Abstract
A modified Blalock–Taussig shunt (mBTS) is often employed to provide pulmonary blood flow in neonates that are born with cyanotic congenital heart defects. However, acute shunt thrombosis can occur in the postoperative period, resulting in profound cyanosis. In this case report, we describe the utility of computed tomographic angiography (CTA) in the management of a neonate with extreme cyanosis after placement of a mBTS while on extracorporeal membrane oxygenation. Using CTA, several small clots were identified in the shunt as well as stenosis of the left pulmonary artery; neither of which were identified with echocardiography. The CTA allowed for quick identification of the disorder and helped direct prompt surgical intervention.
Keywords: Blalock–Taussig shunt, computed tomographic angiography, cyanotic congenital heart disease, extracorporeal membrane oxygenation, postoperative care
INTRODUCTION
A modified Blalock–Taussig shunt (mBTS) is often utilized to provide pulmonary blood flow in neonates that are born with cyanotic congenital heart defects. In this case report, we describe a patient who displayed extreme cyanosis in the Intensive Care Unit (ICU) after placement of a mBTS, necessitating initiation of extracorporeal membrane oxygenation (ECMO). Traditionally, if echocardiography is unable to determine the etiology, such patients are sent for diagnostic catheterization (DC) or directly to the operation room for empiric surgical management. We highlight the utility of computed tomographic angiography (CTA) in this scenario. Although CTA has been shown to be useful in many patients with congenital heart disease, reports of its use while on ECMO are very limited, and its use in this clinical scenario has not been reported. Using CTA, several small clots were identified in the shunt as well as stenosis of the left pulmonary artery (LPA), both of which had been missed in a previous echocardiogram.
CASE REPORT
A 3.9 kg term, female infant was born with pulmonary atresia with an intact ventricular septum (PA/IVS). She was taken to the operation room the following week for a right-sided mBTS. After placement of a 3.5 mm shunt, the ductal tissue was resected from the LPA, which was augmented with a pulmonary homograft patch. At the conclusion of the operation, the patient had acceptable hemodynamics with systolic blood pressures ranging from 70 to 85 mmHg and oxygen saturation levels of 75%–85%.
After several hours of stability in the ICU, the infant experienced profound cyanosis and hypotension. An echocardiogram was performed, which revealed obstruction of the shunt. Her chest was reopened in the ICU and the shunt was stripped toward the pulmonary artery. Since there was insufficient improvement in oxygenation, she was placed on ECMO. Extensive thrombus was then removed from the shunt and proximal pulmonary arteries.
The patient was left on ECMO overnight, with flow maintained between 75% and 85% to allow for pulsatility. The following morning, the patient could not be weaned from ECMO due to cyanosis at lower flow rates. Another echocardiogram was performed, revealing a patent shunt and proximal pulmonary arteries. A decision was made to perform a CTA to further evaluate the pulmonary blood supply. The patient was transferred to the scanner (Somatom Definition Flash, Siemens Healthcare, Forchheim, Germany) on ECMO. The ECMO circuit was briefly clamped, allowing for hand injection of 7 cc (2 ml/kg) of contrast medium. Prospectively gated images were taken at peak systole using low radiation dose settings (80 kV, 53 mAs, DLP 9 mGy-cm) from the lower neck to the diaphragm. The CTA images revealed severe stenosis of the mid-LPA [Figure 1]. In addition, small clots were identified in the proximal shunt and the distal anastomosis.
Figure 1.
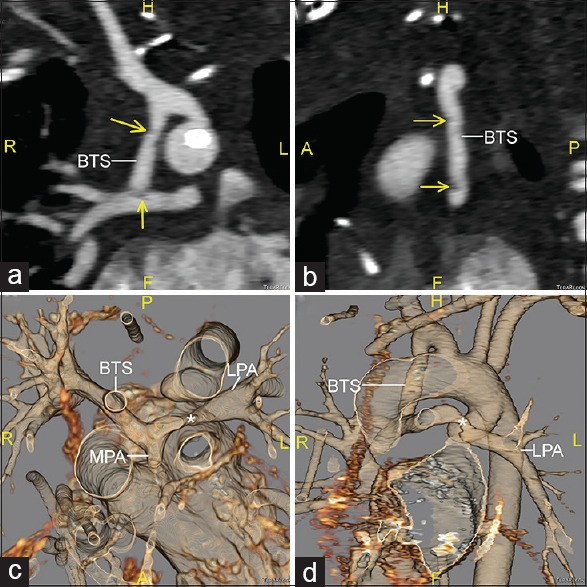
Computed tomographic angiography images reconstructed in oblique coronal (a) and sagittal (b) planes, along with 3D volume-rendered reconstructions viewed from superior (c) and anterior-leftward (d) perspectives. The Blalock–Taussig shunt is seen inserting into the right pulmonary artery. Small thrombi are seen in the mid-portion of the shunt and its distal insertion into the right pulmonary artery (arrows). There is a small main pulmonary artery stump present, and an area of focal stenosis is seen in the left pulmonary artery (*). BTS: Blalock–Taussig shunt; RPA: Right pulmonary artery; LPA: Left pulmonary artery; MPA: Main pulmonary artery
The patient was taken back to the operation room. The proximal end of the main pulmonary artery was divided and oversewn. An incision was made down the LPA beyond the originally placed patch and rightward to the base of the mBTS. A thrombus was identified in the distal shunt, which was extracted with a Fogarty balloon catheter. The branch pulmonary arteries were augmented with a homograft patch. At the conclusion of the surgery, the patient was successfully weaned off bypass, with oxygen saturations between 75% and 85%. Another CTA was performed 2 weeks later, which revealed widely patient pulmonary arteries and mBTS [Figure 2].
