Abstract
Nerve growth factor (NGF) is a key regulator of the development and differentiation of neuronal and non-neuronal cells. In the present study we examined the distribution of NGF and its low and high-affinity receptors, p75NTR and TrkA respectively, in permanent human teeth under normal and pathological conditions. In intact functional teeth, NGF, p75NTR and TrkA are weakly expressed in dental pulp fibroblasts and odontoblasts that are responsible for dentine formation, while the NGF and p75NTR molecules are strongly expressed in nerve fibres innervating the dental pulp. In carious and injured teeth NGF and TrkA expression is upregulated in a selective manner in odontoblasts surrounding the injury sites, indicating a link between NGF signalling and dental tissue repair events. Accordingly, NGF and TrkA expression is strongly upregulated in cultured primary human dental mesenchymal cells during their differentiation into odontoblasts. Targeted release of NGF in cultured human tooth slices induced extensive axonal growth and migration of Schwann cells towards the NGF administration site. These results show that NGF signalling is strongly linked to pathological and regenerative processes in human teeth and suggest a potential role for this neurotrophic molecule in pulp regeneration.
Introduction
Nerve growth factor (NGF) is the first identified member of a family of neurotrophic molecules that also comprises the brain derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), NT-4, and NT-6. All these molecules have essential roles in the fate, development, survival, outgrowth and maintenance of selected group of neurons1–4. NTs bind with either high or low affinity to a variety of specific receptors that are expressed on both neurons and non-neuronal target cells. The transmembrane glycoprotein receptor p75NTR binds all NTs with low affinity1, 4, and its activation can induce either cell survival or apoptosis depending on the identity of the attached ligand4–6. The tyrosine kinases receptors TrkA, TrkB and TrkC also bind NTs with different affinities1, 4. The 140 kDa glycoprotein TrkA acts as high affinity receptor for NGF and its function is influenced by the concomitent presence of p75NTR, which can either inhibit or increase TrkA activation1, 4, 7, 8.
Apart their known functions in the nervous system, additional roles for NTs were suggested based on the expression patterns of p75NTR and Trk receptors during organogenesis and differentiation of non-neuronal cells such as melanocytes, parafollicular cells of the thyroid gland, secretory and pillar cells of the cochlea, glomeruli of the kidney, somites, and odontoblasts and ameloblasts of the teeth4, 9–15. Moreover, in the last years the NTs/p75NTR signalling was extensively studied in the context of stem cell function, and p75NTR was identified as a marker of selected stem cell populations2, 16. NGF may act as a proliferative, differentiation, survival and apoptotic agent in non-neuronal cells, depending on its concentration and the presence of NTs receptors in these cells1, 4, 17.
Previous studies from our group and from others have demonstrated that NGF, p75NTR and TrkA are expressed in the developing rodent and human teeth, thus suggesting that dental cell functions are dependent upon NTs signalling13, 14, 18–24. The tooth develops as a result of sequential and reciprocal interactions between the oral ectoderm and the cranial neural crest-derived mesenchyme25, 26. Differentiation of dental cells gives rise to the mesenchymal-derived odontoblasts that produce the dentine matrix and the epithelial-derived ameloblasts that form the enamel. In developing teeth, expression of NGF, p75NTR and TrkA in mesenchyme correlates with cytodifferentiation events, while epithelial expression is linked to proliferative phenomena13, 14, 20, 24. In vitro studies have shown that NGF produced by dental pulp fibroblasts acts as a neuroattractive agent27, and therefore NGF could have a role in the attraction of neurons into the pulp chamber during odontogenesis13. Studies in rodents and dogs have shown that expression of NGF and p75NTR increased after dental injury, suggesting additional roles for NGF in regenerative processes18, 19, 28, 29. Although all these findings illustrate the importance of NGF signalling in a variety of biological processes linked to tooth development, homeostasis and repair, only very few studies have been realized in human teeth to date. While these studies reported on the expression of TrkA24, p75NTR 22, and NGF mRNA23 during odontogenesis, there is not yet data concerning their expression in adult human teeth under normal and pathological conditions.
The present study was conducted to analyze the in vivo expression of the NGF, p75NTR and TrkA proteins in intact, carious and injured adult human teeth, as well as in cultured human dental pulp cells in vitro. Furthermore, we examined the effects of NGF on neuronal sprouting in an in vitro dental slice culture model.
Results
NGF, p75NTR and TrkA expression in healthy human teeth
In the dental pulp of permanent intact teeth, very little, if not at all, NGF and TrkA immunoreactivities could be observed in decalcified specimens (Fig. 1C,F,I). In freshly extracted non-decalcified pulps, moderate NGF immunoreactivity was observed in odontoblasts and nerve-related cells (Fig. 1A,D,G), while p75NTR staining was strong in nerve fibers and very faint in odontoblasts (Fig. 1B,E,H). The most intense NGF labelling was detected in the odontoblastic processes, while the staining was weaker in the odontoblastic bodies (Fig. 1D). In general, NGF staining was absent in dental pulp fibroblasts (Fig. 1D,G), although NGF immunoreactivity was seen sporadicly in some of these cells (Fig. 1I). In contrast, p75NTR immunoreactivity was absent from all pulp fibroblasts (Fig. 1E,H). Immunostaining for NGF, p75NTR and TrkA was not detected in the sections used as controls (Fig. 1J).
Figure 1.
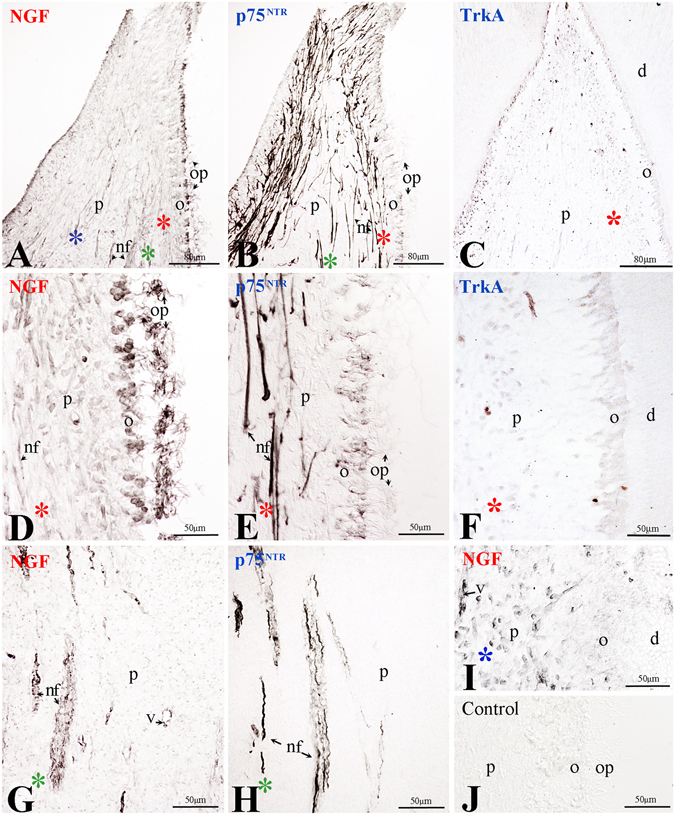
Expression of NGF, p75NTR and TrkA in healthy human teeth. (A–C) Overview of NGF (A), p75NTR (B) and TrkA (C) expression in the pulp of healthy teeth. (D,G,I) NGF, (E,H) p75NTR and (F) TrkA expression in selected regions of healthy intact human teeth. (J) Negative control. Abbreviations: d, dentine; nf, nerve fibres; o, odontoblasts; op, odontoblastic processes; p, pulp; v, vessel. Asterisks in different colours in Fig. 1A–C indicate higher magnifications of the corresponding areas in Fig. 1D–I.
NGF, p75NTR and TrkA expression in carious human teeth
In teeth with carious lesions, the secretory activity of the odontoblasts is stimulated to produce tertiary dentine (Fig. 2A–C). Inflammatory events are often observed in the pulp of teeth with advanced carious lesions (Fig. 2A). In these case, odontoblasts beneath the bacterially infected dentine die and are replaced by newly differentiated odontontoblasts that form the tertiary dentine30. In decalcified carious teeth, NGF immunostaining was detected in both apoptotic and newly formed odontoblasts facing the decay (Fig. 2D,E,F,J,L). Notably, NGF expression could be observed also in nerve fibers and in perivascular regions within the pulp (Fig. 2G), where p75NTR was also detected (Fig. 2I). Very weak p75NTR expression was observed in odontoblasts, both in proximity and at a distance from the site of injury (Fig. 2H,I,K,L). TrkA staining was obvious in odontoblasts facing the carious lesion (Fig. 2M) as well as in odontoblasts that actively produce the tertiary dentine (Fig. 2N,O). TrkA immunoreactivity was detected in the bodies of the odontoblasts as well as in their processes (Fig. 2O). These results show that expression of both NGF and TrkA is strongly upregulated in odontoblasts and newly-formed odontoblasts in carious human teeth.
