Abstract
Purpose of review
High dietary salt intake is detrimental in hypertensive and/or salt sensitive individuals, however there are a large number of normotensive salt resistant individuals for whom dietary salt may also be harmful as a result of salt's blood pressure independent effects. This review will focus on the growing evidence that salt has adverse effects on the vasculature independent of blood pressure.
Recent findings
Data from both animal and human studies provide evidence that salt impairs endothelial function and increases arterial stiffness independent of blood pressure. High dietary salt results in oxidative stress and increased endothelial cell stiffness which impair endothelial function whereas transforming growth factor beta promotes increased arterial stiffness in the presence of endothelial dysfunction.
Summary
Health Policies and most clinical research are focused on the adverse effects of dietary salt on blood pressure however there is an increasing body of evidence to support a deleterious effect of dietary salt on endothelial function and arterial stiffness independent of blood pressure. Endothelial dysfunction and increased arterial stiffness are predictors of cardiovascular disease, therefore reducing excess dietary salt should be considered important for overall vascular health in addition to blood pressure.
Keywords: salt, sodium, endothelial function, arterial stiffness
Introduction
The average intake of sodium for most Americans exceeds 3200 mg per day (1) and is considered an important factor in the development of hypertension (2, 3). Approximately 1 out of 3 US adults are hypertensive and the prevalence of hypertension increases with age (4). Dietary sodium restriction is considered an important lifestyle modification for individuals with hypertension (5). Many organizations provide information on the benefits of reducing sodium in the diet including the National Institutes of Health, Centers for Disease Control, American Heart Association, and the World Health Organization.
Salt sensitivity of blood pressure (BP) can be described as an increase in BP going from a low salt to a high salt diet. Although there are many ways in which salt sensitivity has been determined and defined, research has demonstrated that older adults are more salt sensitive than younger adults and African Americans are more salt sensitive than Caucasians (6, 7). Further, both normotensive and hypertensive individuals can be salt sensitive (8). Indeed, salt sensitivity in normotensive individuals predicts future hypertension (9) and salt sensitive BP is associated with increased mortality in both normotensive and hypertensive adults (8, 9).
Clearly there is a large proportion of the population for which high levels of dietary consumption of sodium is detrimental (i.e. hypertension and/or salt sensitive individuals). However, the majority of younger individuals are normotensive and salt resistant which raises the question as to whether dietary sodium consumption matters in these individuals. The Trials of Hypertension suggests that sodium may have detrimental effects beyond its effect on BP. In these studies sodium reduction reduced long-term risk of cardiovascular events with very little change in BP (SBP -1.7 mmHg, DBP -0.8 mmHg) (10). The appreciation that sodium may have detrimental effects in addition to raising BP is not new (11, 12) but there has been a recent increased interest on the vascular effects of sodium apart from BP (12). This review will focus on the BP independent effects of sodium on the vasculature that is particularly relevant given the current debate over recommended levels of dietary sodium consumption. The purpose of this review however is not to discuss the appropriate level of sodium consumption but the deleterious vascular effects of high salt.
Dietary Salt and Endothelial Function
The endothelium has been the focus of much research since it was shown that it was more than a simple barrier and plays a critical role in mediating vascular relaxation (13). The most important and studied endothelial derived vasodilator is nitric oxide (NO). In addition to vasodilation, NO has anti-atherogenic properties that include inhibition of: platelet adhesion and aggregation, leukocyte adhesion and migration, smooth muscle cell proliferation, and inflammation. It is now recognized that impaired endothelial function is a primary event in the development of atherosclerosis. Endothelial dysfunction has been linked to the pathogenesis of atherosclerosis and acute cardiovascular events, occurs early before angiographic evidence of disease (14), and has been shown to be a predictor of future cardiovascular events in patients with coronary artery disease (15, 16) and in a population-based study of adults (17). Endothelial function is impaired in cardiovascular disease (15, 16) as well as by the presence of cardiovascular risk factors such as diabetes (18) and aging (19). Since endothelial dysfunction is predictive of cardiovascular events, an understanding of factors that may contribute to reduced endothelial function – such as dietary salt – is important. Hypertension is associated with reduced endothelial function (20) so elucidating any effect of salt on the endothelium independent of BP is essential as it may shift our understanding to where dietary salt is no longer linked solely to BP, but rather, to vascular health, which has potentially significant Public Health implications.