Figure 2.
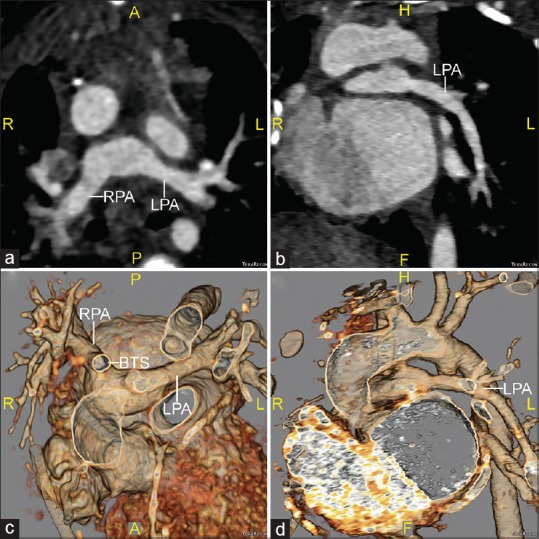
Computed tomographic angiography images taken after surgical reintervention, which are shown in axial (a) and oblique sagittal (b) planes, along with 3D volume-rendered reconstructions viewed from superior (c) and anterior-leftward (d) perspectives. The main pulmonary artery stump has been divided and the left pulmonary artery is now widely patent. BTS: Blalock–Taussig shunt; RPA: Right pulmonary artery; LPA: Left pulmonary artery
DISCUSSION
Most patients with PA/IVS require placement of a mBTS. Complications involving the mBTS are not uncommon, and are associated with a significant risk of mortality.[1] Increased cyanosis after a mBTS can be due to many causes, including shunt malfunction, pulmonary embolus, decreased cardiac function, infection, anemia, and lung disease. When managing such patients, it is important to choose diagnostic modalities that are accurate, quick, and safe.
In the clinical scenario above, the initial imaging modality is usually echocardiography. However, its utility is limited in patients with poor acoustic windows, a common limitation in patients cannulated for ECMO through the chest. In addition, the abnormal loading conditions and pulsatility inherent to ECMO can make echocardiography challenging in this situation. When echocardiography is unable to provide adequate information, these patients are often taken for DC. While DC can be safely and accurately performed in patients on ECMO, it can be challenging in this scenario and can result in prolonged bleeding or vascular injury.[2]
CTA is being increasingly utilized in patients with congenital heart disease,[3] and its use in neonates on ECMO before surgery has been reported.[4] Unlike DC, CTA can be performed with any type of venous access. It requires fewer resources than DC, and can be obtained in a much more timely fashion. Furthermore, we have previously shown that CTA exposes infants to 15 times lower radiation dose than DC.[5] While transportation to the computed tomography (CT) scanner on ECMO is a logistical challenge, this is an issue common to both CTA and DC, and emergent access to the CT scanner is generally better than access to the catheterization laboratory in most pediatric centers. In centers that have adequate experience, we recommend considering CTA in lieu of DC in similar clinical scenarios.
Financial support and sponsorship
UJS is a consultant for and receives research support from Astellas, Bayer, Bracco, General Electric, Guerbet, Medrad, and Siemens. AMH receives research support from Siemens.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bove T, Vandekerckhove K, Panzer J, De Groote K, De Wolf D, François K. Disease-specific outcome analysis of palliation with the modified Blalock-Taussig shunt. World J Pediatr Congenit Heart Surg. 2015;6:67–74. doi: 10.1177/2150135114558690. [DOI] [PubMed] [Google Scholar]
- 2.Panda BR, Alphonso N, Govindasamy M, Anderson B, Stocker C, Karl TR. Cardiac catheter procedures during extracorporeal life support: A risk-benefit analysis. World J Pediatr Congenit Heart Surg. 2014;5:31–7. doi: 10.1177/2150135113505297. [DOI] [PubMed] [Google Scholar]
- 3.Meinel FG, Henzler T, Schoepf UJ, Park PW, Huda W, Spearman JV, et al. ECG-synchronized CT angiography in 324 consecutive pediatric patients: Spectrum of indications and trends in radiation dose. Pediatr Cardiol. 2015;36:569–78. doi: 10.1007/s00246-014-1051-y. [DOI] [PubMed] [Google Scholar]
- 4.Friedman BA, Schoepf UJ, Bastarrika GA, Hlavacek AM. Computed tomographic angiography of infants with congenital heart disease receiving extracorporeal membrane oxygenation. Pediatr Cardiol. 2009;30:1154–6. doi: 10.1007/s00246-009-9488-0. [DOI] [PubMed] [Google Scholar]
- 5.Watson TG, Mah E, Joseph Schoepf U, King L, Huda W, Hlavacek AM. Effective radiation dose in computed tomographic angiography of the chest and diagnostic cardiac catheterization in pediatric patients. Pediatr Cardiol. 2013;34:518–24. doi: 10.1007/s00246-012-0486-2. [DOI] [PubMed] [Google Scholar]