Figure 2.
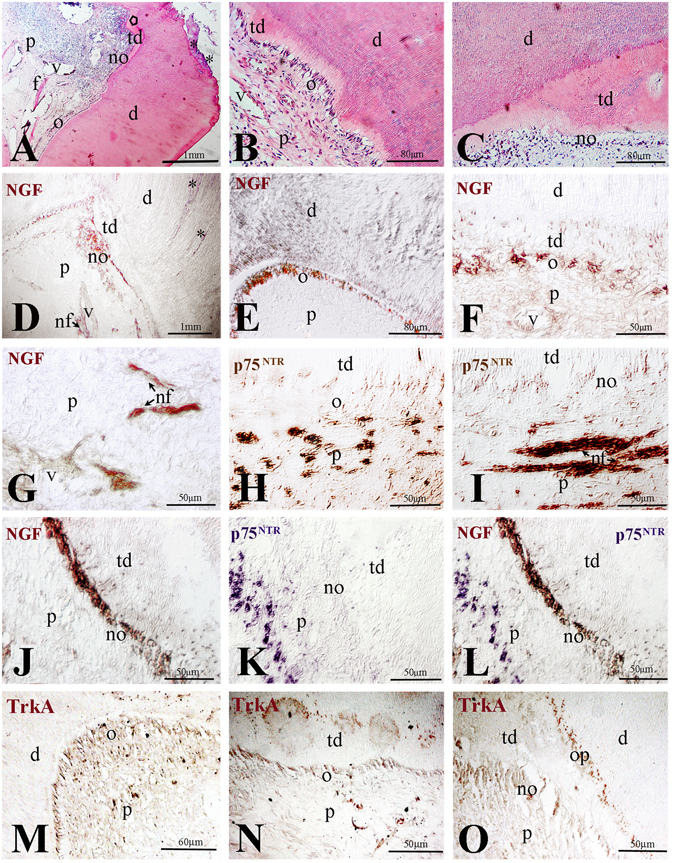
Expression of NGF, p75NTR and TrkA in carious human teeth. (A–C) Histology of carious teeth. (B,C) higher magnifications of regions of production of tertiary dentine. (D–F) NGF expression (brown colour) in regions underlying the carious lesion (D), in proximity of the lesion (E), in odontoblasts producing tertiary dentine (F). (G) Expression of NGF in nerve fibres within the pulp. (H,I) p75NTR expression (brown colour) in nerve fibres and vessels underlying newly formed odontoblasts. (J–L) Co-staining showing NGF (brown colour) and p75NTR (violet colour) expression in the region underlying the carious lesion. (M–O) TrkA expression (brown colour) in odontoblasts facing the carious region (M) and in odontoblasts producing the tertiary dentin (N,O). Abbreviations: d, dentine; nf, nerve fibres; no, newly-formed odontoblasts; o, odontoblasts; op, odontoblast processes; p, pulp; td, tertiary dentine; v, vessel; Asterisks indicate the progression of the carious lesion in the dentine.
NGF, p75NTR and TrkA expression in permanent human teeth after cavity preparation
Injury caused by tooth cavity preparation invokes pulp responses that are accompanied by apoptotic events30. Apoptotic odontoblasts are substituted by newly-formed odontoblasts, which are aligned into a single cell-layer and elaborate the tertiary dentine (Fig. 3A–C). These events were not observed in other pulp areas that are located at a distance from the cavity preparation site (Fig. 3A,B). Nine weeks post-surgery, a thick layer of tertiary dentine was seen beneath the injury site (Fig. 3C). Strong NGF immunoreactivity was detected in the newly-formed odontoblasts that secrete the tertiary dentine matrix (Fig. 3D(a)), but also in apoptotic odontoblasts (Fig. 3D(b)) as well as in odontoblasts located far away from the cavity (Fig. 3c). NGF staining was mainly distributed in the bodies of the odontoblasts, while a weak staining was found in their processes (Fig. Da–c). Quite often, cavity preparations generate hyperemic conditions within the pulp that affect blood vessel physiology. NGF staining was observed in cells of the dilated blood vessels, as well as in few pulp fibroblasts and neurons in the proximity of the vessels (Fig. 3D(a–c)). Strong p75NTR expression was observed in nerve fibres, while weak expression was detected in odontoblasts (Fig. 3E–G). Intense TrkA labelling was observed in odontoblasts located near or far away from the cavity (Fig. 3H,I), while the staining was very weak in dying odontoblasts beneath the cavity (Fig. 3H). No staining was detected in control sections (Fig. 3J).
Figure 3.
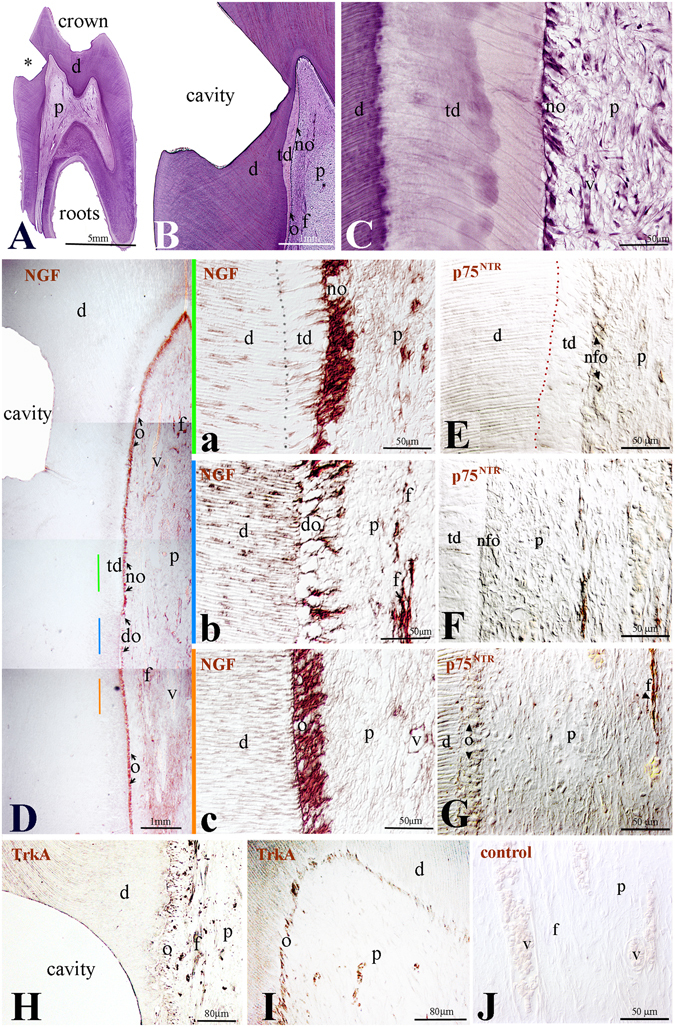
Expression of NGF, p75NTR and TrkA in permanent human teeth after cavity preparation. (A–C) Histology of dental tissues after cavity preparation. (D) Overview of NGF staining (brown colour) in tooth after cavity preparation. (D(a–c)) Higher magnifications of D. (E) p75NTR expression (brown colour) in newly formed odontoblasts underlying tertiary dentin. (F) p75NTR expression in nerve fibres in proximity of tertiary dentin. (G) p75NTR expression in odontoblasts (arrowheads) and in nerve fibres within the pulp. (H,I) TrkA expression (brown colour) in odontoblasts and nerve fibres adjacent to the cavity (H) and far away from the injury site (I). (J) Negative control. Abbreviations: d, dentine; do, dying odontoblasts; f, nerve fibres; no, newly-formed odontoblasts; o, odontoblasts; p, pulp; v, vessel. Asterisk indicates the cavity.
