spleens can exhibit splenic dysfunction.[17,18,19] Double inlet to a functionally univentricular heart through a common atrioventricular valve is much more frequent in association with the right than the left isomerism.[10,20] In our previous case series of patients with dominant right ventricles and common inlet,[20] all of the incomplete left ventricles were lying posterior to the dominant chamber, irrespective of whether they were right or left sided. As a general rule, incomplete morphologically left ventricles can be expected to be located in the “hip pocket” of the morphologically right ventricle, either to the right, or to the left. When incomplete ventricles have been positioned anteriorly, on the “shoulders” of the ventricular mass, then they have always been of right morphology.[20] In the present case, however, it was evident that the incomplete left ventricle was located anterior to the morphologically right ventricle. Despite this anterior location, nonetheless, the incomplete left ventricle remained in the left-sided “hip pocket” of the ventricular mass, and was distant from the origin of the anterior and right-sided aorta. The location of the incomplete left ventricle, therefore, remains as expected when assessed in terms of the ventricular mass, rather than the overall location of the heart.[20]
DISCUSSION
Appropriate terminology to describe isomerism
When used in the literal context, the term “heterotaxy” means no more than an abnormal or irregular arrangement. In this respect, all congenital malformations could be considered heterotaxic.[10] In addition, as demonstrated in the present cases, isomeric features are not necessarily uniform throughout the body. It is now well established that not all patients with right isomerism have absence of the spleen, just as not all those with left isomerism have multiple spleens.[10] Furthermore, the anatomic findings do not always correlate with function, as demonstrated by the fact that patients with multiple spleens, or even a normally located solitary spleen, can be associated with abnormal splenic function in the setting of bodily isomerism.[1,12] Taking all these facts into consideration, it follows that an abnormal location of the bodily organs should not simply be represented by terms such as “splenic syndromes,” or “heterotaxy.” Moreover, as is now demonstrated by our images, all acquired during life in adults using standard computer tomographic datasets, simple description removes any potential for presumed ambiguity when the arrangements within the different systems of organs are themselves disharmonious.[10] By the same token, this approach renders gratuitous the alternative term “situs ambiguous.”
Computed tomography for demonstration of congenitally cardiac malformations
By virtue of its excellent temporal and spatial resolutions, combined with the ability to repeatedly dissect the living heart in every desired plane from every desired direction, without distorting the structures and relationships, computed tomography has now become the gold standard for delineating the subtle details of all aspects of cardiac anatomy.[21] Indeed, it is difficult, if not impossible, to replicate with comparable accuracy, the analysis of the hearts in either the dissection room or the autopsy suite. The very process of death distorts the cardiac anatomy, even before the hearts are opened to display the anatomic features. Moreover, once opened or sectioned, it is difficult to obtain alternative “cuts” should the original options have proved unsuitable.
Our current experience now demonstrates the usefulness of three-dimensional reconstruction, based on computed tomography, in revealing even the most subtle anatomic nuances. It reinforces previous findings in the settings of the normal adult heart,[22,23,24,25] valvar diseases,[26] and in some structurally abnormal hearts.[27,28,29] The utility of three-dimensional imaging techniques has already been demonstrated in the setting of congenitally cardiac disease, including isomerism.[10,12,13] It is noteworthy, as evidenced by the study of Figures 2, 3, 8, and 9, nonetheless, that the complex three-dimensional anatomy is hard to visualize when using only conventional two-dimensional multiplanar reconstruction images. It is the possibility to produce three-dimensional reconstructions, based on the volume-rendering method, including dye-cast images [Figures 4 and 10] and virtual dissection images [Figures 5 and 11], that permits more precise evaluation of complex anatomical arrangements as encountered in the setting of isomerism. In particular, the three-dimensional reconstructions reveal in unequivocal fashion the extent of the pectinate muscles within the atrial appendages relative to the atrioventricular junctions [Figures 5 and 11]. It is this feature that permits the recognition of cardiac isomerism,[4,9] a feature which had been doubted by some distinguished authorities.[3] In addition, our current images show how it is possible to recognize the individual leaflets of the common atrioventricular valve [Figure 11],[30] the coarse apical trabeculations of the morphologically right ventricle [Figure 11], and the location of the incomplete left ventricle, even when solidified and contained within the wall of the ventricular mass [Figures 8–11]. The accuracy in diagnosis, furthermore, is not confined to the heart. Since the computed tomographic dataset encompasses the entire body, it is possible to recognize bronchial morphology, including the important relationship to the pulmonary arteries, to determine the precise pulmonary lobation, and to reveal the overall arrangement of the abdominal organs. Thus, with appropriate enhancement and scanning, the location and arrangement of all the thoracoabdominal organs, including the heart, is determined with a single examination.