Basal synthesis of NO is increased by short term high salt intake that is important in facilitating sodium excretion (21, 22) but there is evidence that stimulated NO synthesis is reduced by high salt intake that likely has implications for local blood flow regulation (12). Rodent studies have provided evidence that sodium impairs endothelial function without alterations in BP. Deleterious changes in the vasculature from excess salt have been documented in spontaneously hypertensive rats, normotensive Wistar-Kyoto rats, and Sprague-Dawley rats, in which BP is unaltered during the early stages of exposure to a high salt diet (23-25). A high salt diet impairs aortic and mesenteric endothelial function in Sprague-Dawley rats without altering resting BP (26-28). Similar findings have been observed in mice (29).
Data from human studies support the aforementioned work done in rodents. A reduced vasoconstrictor response to L-NMMA (a competitive inhibitor of endothelial NO synthase) after 5 days of high dietary salt was reported in healthy, normotensive men indicating reduced basal levels of endothelial-derived NO (30). Additionally, impairments in endothelial function, assessed via acetylcholine (Ach)-induced increases in forearm blood flow, were also observed indicating reduced stimulated NO production (30). However, increases in systolic BP during acute salt loading were also observed (30) making it difficult to separate any direct detrimental effects of salt intake on endothelial function from the effects on BP. In order to investigate the effects of high dietary salt intake on endothelial function independent of BP we studied normotensive salt resistant individuals in a randomized controlled feeding studies in which subjects consumed 7 days each of a high and low salt diet which was provided to them. For the purposes of these studies salt resistance was defined as a change in 24-hour MAP ≤ 5 mmHg between low and high salt diets. Seven days of the high salt diet resulted in reduced brachial artery flow mediated dilation (FMD) (31), the most common and widely accepted non-invasive measure of conduit artery endothelial dependent dilation in humans (32). Additionally, high salt reduced cutaneous vasodilation in response to local heating, a measure of microvascular function (33) which is largely an endothelial derived NO mediated response (34, 35). Taken together these studies demonstrate that dietary salt loading impairs endothelial function independent of BP in healthy, normotensive adults.
Data from populations at higher risk for cardiovascular disease also provide evidence for a deleterious effect of salt on endothelial function. Brachial artery FMD has been shown to be greater in overweight and obese normotensive adults following 2 weeks on a low salt diet compared to 2 weeks of a usual salt diet in a randomized crossover study (36). BP also decreased in this study therefore the beneficial effects of salt reduction on FMD cannot be separated from the effects of lower BP, however more recently Dickinson and colleagues reported a modest reduction of dietary salt (3g/day) from a usual salt diet for 6 weeks resulted in improved FMD in normotensive overweight and obese individuals independent of BP (37). Cross-sectional data from middle-aged and older adults with elevated systolic BP suggests that habitual low salt intake is associated with higher FMD (38). Jablonski et al (39) followed up on these findings and reported that dietary sodium restriction in middle age and older adults with moderately elevated systolic BP (130-159 mmHg) improved both brachial artery FMD and the forearm blood flow response to Ach. Systolic BP was reduced by ∼12 mmHg in this study however the improvement in endothelial function remained after correction for systolic BP suggesting the improvement in endothelial function occurred beyond the effect of lower BP.
The studies described above were composed of both men and women however there is evidence that sex differences may exist in the vascular response to dietary salt. In a study of two groups of men and women who followed either a low salt or high salt diet for 5 days, the NO component of the forearm blood flow response to Ach was reduced in the group of men who consumed the high salt diet compared to the group that consumed the low salt diet whereas there was no difference between the 2 groups of women (40). More recently, 7 days of high salt reduced FMD in both normotensive salt resistant men and women however FMD was lower in the men compared to women on the high salt diet (41). These two studies suggest a potentially greater sensitivity of the vasculature to high salt in men.