NGF, p75NTR and TrkA expression in human dental pulp cells in vitro
In order to better understand the role of NGF signalling in homeostasis and repair of human dental pulp we have analysed NGF, p75NTR and TrkA expression in primary dental pulp cell cultures and in dental pulp stem cells (hDPSCs). In cultures of hDPSCs, only a very tiny group of cells expressed NGF (Fig. 4A–C), p75NTR (Fig. 4D–F) and TrkA (Fig. 4G–I). We then studied the expression of these molecules in dental pulp cells cultured in mineralizing conditions that require the presence of ß-glycerophosphate (Fig. 5). All dental pulp cells cultured in presence of ß-glycerophosphate for different periods of time up to 4 weeks exhibited NGF immunoreactivity (Fig. 5B–G). The strongest NGF staining was observed in cells forming the mineralized nodules (Fig. 5F,G). p75NTR staining was weak in cultured pulp cells but its expression increased in cells producing mineral matrix (Fig. 5H,I). Intense TrkA labelling was observed in cultured pulp cells forming the mineralized nodules (Fig. 5J–L).
Figure 4.
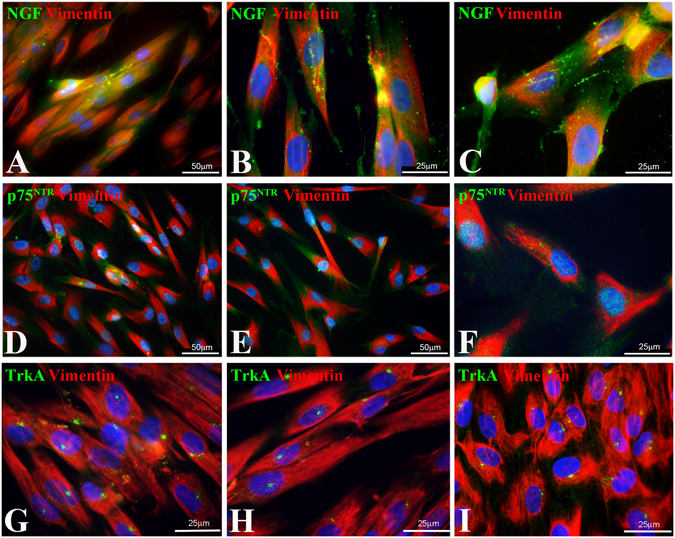
Expression of NGF, p75NTR and TrkA in combination with vimentin in human dental pulp stem cells (hDPSCs). (A–C) NGF, (D–F) p75NTR and (G–I) TrkA expression in hDPSCs.
Figure 5.
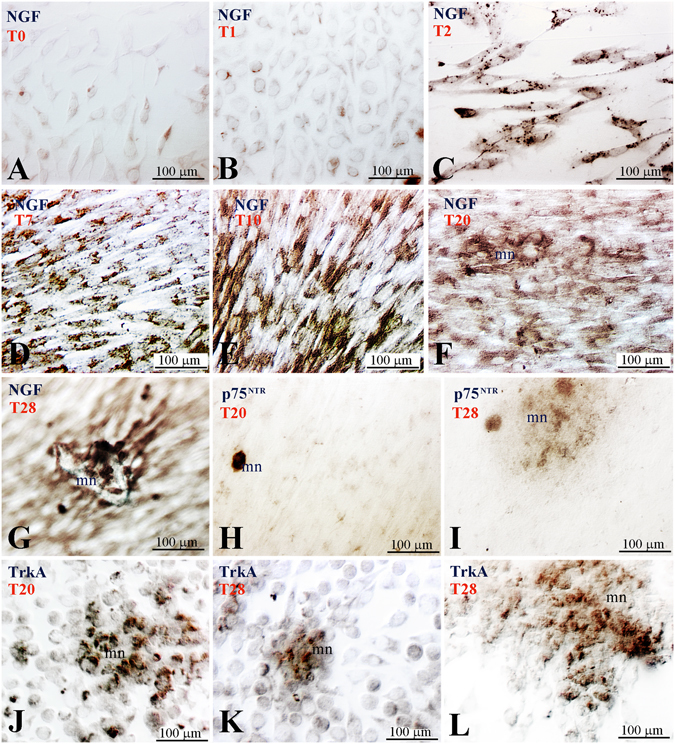
Expression of NGF and p75NTR in human pulp cells cultured in the presence of β-glycerophosphate. (A) NGF expression at time 0 (T0). (B–G) NGF expression cultured in mineralizing conditions at successive time points (T1–T28). (H,I) p75NTR expression in cells forming a mineral nodule (T20–T28). (J–L) TrkA expression in cells forming the mineral nodules (T20–T28). Abbreviation: mn, mineralized nodule; T1, one day of culture.
TGFβ1 deregulates NGF expression in human dental pulp
Based on the observation than NGF expression is upregulated in differentiating dental pulp cells, it is possible that TGFβ1 plays a role in this process, since it has been shown that TGFβ1 is involved in odontoblast differentiation31. To test this hypothesis, we followed the expression of NGF and p75NTR in presence or absence of recombinant TGFβ1 protein in thick dental explants cultured in vitro for 4 days (Fig. 6). In absence of TGFβ1, weak NGF immunoreactivity was found in the odontoblasts from the first day of culture and this expression was maintained for the whole culture period (Fig. 6A,B). The presence of TGFβ1 did not affect NGF expression in odontoblasts (Fig. 6C). In absence of TGFβ1, p75NTR immunoreactivity was detected in the odontoblasts and in nerve fibers for the whole culture period (Fig. 6D,E). Addition of TGFβ1 in the culture medium did not affect p75NTR expression in both odontoblasts and nerve fibres (Fig. 6F). However, the structure of the nerve fibers appeared disorganized and fragmented (compare Fig. 6D and E with Fig. 6F).
Figure 6.
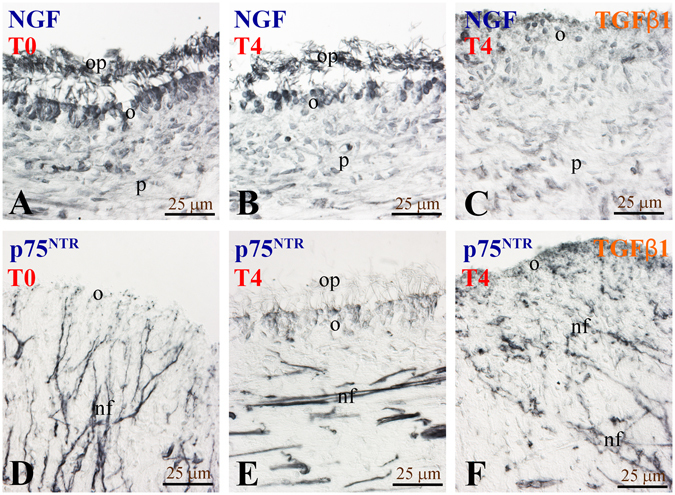
Effects of TGFβ1 on NGF and p75NTR expression in cultured tooth slices in vitro. (A) NGF expression at T0 (starting point, time 0). (B) NGF expression at T4. (C) NGF expression at T4 upon treatment with TGFβ1. (D) p75NTR expression at T0. (E) p75NTR expression at T4. (F) p75NTR expression at T4 upon treatment with TGFβ1. Abbreviations: nf, nerve fibres; o, odontoblasts; op, odontoblastic processes; p, pulp.
Administration of NGF in dental tissues induces axonal sprouting and migration of Schwann cells
Since NGF has a pivotal role in axonal guidance, growth and sprouting we wish to know whether it is able to affect neurites within dental pulp and atract them towards the injured/pathologic area where NGF upregulation was seen in vivo. For this purpose we used an in vitro human dental pulp slice model, where we applied radioactive NGF (125I-NGF) within the dentine via a propylene tube (Fig. 7A). 125I-NGF diffused through the dentineal tubules and after seven days of culture could be detected within the dental pulp (Fig. 7B). In correspondance to the region where 125I-NGF was released, we observed a significant increase in the expression of p75NTR within the dental pulp, when compared to the non treated pulp horn (Fig. 7C). Furthermore, a considerable increase of S-100 (a specific marker for Schwann cells) (Fig. 8A,B) and NFP (a general marker of nerve fibres) (Fig. 8C,D) staining was observed at the sites of 125I-NGF release. Interestingly, labelling with laminin-α2, which promotes axonal growth32, also increased at the site of NGF delivery (Fig. 8E,F).