Although we have shown the benefits of computed tomography in clarifying the complex living anatomy of patients with congenitally malformed hearts, we recognize that both of our current patients were adults. Multiple specific features, including the small size of the heart, the high heart rate, and difficulties in controlling respiratory and body movements, can prove problematic when imaging neonates, infants, and children. We also recognize that, especially in the young patients, every study obtained using computed tomography must be justified in view of the risk of radiation and the use of contrast material. As demonstrated in our second patient, even though the current high-end third-generation dual-source scanners have the capability of providing optimal images at extremely low doses of radiation around 1 mSv,[28] which is equivalent to about fifty standard chest radiographs, and with minimal use of contrast, the procedure is not free from exposure to radiation and does require the use of some contrast material. The ease and quality of the reconstruction depends on the quality of the original datasets. Homogenous enhancement of chambers of interest would be difficult in cases with intracardiac shunting due to mixing, especially in younger patients. Thus, preprocedural communication and discussion with the radiologist should be undertaken so as to construct an appropriate and tailored individual strategy for scanning with justifiable exposure to radiation and appropriate use of contrast material. It will be a multidisciplinary approach, involving cardiologists, cardiac radiologists, anatomists, and radiological technologists, which will produce optimal results.
Pediatric cardiologists, nonetheless, should now be keeping abreast of all the current advance in the field of three-dimensional reconstruction. It is beyond our current purpose to discuss the detailed indication and feasibility of the use of computed tomographic analysis for each congenital cardiac lesion, however, the images currently presented show that all features in cases with isomerism, no matter how complex, can be precisely determined during life, including morphology of the atrial appendage, bronchi, lungs, venoatrial, atrioventricular, and ventriculo-arterial connections, as well as abdominal organs, with the appropriate and tailored use of current computed tomography under a multidisciplinary approach. Since the correct anatomic diagnoses, during life, are key to the management of patients with congenital cardiac malformation, we suggest that at least a single examination using electrocardiographically gated contrast-enhanced computed tomography with a low-dose protocol for radiation can now be justified in many who are candidates for surgery. It is the case that all clinicians with access to standard computed tomographic scanners and workstations should now have the capacity to match the images we have produced. If young patients with congenitally malformed hearts are now submitted to contrast-enhanced computed tomography, therefore, every effort should be made to obtain full three-dimensional information from the obtained dataset. As our current images demonstrate, the rewards can be considerable.
CONCLUSION
Currently, based on a multidisciplinary approach, the complex anatomic features of the congenitally malformed heart can be accurately recognized during life by appropriate use of computed tomography. In the setting of isomerism, furthermore, the overall arrangement of the thoracoabdominal organs, including the heart, can be determined by a single computed tomographic examination. Then with the provided living-heart images, if each system of organs is described in independent fashion, any suggestion of ambiguity can be excluded.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Prof. Ken-Ichi Hirata from the Division of Cardiovascular Medicine, Department of Internal Medicine, Kobe University Graduate School of Medicine, for placing his scientific expertise at our disposal during the preparation of the manuscript. We also thank our radiological technologists for their assistance in acquiring and reconstructing our images, specifically Erina Suehiro, Wakiko Tani, Toshinori Sekitani, Kiyosumi Kagawa, and Noriyuki Negi.