Potential Mechanisms of Reduced Endothelial Function by High Salt
Oxidative stress, an imbalance between reactive oxygen species (ROS) production and scavenging is widely thought to contribute to the development of cardiovascular disease and an increase in ROS generation is one mechanism by which a high salt diet appears to impair endothelial function. A well accepted mechanism for reduced NO bioavailability is the rapid reaction of NO with elevated levels of superoxide (Figure 1). A number of rodent studies have demonstrated that superoxide levels are higher in resistance arteries (26-28) and venules (26, 27) on a high salt diet. Indeed, rodent studies have reported that the decline in stimulated NO during dietary salt loading result from an increase in ROS (27, 28). Ach-induced endothelium-dependent dilation was reduced in normotensive rats fed a high-salt diet compared with rats fed a low-salt diet and exposure to ROS scavengers improved arteriolar responsiveness to Ach (27). In humans, local infusion of ascorbic acid reversed the impaired cutaneous vasodilation to local heat observed following 7 days of high salt by increasing the NO component of vasodilation (33). In middle age and older adults with elevated SBP on a normal sodium diet, ascorbic acid improved both conduit and resistance vessel endothelial function but this effect was abolished by dietary sodium restriction (39). In addition to scavenging NO, ROS may lead to oxidation of tetrahydrobiopterin (BH4), a critical cofactor for eNOS (Figure 1). This leads to reduced NO synthesis and increased generation of superoxide. In a rodent study, a high salt diet reduced BH4 levels compared to a normal diet as a result of increased BH4 oxidation as indicated by an increased ratio of BH2/BH4 (42). In the aforementioned study in middle age and older adults, oral BH4 improved both conduit and resistance vessel endothelial function on a normal sodium diet but this effect was abolished by dietary sodium restriction (39) providing additional support for salt-induced declines in BH4.
Figure 1.
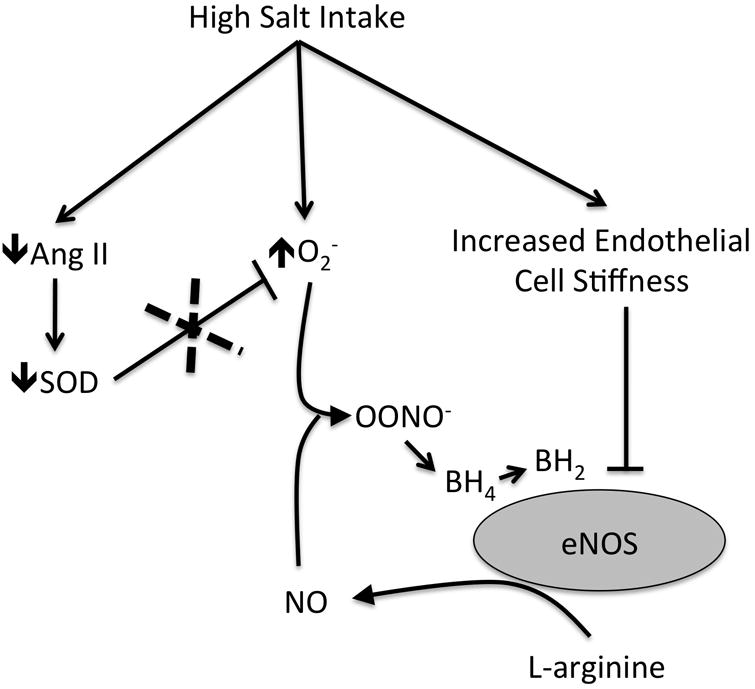
Proposed mechanisms by which high salt intake leads to reduced nitric oxide (NO) bioavailablilty. High salt leads to increased superoxide (O2-) production and suppression of angiotensin II (Ang II) which leads to decreased superoxide dismutase (SOD) expression and activity reducing scavenging of O2-. NO bioavailability is decreased by the following: 1) via reaction of NO with O2- to form peroxynitrite (OONO-); 2) oxidation of endothelial nitric oxide synthase (eNOS) cofactor tetrahydrobiopterin (BH4) reducing NO synthesis; and 3) an increase in endothelial cell stiffness which leads to decreased synthesis of NO.
The primary source of ROS during dietary salt loading appears to be superoxide (28, 29), and there is evidence for its production via NADPH oxidase (27, 28), xanthine oxidase (27, 28), and eNOS (29). In addition to increased ROS production, a reduction in endogenous antioxidant capacity for scavenging ROS may also play a role in the deleterious effects of high salt. Superoxide dismutase (SOD) is important for scavenging superoxide and a high salt diet has been shown to reduce SOD expression and activity (43, 44). The mechanism by which salt reduces SOD has been demonstrated to be through the suppression of angiotensin II (Ang II) that accompanies a high salt diet (Figure 1). When subpressor doses of Ang II are given to rodents on a high salt diet, oxidative stress and endothelial dysfunction are prevented (45, 46). Recently Durand and Lombard examined the effect of subpressor Ang II in high salt fed congenic Ren1-BN rats which have rescued Ach induced dilation and increased Cu/Zn SOD compared to their parental Dahl salt sensitive strain (43). They found that high salt prevented Ach induced dilation of the middle cerebral artery and reduced both Cu/Zn and Mn SOD expression whereas Ang II restored MCA dilation and increased Cu/Zn SOD (43). These results lend additional support the idea that prevention of Ang II suppression during high salt prevents endothelial dysfunction via maintenance of SOD. There are no data in humans that have examined the role of high salt-induced suppression of Ang II on oxidative stress and endothelial function. This may be an important mechanism in studies where Ang II levels are suppressed (31, 33) however endothelial function also has been shown to improve during more moderate reductions in sodium when Ang II levels do not change (37, 39).