Figure 7.
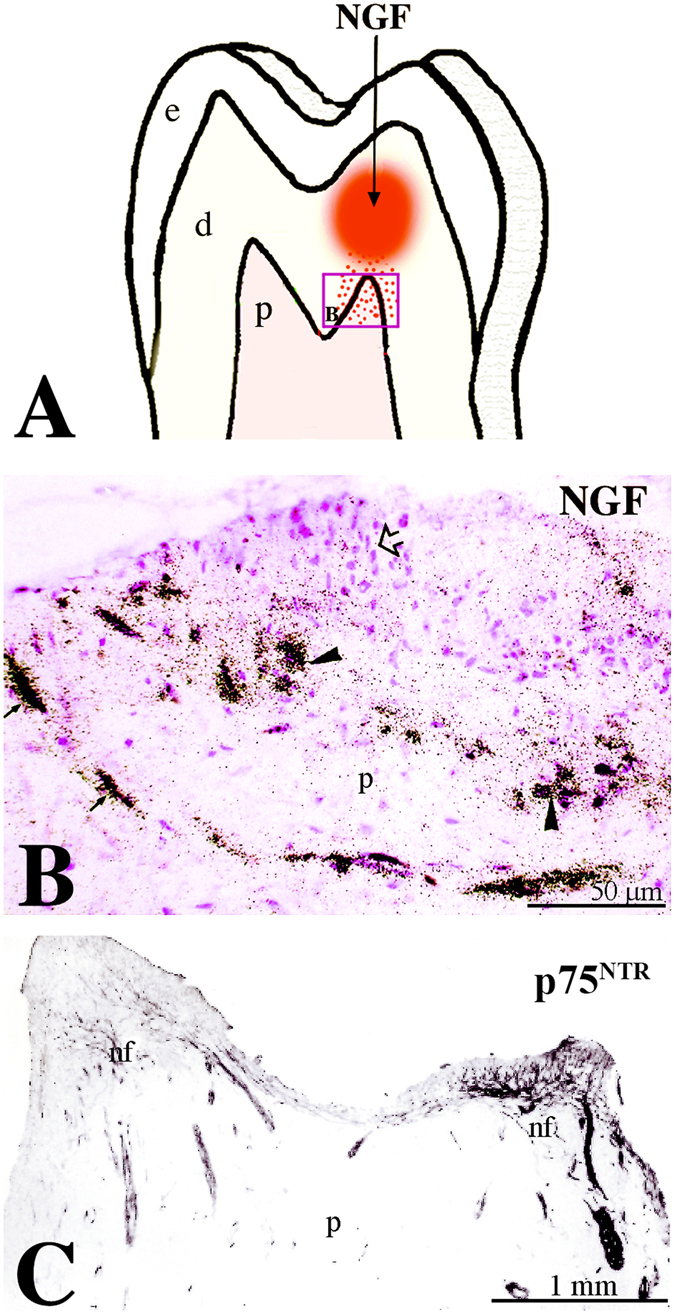
Administration of radioactive NGF (125I-NGF) induces nerve growth towards the release site. (A) Schematic representation of the experimental approach for 125I-NGF infusion. (B) Detection of 125I-NGF diffusion in the pulp. (C) p75NTR expression marking neurites growth towards the site of NGF infusion. Abbreviations: nf, nerve fibres; p, pulp.
Figure 8.
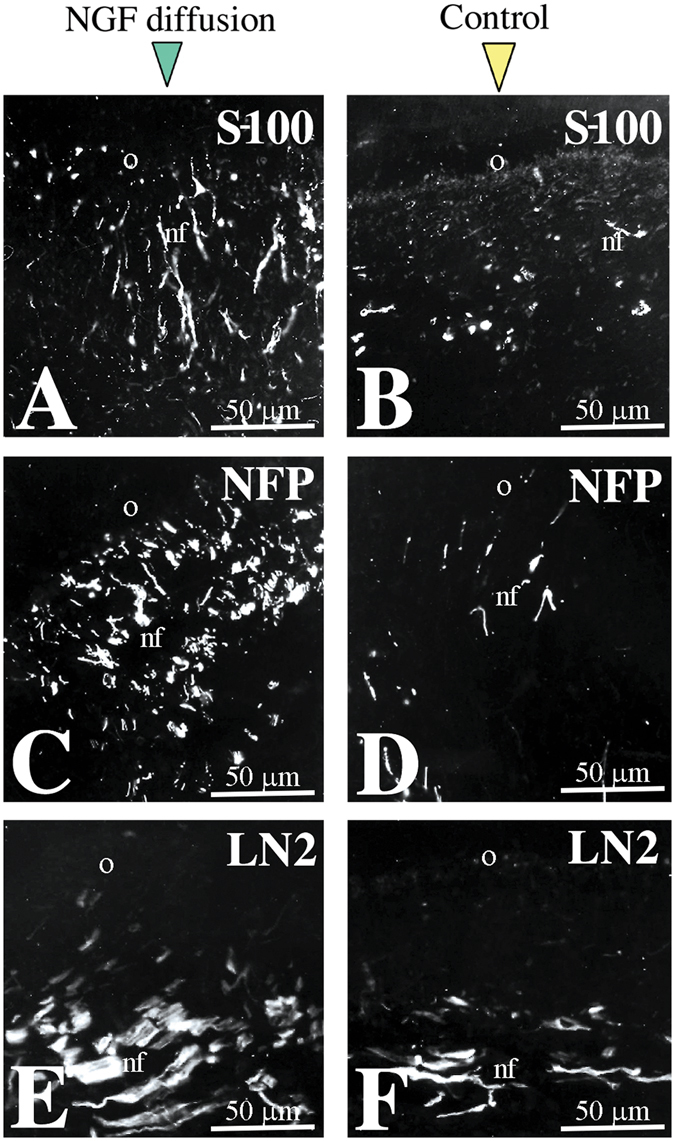
NGF administration induces growth of axons and migration of glial cells. (A,B) S-100 staining marking glial cells in NGF-treated (A) and control (B) pulps. (C,D) Neurofilament (NFP) staining marking axons in NGF-treated (C) and control (D) pulps. (E,F) Laminin-α2 (LN2) expression in NGF-treated (E) and control (F) pulps. Abbreviations: nf, nerve fibres; o, odontoblasts.
Discussion
Tooth development, homeostasis and pathology are controlled by a complex network of molecules, which guarantee tissue integrity and function26, 33. Among these molecules, neurotrophic factors are important for tooth innervation as well as for cytodifferentiation and mineralization events. Our recent studies have shown that NGF and its receptors p75NTR and TrkA are expressed in the developing human teeth24. More specifically, these proteins are distributed in differentiating odontoblasts secreting the mantle dentine matrix, illustrating a close correlation between the appearance of NGF-related molecules and the process of odontoblast differentiation. While numerous studies are undertaken in rodents to understand the role of NGF signalling in injured teeth, there are not equivalent studies in humans. Our results revealed that NGF, p75NTR and TrkA are expressed in human functional intact teeth as well as in carious and injured teeth. However, there were noticeable differences in the expression patterns of these molecules. In the pulp of healthy adult teeth, NGF is faintly expressed in functional odontoblasts, while expression of both p75NTR and TrkA in these cells is very weak. A number of dental pulp fibroblasts and cells correlated with neuronal structures express NGF, whereas p75NTR is distributed to the dense neuronal plexus of the pulp. The same expression patterns have been observed previously in rodent teeth13, 14, 20, illustrating a close correlation between NGF expression and odontoblast differentiation and function. Continuous expression of NGF by odontoblasts of permenant human teeth indicates that this molecule, in addition to its neuroattractive role, may be implicated in the processes of dentine matrix synthesis and mineralization. This hypothesis can be reinforced by the fact that NGF is strongly expressed in differentiating odontoblast-like cells that form mineralization nodules, which also express TrkA and low levels of p75NTR in cultures of human dental pulp cells in vitro.
In contrast to the previous findings in developing human teeth showing no p75NTR immunoreactivity in dental pulp22, 24, we found weak p75NTR labelling in the odontoblastic layer suggesting that odontoblasts express also the p75NTR receptor. It is equally possible that this staining may be due to the existence of small nerve endings at the odontoblastic layer that intermingle with the odontoblasts. However, the presence of p75NTR in pulp cells forming the mineralization nodules in vitro indicates that this receptor, acting together with TrkA, could be also involved in cytodifferentiation events. Further studies using electron microscopy will be needed to elucidate questions about p75NTR expression in odontoblasts.