REFERENCES
- 1.Jacobs JP, Anderson RH, Weinberg PM, Walters HL, 3rd, Tchervenkov CI, Del Duca D, et al. The nomenclature, definition and classification of cardiac structures in the setting of heterotaxy. Cardiol Young. 2007;17(Suppl 2):1–28. doi: 10.1017/S1047951107001138. [DOI] [PubMed] [Google Scholar]
- 2.Vanpraagh R, Vanpraagh S, Vlad P, Keith JD. Anatomic types of congenital dextrocardia: Diagnostic and embryologic implications. Am J Cardiol. 1964;13:510–31. doi: 10.1016/0002-9149(64)90159-6. [DOI] [PubMed] [Google Scholar]
- 3.Van Praagh R. Segmental anatomy and heterotaxy syndromes. In: Ezon DS, Goldberg JE, Kyle WB, editors. Atlas of Congenital Heart Disease Nomenclature. Houston: Baylor College of Medicine; 2016. pp. 18–9. [Google Scholar]
- 4.Anderson RH. Segmental anatomy and heterotaxy syndromes. In: Ezon DS, Goldberg JE, Kyle WB, editors. Atlas of Congenital Heart Disease Nomenclature. Houston: Baylor College of Medicine; 2016. pp. 20–1. [Google Scholar]
- 5.Putschar WG, Manion WC. Congenital absence of the spleen and associated anomalies. Am J Clin Pathol. 1956;26:429–70. doi: 10.1093/ajcp/26.5.429. [DOI] [PubMed] [Google Scholar]
- 6.Van Mierop LH, Eisen S, Schiebler GL. The radiographic appearance of the tracheobronchial tree as an indicator of visceral situs. Am J Cardiol. 1970;26:432–5. doi: 10.1016/0002-9149(70)90743-5. [DOI] [PubMed] [Google Scholar]
- 7.Van Mierop LH, Wiglesworth FW. Isomerism of the cardiac atria in the asplenia syndrome. Lab Invest. 1962;11:1303–15. [PubMed] [Google Scholar]
- 8.Van Mierop LH, Gessner IH, Schiebler GL. Asplenia and polysplenia syndromes. Birth Defects Orig Artic Ser. 1972;8:36–52. [Google Scholar]
- 9.Uemura H, Ho SY, Devine WA, Kilpatrick LL, Anderson RH. Atrial appendages and venoatrial connections in hearts from patients with visceral heterotaxy. Ann Thorac Surg. 1995;60:561–9. doi: 10.1016/0003-4975(95)00538-V. [DOI] [PubMed] [Google Scholar]
- 10.Loomba RS, Hlavacek AM, Spicer DE, Anderson RH. Isomerism or heterotaxy: Which term leads to better understanding? Cardiol Young. 2015;25:1037–43. doi: 10.1017/S1047951115001122. [DOI] [PubMed] [Google Scholar]
- 11.Uemura H, Ho SY, Devine WA, Anderson RH. Analysis of visceral heterotaxy according to splenic status, appendage morphology, or both. Am J Cardiol. 1995;76:846–9. doi: 10.1016/s0002-9149(99)80243-4. [DOI] [PubMed] [Google Scholar]
- 12.Loomba RS, Shah PH, Anderson RH, Arora Y. Radiologic considerations in heterotaxy: The need for detailed anatomic evaluation. Cureus. 2016;8:e470. doi: 10.7759/cureus.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loomba RS, Ahmed MM, Spicer DE, Backer CL, Anderson RH. Manifestations of bodily isomerism. Cardiovasc Pathol. 2016;25:173–80. doi: 10.1016/j.carpath.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Loomba RS, Aggarwal S, Gupta N, Buelow M, Alla V, Arora RR, et al. Arrhythmias in adult congenital patients with bodily isomerism. Pediatr Cardiol. 2016;37:330–7. doi: 10.1007/s00246-015-1281-7. [DOI] [PubMed] [Google Scholar]
- 15.Loomba RS, Willes RJ, Kovach JR, Anderson RH. Chronic arrhythmias in the setting of heterotaxy: Differences between right and left isomerism. Congenit Heart Dis. 2016;11:7–18. doi: 10.1111/chd.12288. [DOI] [PubMed] [Google Scholar]