The capacity of the pulp to resist and repair injuries is fundamental for maintaining tooth integrity and homeostasis. Although in the pulp of healthy teeth cell divisions and dentine matrix secretion by odontoblasts are reduced, these processes are stimulated in carious lesions or traumatic injuries33, 34. The cellular responses to various degrees of tooth injury are wide-ranging and target to prevent bacterial invasion into the pulp chamber, inhibit the lesion, and induce wound healing. Therefore, all the dental pulp tissue is involved in this reparative process and not only the areas neighbouring the dental pathologic territory. In mild injuries such as slow progressing caries, the transiently damaged odontoblasts do survive, their morphology remains normal and their activity is stimulated leading to the production of a tertiary dentine called reactionary dentine33, 34. In these conditions, integrity of the injured odontoblasts is ensured by increased protein synthesis that targets to the reorganization of their cytoskeleton35, which is an important process during wound healing36. For example, activation of nestin expression in odontoblasts facing the carious decay has been associated with odontoblast cytoskeletal re-organization37. NGF and TrkA are also upregulated in the odontoblasts beneath the carious front, suggesting that NGF may play a role in this process. Indeed, numerous studies have linked NGF signalling with reorganization of the cytoskeleton in a variety of neuronal and non-neuronal cell types38, inclunding odontoblasts39. Therefore, an additional function for NGF in tertiary dentine matrix synthesis and secretion should not be excluded.
Fast progressing caries and operative procedures involving the dentine lead to the apoptosis of the primary odontoblasts30, 33. Formation of tertiary dentine (also called reparative dentine) at the pathologic site results from the proliferation and recruitment of dental pulp stem cells and their differentiation into a second-generation of odontoblasts30, 40. In pathological conditions, NGF can exert a variety of effects that promote cell survival and/or apoptosis4. Expression of NGF and TrkA in both apoptotic and newly-formed odontoblasts indicates that NGF signalling may be involved in apoptotic, cytodifferentiation and secretory events after severe tooth injury. NGF and TrkA are also activated in odontoblasts far away from the injury site, indicating their interconnectivity in reparing damaged dental tissues. It is worth noting that NGF expression is also activated in severe pathologies of broad interest such as injuries of the central nervous system and tumorigenic conditions within and outside the nervous system2. In dental injuries of strong intensity, the blood vessels of the pulp dilate and inflammatory responses are triggered in order to limit pulp damage. NGF is expressed in cells related to these vascular structures, thus indicating a correlation between NGF upregulation and pulp inflammatory events, similarly to what is known in the nervous system41.
It has been shown that the TrkA has a pivotal role in the pain perception in several organs, including teeth24, 42, 43. In humans, NTRK1 mutations (the gene coding for the TRKA protein) lead to pain insensitivity44, 45. The observed upregulation of NGF and TrkA following tooth injury suggests that NGF signalling is involved in pain perception after dental injury. TrkA inhibition could be a valuable therapeutic tool to reduce dental pain perception. Indeed, NGF signalling gained great attention as targets for treatments against cancer-associated pain during the last years46.
Signalling molecules that are expressed by dental pulp cells could play a role in tooth repair events33. A variety of in vitro findings and genetic studies in mice have demonstrated the importance of Transforming Growth Factor-beta1 (TGFβ1) in dental tissue homeostasis and regeneration33, 36, 47, 48. In vitro studies using human dental tissues have shown that TGFβ1 induces proliferation and migration of pulp fibroblasts at the injury area as well as odontoblast differentiation31. Our results show that NGF is also upregulated in differentiating odontoblast-like cells in cultured human dental pulp cells. It is thus possible that NGF may interact with TGFβ1 to stimulate reparative events within the pulp.
Several studies showed that p75NTR is expressed by a number of stem cell populations of different origins2, including dental pulp stem cells49. Our results show that p75NTR is upregulated only in the sub-odontoblastic cell layer in human tooth slices after TGFβ1 administration, thus suggesting that the NTFs/p75NTR signalling regulates the fate of odontoblast progenitors. Surprisingly, the organization of nerve fibres within the pulp was disturbed in the presence of TGFβ1.
Sprouting nerves may have stimulatory roles in the healing process by delivering neuropeptides and other substances to the wounded site50. The sensory nerve endings are perceptive to signals released during dental injury, resulting in nerve sprouting at the site of the lesion. It has been already established that NGF is one of the wound-induced signals controlling neuronal regeneration and sprouting51. The occurrence of sensory nerve outgrowth in relation to pulp injury has been already described in rodent teeth18, 52. Similar effects have been observed in studies using transgenic mice overexpressing NGF53, 54 as well as on NGF-infused brain55 and skin56. Our in vitro study of NGF release through the dentinal tubules of human tooth slices clearly demonstrates a localised outgrowth and sprouting of pulp nerves towards the NGF-releasing source. However, such neuro-attractive phenomenon could not be observed in vivo after cavity preparation, most probably because of the slow process of neuronal regrowth in humans and the much lower quantities of NGF secreted by odontoblasts when compared to the higher doses of administrated NGF in vitro. Schwann cells of the pulp, which are characterised by the expression of the p75NTR and S-100 proteins, are involved in the process of nerve sprouting. These cells produce laminin-α2 that creates a favourable environment for axonal regrowth32, 57 and odontoblast differentiation58. The enrichment with Schwann cells at the site of NGF release suggests that this neurotrophic molecule might have an additional chemotrophic effect on glial cells by activating p75NTR expression, as it has been demonstrated in other models of peripheral nerve regeneration59, 60. It is tempting to hypothesize that, upon injury in human teeth, Schwann cells can be reprogrammed to form odontoblasts, as it has been recently shown for glial cells of incisors in rodents61. Taken together, the above-mentioned observations indicate that NGF signalling is important for the recruitment of specific dental pulp cell populations and their subsequent differentiation into odontoblasts upon tooth injury in humans. A possible involvement of other neurotrophic factors (i.e., BNDF, NT-3, NT-4, NT-6) in these processes has not to be excluded.
In conclusion, the present results demonstrate that NGF signalling in dental pulp is associated with odontoblast differentiation, dentine matrix synthesis and neuronal attraction, and suggest that NGF plays a crucial role in regenerative events of pathological and injured human teeth.
Materials and Methods
Collection of human pulps and teeth
All procedures were performed in accordance with the current guidelines. Cavity preparation and tooth extraction were performed by professional dentists. Experimental procedures involving the use of human specimens were approved by the Kantonale Ethikkommission of Zurich (reference number 2012-0588). All procedures were performed after obtainance of informed written consent from all patients.
Materials
Preparation, purification, and characterization of polyclonal anti-NGF antibodies have been described previously13, 20. Affinity purified mouse anti-human p75NTR monoclonal antibody (20.4) was the kind gift of Dr E.M. Johnson Jr. and Dr C. Osborn (St. Louis, USA). The purification and characterization of the 20.4 antibody, which recognizes the human p75NTR, has been already described62, 63. Preparation and characterization of the rabbit anti-NGF antiserum has been already described13. This antiserum specifically identifies NGF in immunohistochemistry and in Western blots and does not cross react with other known neurotrophins. The rabbit anti-human/mouse/rat TrkA monoclonal antibody was purchased from Abcam (ab76291 – Cambridge, UK). Murine 2.5S NGF was obtained from Promega Corportation (Madison, WI, USA). Vector Vectastain ABC kit was purchased from Biosys (Compiègne, France). For the preparation of the culture media, all materials were purchased from Gibco BRL (Life Technologies Inc., NY, USA). Other chemicals were obtained from Sigma (St. Louis, MO, USA).
For cultures, Minimum Essential Medium (MEM) was supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 UI/mL penicillin, 100 µg/mL streptomycin (Biowhittaker, Gagny, France), and 0.25 µg/mL amphotericin B (Fungizone®).
Tissue preparation
Freshly extracted teeth used in this study comprised: a) ten premolars of 11–15 years old adolescents, b) ten intact and ten carious teeth (molars) of 40–45 years old patients.
For studies on injured dental tissues, cavities were prepared in intact first premolars scheduled for extraction at the Dental Care Center of Marseille. The pulp chambers were not exposed during the preparation of the cavities. The teeth were extracted after a post-operative interval of 9 weeks with the patients informed consent. Cavities were prepared in the flank of the crown (Fig. 3A) first by means of an intermittent application of an Airotor with water coolant to remove the enamel. Cavities 2–3 mm wide and 1.3–1.8 mm deep were then cut into the tooth dentine with a bur using the least possible pressure. The walls of the cavities were immediately conditioned with a 3% hydrogen peroxide solution and dried with a light stream. The cavities were then restored with the calcium hydroxide product Dycal (Dentsplay, USA).
Extracted teeth were fixed in 10% neutral-buffered formalin for 24 hours, demineralized in sodium formiate for 21 days, and then embedded in paraffin wax. Thereafter teeth were serially sectioned (6 µm thick sections) and processed for immunohistochemistry. For the detection of extremely low levels of NGF we proceeded with immediate pulp extraction from intact healthy teeth, followed by 10% neutral-buffered formalin fixation for 24 hours and embedded in paraffin without prior decalcification.
Cultures of human dental pulp cells
Immediately after extraction of third molars from 17 years old patients, teeth were swabbed with 70% (v/v) alcohol. Teeth were then washed with sterile PBS and transferred into a laminar flow tissue culture hood in order to perform the rest of the procedures under sterile conditions. Dental pulps were gently removed with forceps, minced with scalpels, and rinsed with PBS. After mincing, each tooth pulp explant was cultured in 100-mm diameter culture dishes (Becton Dickinson Labware, Lincoln Park, NJ, USA) in MEM medium supplemented with 2 mM ß-glycerophosphate (Sigma Chemical Co., St Louis, USA). The explants were cultured at 37 °C in a humidified atmosphere of 5% CO2, 95% air and the culture medium was changed every other day. Confluent cultures were collected by trypsinization (0.2% trypsin and 0.02% EDTA). The cells were plated at 3 × 103/cm2 on tissue culture treated 8-chambers glass slides (Becton Dickinson Labware, Lincoln Park, NJ, USA). After 4 weeks of culture, cells were fixed with 70% ethanol for one hour at 4 °C and processed for immunohistochemistry.
Cultures of human dental pulp stem cells
Human dental pulp stem cells (hDPSCs) were isolated from the dental pulp of extracted impacted wisdom teeth of healthy patients as previously described64. The dental pulps were enzymatically digested for one hour at 37 °C in a solution of collagenase (3 mg/mL; Life Technologies Eυρoπε BV, Zug ZG, Switzerland) and dispase (4 mg/mL; Sigma-Aldrich Chemie GmbH, Buchs SG, Switzerland). A filtered single-cell suspension was plated in a 40 mm Petri dish with hDPSC growth medium containing DMEM/F12 (Sigma-Aldrich Chemie GmbH, Buchs SG, Switzerland) with 10% fetal bovine serum (FBS) (PAN Biotech GmbH, Aidenbach, Germany), 1% penicillin/streptomycin (P/S) (Sigma-Aldrich Chemie GmbH, Buchs SG, Switzerland), 1% L-glutamine (Sigma-Aldrich Chemie GmbH, Buchs SG, Switzerland), and 0.5 μg/ml fungizone (Life Technologies Europe BV, Zug ZG, Switzerland) after washing away the enzyme solution. Cells were passaged at 80–90% confluence64. Osteogenic, chondrogenic and adipogenic differentiation potential of hDPSCs was tested via standard differentiation protocols65.
In vitro cultures using human tooth slices
Freshly extracted third molars were sliced in 750 µm thick sections as described previously66. Selected slices were washed and the dentine carefully dried by means of a sterile paper. A small propylene tube was glued to the dentine to allow diffusion of the factor-containing solution through the dentineal tubules as described previosly67. Tubes were filled only once with either 50 µL serum free medium containing NGF (50 ng/mL) or TGFβ1 (5 ng/mL) and the slices were cultured from 4 (for TGFβ1 administration) to 7 (for NGF administration) days. After culture, thick slices were rinsed in PBS, fixed in 4% paraformaldehyde (PFA)-PBS solution for 30 min. Some of the dental slices were equilibrated with 30% sucrose-PBS at 4 °C overnight, and then were embedded in Tissue-Tek OCT (Miles Lab., Elkhart, USA) and frozen in a liquid nitrogen/isobutane bath. 14 micrometer cryostat sections were mounted on slides and stored at −20 °C until staining.
Immunostainings
Immunoperoxidase staining on sections was performed as previously described13, 20. Briefly, the sections were first deparaffinized, treated with 0.4% pepsin, exposed to a 0.3% solution of hydrogen peroxide in methanol, and then were incubated overnight at 4 °C with primary antibodies diluted in PBS/0.2% BSA. The mouse anti-human S-100 and p75NTR primary antibodies (Boehringer Mannheim, Germany) were used at dilution 1:20 and 1:40 respectively. The polyclonal anti-NGF antibody was used at dilution 1:300, the monoclonal antibody 20.4 was diluted 1:200 to 1:1000 and the anti-TrkA antibody was diluted 1:70. Mouse anti-neurofilament proteins (NFP; Zymed labs, San Francisco, CA, USA) and anti-human laminin-α2 (merosin) (Chemicon International, Temecula, CA, USA) were diluted 1:50 and 1:2500, respectively. Peroxidase was revealed by incubation with 3.3-diaminobenzidine tetrahydrochloride (DAB) reaction solution. After staining the sections were mounted with Eukit. In control sections the primary antibodies were ommited.
For immunohistochemistry on cultured dental pulp cells, the incubation with primary antibodies was performed overnight at 4 °C after permeabilizion of cells for 15 min with 0.5% Triton X-100 in PBS. Peroxidase was revealed by incubation with 3-amino-9-ethylcarbazole (AEC) reaction solution and then the slides were mounted with Aquamount (BDH, Gurr, England). Controls were performed by incubations with unrelated primary antibodies.
For immunohistochemistry on thick slices, after blocking with 10% normal goat serum (NGS) in PBS for 30 min, slides were incubated in primary antibodies diluted in PBS overnight at room temperature, then extensively washed in PBS and incubated with goat anti-rabbit or sheep anti-mouse-IgG peroxidase conjugate diluted 1:50 (Sanofi Diagnostics Pasteur, France) and routinely subjected to the DAB (3-3′diaminobenzidine) procedure.
For immunofluorescence identification of S-100, NFP and laminin-α2 antigens, cryosections were incubated with primary antibodies and counterstained with a 1:70 dilution of anti-rabbit IgG FITC conjugate (Sanofi Diagnostics Pasteur, France). Thereafter sections were washed extensively and sealed with glycerol-phosphate buffered saline before examination with a Zeiss microscope. Negative controls were carried out with conjugates alone in place of the primary antibodies, the other steps remaining unchanged. For immunofluorescence identification of NGF and p75NTR antigens on hDPSCs, cells were fixed in 4% PFA, washed extensively with PBS, blocked with PBS/2% BSA/0.5% Tween-20 and then incubated for 2 hours at room temperature with primary antibodies (polyclonal rabbit anti-NGF 1:100, monoclonal anti-p75NTR 1:100, monoclonal anti-vimentin 1:300, monoclonal anti-TrkA 1:70) diluted in PBS/2% BSA/0.5% Tween-20. Samples were then washed extensively and incubated with secondary antibodies (alexa 488-conjugated anti-rabbit 1:250, alexa 568-conjugated anti-mouse 1:250; Life Technologies/Invitrogen) diluted in blocking buffer for 2 hours at room temperature. Cells were then counterstained with DAPI and mounted with ProLong© Diamond Anti-Fade Mountant (Thermo Fischer).
Light microscopic autoradiography
Thick slices cultured under NGF tube diffusion were washed and incubated in serum free medium supplemented with 50 ng/mL 125I-NGF (specific activity 1320 Ci/mmol; final concentration 10 mCi/mL; Amersham, Buckinghamshire, England) for 2 hours at 37 °C. The specificity of the iodinated NGF binding was established by adding 50 μg/mL of unlabelled NGF. After being rinsed with cold culture medium, the slices were fixed in a 4% PFA solution for 48 hours, washed in PBS, osmicated, dehydrated in ethanol and embedded in Epon 812. Five µm sections were cut, coated with a monolayer of LM-1 emulsion (Amersham) and exposed for one month at 4 °C. They were developed with Kodak D-19 developer for 4 min, counterstained with 0.1% safranin, dehydrated and mounted in Eukitt.