- 16.Calcaterra G, Anderson RH, Lau KC, Shinebourne EA. Dextrocardia – Value of segmental analysis in its categorisation. Br Heart J. 1979;42:497–507. doi: 10.1136/hrt.42.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedom RM, Jaeggi ET, Lim JS, Anderson RH. Hearts with isomerism of the right atrial appendages – One of the worst forms of disease in 2005. Cardiol Young. 2005;15:554–67. doi: 10.1017/S1047951105001708. [DOI] [PubMed] [Google Scholar]
- 18.Nagel BH, Williams H, Stewart L, Paul J, Stümper O. Splenic state in surviving patients with visceral heterotaxy. Cardiol Young. 2005;15:469–73. doi: 10.1017/S1047951105211320. [DOI] [PubMed] [Google Scholar]
- 19.Loomba RS, Geddes GC, Basel D, Benson DW, Leuthner SR, Hehir DA, et al. Bacteremia in patients with heterotaxy: A review and implications for management. Congenit Heart Dis. 2016;11:537–47. doi: 10.1111/chd.12395. [DOI] [PubMed] [Google Scholar]
- 20.Keeton BR, Macartney FJ, Hunter S, Mortera C, Rees P, Shinebourne EA, et al. Univentricular heart of right ventricular type with double or common inlet. Circulation. 1979;59:403–11. doi: 10.1161/01.cir.59.2.403. [DOI] [PubMed] [Google Scholar]
- 21.Anderson RH. Re-setting the gold standard. J Cardiovasc Electrophysiol. 2015;26:713–4. doi: 10.1111/jce.12696. [DOI] [PubMed] [Google Scholar]
- 22.Mori S, Fukuzawa K, Takaya T, Takamine S, Ito T, Fujiwara S, et al. Clinical structural anatomy of the inferior pyramidal space reconstructed within the cardiac contour using multidetector-row computed tomography. J Cardiovasc Electrophysiol. 2015;26:705–12. doi: 10.1111/jce.12687. [DOI] [PubMed] [Google Scholar]
- 23.Mori S, Fukuzawa K, Takaya T, Takamine S, Ito T, Fujiwara S, et al. Clinical cardiac structural anatomy reconstructed within the cardiac contour using multidetector-row computed tomography: The arrangement and location of the cardiac valves. Clin Anat. 2016;29:364–70. doi: 10.1002/ca.22549. [DOI] [PubMed] [Google Scholar]
- 24.Mori S, Spicer DE, Anderson RH. Revisiting the anatomy of the living heart. Circ J. 2016;80:24–33. doi: 10.1253/circj.CJ-15-1147. [DOI] [PubMed] [Google Scholar]
- 25.Anderson RH, Mori S. How can we best describe the cardiac components? J Cardiovasc Electrophysiol. 2016;27:972–5. doi: 10.1111/jce.13013. [DOI] [PubMed] [Google Scholar]
- 26.Soga F, Takaya T, Mori S, Nishii T, Hirata K. Bending of the aortic valvar leaflet causing severe aortic regurgitation in a patient with osteogenesis imperfecta. Eur Heart J Cardiovasc Imaging. 2016;17:708. doi: 10.1093/ehjci/jew057. [DOI] [PubMed] [Google Scholar]
- 27.Ichibori H, Mori S, Takaya T, Kiuchi K, Ito T, Fujiwara S, et al. Slit-like deformation of the coronary sinus orifice due to compression of the inferior pyramidal space by the severely dilated left ventricle. Pacing Clin Electrophysiol. 2016;39:1026–9. doi: 10.1111/pace.12881. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa K, Takaya T, Mori S, Ito T, Fujiwara S, Nishii T, et al. Compression of the right ventricular outflow tract due to straight back syndrome clarified by low-dose dual-source computed tomography. Intern Med. 2016;55:3279–83. doi: 10.2169/internalmedicine.55.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryo-Koriyama K, Matsumoto K, Nishii T, Tanaka H, Hirata K. A rare case of double-chambered right ventricle apparent on the compression by both pectus excavatum and straight back syndrome. Eur Heart J Cardiovasc Imaging. 2016;17:706. doi: 10.1093/ehjci/jew051. [DOI] [PubMed] [Google Scholar]
- 30.Craig B. Atrioventricular septal defect: From fetus to adult. Heart. 2006;92:1879–85. doi: 10.1136/hrt.2006.093344. [DOI] [PMC free article] [PubMed] [Google Scholar]