Acknowledgements
This work was supported by institutional grants from the University of Zurich.
Author Contributions
T.A.M.: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript, financial support. H.M.: Data analysis and interpretation of the in vitro studies, critical reading and editing of the manuscript, final approval of manuscript. P.P.: Conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2006;361:1545–64. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomellini E, Lagadec C, Polakowska R, Le Bourhis X. Role of p75 neurotrophin receptor in stem cell biology: More than just a marker. Cell. Mol. Life Sci. 2014;71:2467–2481. doi: 10.1007/s00018-014-1564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levi-Montalcini, R. The Nerve Growth Factor 35 Years Later. 237, 1154–1162 (1987). [DOI] [PubMed]
- 4.Lewis, G. R. & Carter, B. D. Neurotrophic factors. Endeavour9 (2014).
- 5.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 6.Ibáñez CF, Simi A. P75 neurotrophin receptor signaling in nervous system injury and degeneration: paradox and opportunity. Trends Neurosci. 2012;35:431–440. doi: 10.1016/j.tins.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti M, Levi a, Chao MV. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc. Natl. Acad. Sci. USA. 1993;90:7859–7863. doi: 10.1073/pnas.90.16.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibel M, Hoppe E, Barde Y. Biochemical and functional interactions between the neurotrophin receptors trk and p75 NTR. EMBO J. 1999;18:616–622. doi: 10.1093/emboj/18.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesa PG, Rettig WJ, Thomson TM, Old LJ, Melamed MR. Immunohistochemical analysis of nerve growth factor receptor expression in normal and malignant human tissues. J. Histochem. Cytochem. 1988;36:383–389. doi: 10.1177/36.4.2831267. [DOI] [PubMed] [Google Scholar]
- 10.Yan Q, Johnson EM. An immunohistochemical study of the nerve growth factor receptor in developing rats. J. Neurosci. 1988;8:3481–98. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Represa J, Bernd P. Nerve growth factor and serum differentially regulate development of the embryonic otic vesicle and cochleovestibular ganglion in vitro. Dev. Biol. 1989;134:21–29. doi: 10.1016/0012-1606(89)90074-2. [DOI] [PubMed] [Google Scholar]
- 12.von Bartheld CS, et al. Expression of nerve growth factor (NGF) receptors in the developing inner ear of chick and rat. Development. 1991;113:455–470. doi: 10.1242/dev.113.2.455. [DOI] [PubMed] [Google Scholar]
- 13.Mitsiadis TA, Dicou E, Joffre A, Magloire H. Immunohistochemical localization of nerve growth factor (NGF) and NGF receptor (NGF-R) in the developing first molar tooth of the rat. Differentiation. 1992;49:47–61. doi: 10.1111/j.1432-0436.1992.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 14.Mitsiadis TA, Luukko K. Neurotrophins in odontogenesis. Int. J. Dev. Biol. 1995;39:195–202. [PubMed] [Google Scholar]
- 15.Ernfors P, Merlio J-P, Persson H. Cells Expressing mRNA for Neurotrophins and their Receptors During Embryonic Rat Development. Eur. J. Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 16.Tomellini E, et al. Nerve growth factor and prongf simultaneously promote symmetric self-renewal, quiescence, and epithelial to mesenchymal transition to enlarge the breast cancer stem cell compartment. Stem Cells. 2015;33:342–353. doi: 10.1002/stem.1849. [DOI] [PubMed] [Google Scholar]
- 17.Lee FS, Kim aH, Khursigara G, Chao MV. The uniqueness of being a neurotrophin receptor. Curr. Opin. Neurobiol. 2001;11:281–286. doi: 10.1016/S0959-4388(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 18.Byers MR, Schatteman GC, Bothwell M. Multiple functions for NGF receptor in developing, aging and injured rat teeth are suggested by epithelial, mesenchymal and neural immunoreactivity. Development. 1990;109:461–71. doi: 10.1242/dev.109.2.461. [DOI] [PubMed] [Google Scholar]
- 19.Byers MR. Segregation of NGF receptor in sensory receptors, nerves and local cells of teeth and periodontium demonstrated by EM immunocytochemistry. J. Neurocytol. 1990;19:765–75. doi: 10.1007/BF01188044. [DOI] [PubMed] [Google Scholar]
- 20.Mitsiadis TA, Couble P, Dicou E, Rudkin BB, Magloire H. Patterns of nerve growth factor (NGF), proNGF, and p75 NGF receptor expression in the rat incisor: comparison with expression in the molar. Differentiation. 1993;54:161–175. doi: 10.1111/j.1432-0436.1993.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 21.Luukko K, et al. Neurotrophin mRNA expression in the developing tooth suggests multiple roles in innervation and organogenesis. Dev. Dyn. 1997;210:117–29. doi: 10.1002/(SICI)1097-0177(199710)210:2<117::AID-AJA5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 22.Christensen LR, Møllgård K, Kjaer I, Janas MS. Immunocytochemical demonstration of nerve growth factor receptor (NGF-R) in developing human fetal teeth. Anat. Embryol. (Berl) 1993;188:247–55. doi: 10.1007/BF00188216. [DOI] [PubMed] [Google Scholar]
- 23.Nosrat I, Seiger A, Olson L. & Nosrat, C. a. Expression patterns of neurotrophic factor mRNAs in developing human teeth. Cell Tissue Res. 2002;310:177–187. doi: 10.1007/s00441-002-0618-8. [DOI] [PubMed] [Google Scholar]
- 24.Mitsiadis TA, Pagella P. Expression of Nerve Growth Factor (NGF), TrkA, and p75NTR in Developing Human Fetal Teeth. Front. Physiol. 2016;7:338. doi: 10.3389/fphys.2016.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsiadis TA, Luder HU. Genetic basis for tooth malformations: from mice to men and back again. Clin. Genet. 2011;80:319–29. doi: 10.1111/j.1399-0004.2011.01762.x. [DOI] [PubMed] [Google Scholar]
- 26.Mitsiadis TA, Graf D. Cell fate determination during tooth development and regeneration. Birth Defects Res. C. Embryo Today. 2009;87:199–211. doi: 10.1002/bdrc.20160. [DOI] [PubMed] [Google Scholar]
- 27.Nosrat IV, Widenfalk J, Olson L, Nosrat CA. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev. Biol. 2001;238:120–32. doi: 10.1006/dbio.2001.0400. [DOI] [PubMed] [Google Scholar]
- 28.Asaumi K, Nakanishi T, Asahara H, Inoue H, Takigawa M. Expression of neurotrophins and their receptors (TRK) during fracture healing. Bone. 2000;26:625–633. doi: 10.1016/S8756-3282(00)00281-7. [DOI] [PubMed] [Google Scholar]
- 29.Byun J-H, Lee J-H, Choi Y-J, Kim J-R, Park B-W. Co-expression of nerve growth factor and p75NGFR in the inferior alveolar nerve after mandibular distraction osteogenesis. Int. J. Oral Maxillofac. Surg. 2008;37:467–72. doi: 10.1016/j.ijom.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Mitsiadis TA, De Bari C, About I. Apoptosis in developmental and repair-related human tooth remodeling: a view from the inside. Exp. Cell Res. 2008;314:869–77. doi: 10.1016/j.yexcr.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, et al. Odontoblast-like cell differentiation and dentin formation induced with TGF-β1. Arch. Oral Biol. 2011;56:1221–1229. doi: 10.1016/j.archoralbio.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Leivo I, Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc. Natl. Acad. Sci. USA. 1988;85:1544–8. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitsiadis TA, Rahiotis C. Parallels between tooth development and repair: conserved molecular mechanisms following carious and dental injury. J. Dent. Res. 2004;83:896–902. doi: 10.1177/154405910408301202. [DOI] [PubMed] [Google Scholar]
- 34.About I, Mitsiadis TA. Molecular aspects of tooth pathogenesis and repair: in vivo and in vitro models. Adv. Dent. Res. 2001;15:59–62. doi: 10.1177/08959374010150011501. [DOI] [PubMed] [Google Scholar]
- 35.Magloire H, Bouvier M, Joffre A. Odontoblast response under carious lesions. Proc. Finnish Dent. Soc. Suom. Hammaslääkäriseuran Toim. 1992;88(Suppl 1):257–74. [PubMed] [Google Scholar]
- 36.Iwata M, et al. Zinc accumulation and metallothionein gene expression in the proliferating epidermis during wound healing in mouse skin. Histochem. Cell Biol. 1999;112:283–290. doi: 10.1007/s004180050449. [DOI] [PubMed] [Google Scholar]
- 37.About I, Laurent-maquin D, Lendahl U, Mitsiadis TA. Nestin Expression in Embryonic and Adult Human Teeth under Normal and Pathological Conditions. Am. J. Pathol. 2000;157:287–295. doi: 10.1016/S0002-9440(10)64539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niewiadomska G, Mietelska-Porowska A, Mazurkiewicz M. The cholinergic system, nerve growth factor and the cytoskeleton. Behav. Brain Res. 2011;221:515–526. doi: 10.1016/j.bbr.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 39.Magloire H, Romeas A, Melin M, Bleicher F. Molecular Regulation of Odontoblast Activity under Dentin Injury. Adv. Dent. Res. 2001;15:46–50. doi: 10.1177/08959374010150011201. [DOI] [PubMed] [Google Scholar]
- 40.Mitsiadis TA, Feki A, Papaccio G, Catón J. Dental pulp stem cells, niches, and notch signaling in tooth injury. Adv. Dent. Res. 2011;23:275–9. doi: 10.1177/0022034511405386. [DOI] [PubMed] [Google Scholar]
- 41.Levi-Montalcini R, Skaper SD, Dal Toso R, Petrelli L, Leon A. Nerve growth factor: From neurotrophin to neurokine. Trends Neurosci. 1996;19:514–520. doi: 10.1016/S0166-2236(96)10058-8. [DOI] [PubMed] [Google Scholar]
- 42.Kumar V, Mahal BA. NGF – the TrkA to successful pain treatment. J. Pain Res. 2012;5:279–287. doi: 10.2147/JPR.S33408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ugolini G, Marinelli S, Covaceuszach S, Cattaneo A, Pavone F. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc. Natl. Acad. Sci. USA. 2006;104:2985–2990. doi: 10.1073/pnas.0611253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao L, et al. Oral and Craniofacial Manifestations and Two Novel Missense Mutations of the NTRK1 Gene Identified in the Patient with Congenital Insensitivity to Pain with Anhidrosis. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0066863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonkowsky JL, Johnson J, Carey JC, Smith AG, Swoboda KJ. An infant with primary tooth loss and palmar hyperkeratosis: a novel mutation in the NTRK1 gene causing congenital insensitivity to pain with anhidrosis. Pediatrics. 2003;112:e237–e241. doi: 10.1542/peds.112.3.e237. [DOI] [PubMed] [Google Scholar]
- 46.Demir IE, Tieftrunk E, Schorn S, Friess H, Ceyhan GO. Nerve growth factor & TrkA as novel therapeutic targets in cancer. Biochim. Biophys. Acta - Rev. Cancer. 2016;1866:37–50. doi: 10.1016/j.bbcan.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 47.D’Souza RN, Cavender A, Dickinson D, Roberts A, Letterio J. TGF-beta1 is essential for the homeostasis of the dentin-pulp complex. Eur. J. Oral Sci. 1998;106(Suppl):185–191. doi: 10.1111/j.1600-0722.1998.tb02174.x. [DOI] [PubMed] [Google Scholar]
- 48.Farges JC, et al. TGF-beta 1 Induces Accumulation of Dendritic Cells in the Odontoblast Layer. J. Dent. Res. 2003;82:652–656. doi: 10.1177/154405910308200816. [DOI] [PubMed] [Google Scholar]
- 49.Martens W, et al. Expression pattern of basal markers in human dental pulp stem cells and tissue. Cells Tissues Organs. 2012;196:490–500. doi: 10.1159/000338654. [DOI] [PubMed] [Google Scholar]
- 50.Pagella P, Jiménez-Rojo L, Mitsiadis TA. Roles of innervation in developing and regenerating orofacial tissues. Cell. Mol. Life Sci. 2014;71:2241–2251. doi: 10.1007/s00018-013-1549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derby A, et al. Nerve Growth Factor Facilitates Regeneration across Nerve Gaps: Morphological and Behavioural Studies in Rat Sciatic Nerve. Exp. Neurol. 1993;119:176–191. doi: 10.1006/exnr.1993.1019. [DOI] [PubMed] [Google Scholar]
- 52.Hildebrand C, Fried K, Tuisku F, Johansson CS. Teeth and tooth nerves. Progesses Neurobiol. 1995;45:165–222. doi: 10.1016/0301-0082(94)00045-J. [DOI] [PubMed] [Google Scholar]
- 53.Kawaja MD, Crutcher Ka. Sympathetic axons invade the brains of mice overexpressing nerve growth factor. J. Comp. Neurol. 1997;383:60–72. doi: 10.1002/(SICI)1096-9861(19970623)383:1<60::AID-CNE5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 54.Ramirez JJ, et al. Adeno-Associated Virus Vector Expressing Nerve Growth Factor Enhances Cholinergic Axonal Sprouting after Cortical Injury in Rats. J. Neurosci. 2003;23:2797–2803. doi: 10.1523/JNEUROSCI.23-07-02797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winkler J, et al. Reversible Schwann cell hyperplasia and sprouting of sensory and sympathetic neurites after intraventricular administration of nerve growth factor. Ann. Neurol. 1997;41:82–93. doi: 10.1002/ana.410410114. [DOI] [PubMed] [Google Scholar]
- 56.Diamond J, Holmes M, Coughlin M. Endogenous NGF and nerve impulses regulate the collateral sprouting of sensory axons in the skin of the adult rat. J. Neurosci. 1992;12:1454–1466. doi: 10.1523/JNEUROSCI.12-04-01454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mirsky R, et al. Schwann cells as regulators of nerve development. J. Physiol. Paris. 2002;96:17–24. doi: 10.1016/S0928-4257(01)00076-6. [DOI] [PubMed] [Google Scholar]
- 58.Yuasa K, et al. Laminin alpha 2 Is Essential for Odontoblast Differentiation Regulating Dentin Sialoprotein Expression. J. Biol. Chem. 2004;279:10286–10292. doi: 10.1074/jbc.M310013200. [DOI] [PubMed] [Google Scholar]
- 59.Anton ES, Weskamp G, Reichardt LF, Matthew WD. Nerve growth factor and its low-affinity receptor promote Schwann cell migration. Proc. Natl. Acad. Sci. USA. 1994;91:2795–2799. doi: 10.1073/pnas.91.7.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torigoe K, Tanaka HF, Takahashi A, Awaya A, Hashimoto K. Basic behavior of migratory Schwann cells in peripheral nerve regeneration. Exp. Neurol. 1996;137:301–308. doi: 10.1006/exnr.1996.0030. [DOI] [PubMed] [Google Scholar]
- 61.Kaukua N, et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551–4. doi: 10.1038/nature13536. [DOI] [PubMed] [Google Scholar]
- 62.Grob PM, Ross aH, Koprowski H, Bothwell M. Characterization of the human melanoma nerve growth factor receptor. J. Biol. Chem. 1985;260:8044–8049. [PubMed] [Google Scholar]
- 63.Ross AH, et al. Characterization of nerve growth factor receptor in neural crest tumors using monoclonal antibodies Biochemistry. Biochemistry. 1984;81:6681–6685. doi: 10.1073/pnas.81.21.6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woloszyk A, Buschmann J, Waschkies C, Stadlinger B, Mitsiadis TA. Human Dental Pulp Stem Cells and Gingival Fibroblasts Seeded into Silk Fibroin Scaffolds Have the Same Ability in Attracting Vessels. Front. Physiol. 2016;7:1–7. doi: 10.3389/fphys.2016.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tirino V, Paino F, Rosa A, De Papaccio G. Identification, isolation, characterization, and banking of human dental pulp stem cells. Stem Cells. 2012;879:443–463. doi: 10.1007/978-1-61779-815-3_26. [DOI] [PubMed] [Google Scholar]
- 66.Magloire H, Joffre A, Bleicher F. An in vitro model of human dental pulp repair. J. Dent. Res. 1996;75:1971–8. doi: 10.1177/00220345960750120901. [DOI] [PubMed] [Google Scholar]
- 67.Melin M, et al. Effects of TGFbeta1 on dental pulp cells in cultured human tooth slices. J. Dent. Res. 2000;79:1689–1696. doi: 10.1177/00220345000790090901. [DOI] [PubMed] [Google Scholar]