In addition to oxidative stress a high salt diet may have a direct effect on endothelial function by altering endothelial cell stiffness. Increased extracellular sodium concentrations in the presence of physiologic levels of aldosterone have been shown to stiffen endothelial cells and reduce NO synthesis, an effect that can be inhibited by the epithelial sodium channel (ENaC) inhibitor amiloride (47). Elevated extracellular sodium levels lead to an increase the abundance of ENaC on endothelial cells (48) (abbreviated EnNaC (49)) in addition to damaging the endothelial glycocalyx (eGC)(50). The eGC is thought to play a role in buffering sodium ions such that when it becomes damaged sodium is permitted to enter endothelial cells via EnNaC resulting in cell stiffening (49). Jeggle and coworkers examined the role of EnNaC in stiffening the endothelium in Liddle mice that have an increase in EnNaC abundance (51). They found that endothelial cells from Liddle mice had increased cortical stiffness in the presence of aldosterone in situ however they did not assess endothelial function in this study. Accompanying cell studies provided further evidence that EnNaC abundance determines cell stiffness (51). Old mice have greater numbers of EnNaC and higher cortical stiffness at baseline and following the addition of high sodium ex vivo that likely contributes to endothelial dysfunction with age (52). It appears that high sodium leads to disturbed eGC, increased EnNaC abundance and reduced NO synthesis (49) however the role these channels on vessel function during high salt has yet to be investigated in animal models or humans.
Dietary Salt and Arterial Stiffness
An increase in arterial stiffness increases systolic BP and left ventricular workload and hypertrophy as well as decreases diastolic pressure that can result in reduced coronary blood flow (53). Measures of arterial stiffness have been shown to be predictors of cardiovascular events in hypertension (54, 55) and kidney disease (56, 57). In apparently healthy subjects, aortic pulse wave velocity (PWV) was shown to be an independent predictor of heart disease and stroke (58). Therefore, similar to endothelial function, understanding mediators of arterial stiffness is of clinical importance.
Animal studies of hypertension demonstrate that elevated dietary salt can increase arterial stiffness and that this effect is independent of BP (reviewed by Safar et al (59)). Reduced dietary sodium has also been shown to lower arterial stiffness in hypertensive humans (60, 61). Cross-sectional studies in humans provide evidence of an independent effect of salt on arterial stiffness. In a study of two groups of Chinese populations, a rural population that consumed lower salt compared to an urban population that consumed higher salt, pulse wave velocity was lower (i.e., better) in the rural group when the groups were compared at similar BPs (62). Similarly, arterial stiffness was lower in a group consuming a low salt diet compared to an age and BP matched group who consumed normal salt (63). The mechanism by which dietary salt increases arterial stiffness appears to be due to the pro-fibrotic effects of transforming growth factor-β (TGF-β)(64). When rats were fed a high salt diet for 7 days endothelial production of TGF-β increased without an increase in BP (22). Basal levels of NO production are increased short term by high salt, via TGF-β signaling, which may help reduce the deleterious effect of TGF-β initially (21, 22) but as has been described above, endothelial function and stimulated NO synthesis are reduced by high salt intake. Thus, high dietary salt likely stiffens arteries via TGF-β that is unrestrained due to high-salt induced reductions in NO bioavailability. The effects of TGF-β may be further amplified in clinical populations with already reduced endothelial function (65).
Potential of Potassium to Combat Deleterious Effects of Salt
It is important to briefly mention the potential importance of potassium in mitigating the deleterious effects of high salt. Large trials have provided evidence that the interaction of sodium and potassium consumption may be important as higher urinary sodium to potassium excretion ratio is associated with increased risk of cardiovascular disease (66, 67). There is evidence that potassium supplementation can reduce BP (68) however there are limited studies of vascular function and potassium consumption. Endothelial cell studies indicate that potassium can soften cells and increase NO synthesis (69) while increased dietary potassium has been shown to inhibit vascular production of TGF-β in rats fed a high salt diet (70). Future studies are warranted to examine the interaction of potassium and sodium on vascular function.
Conclusion
Health Policies and most clinical research are focused on the adverse effects of dietary salt on BP however there is an increasing body of evidence to support a deleterious effect of dietary salt on endothelial function and arterial stiffness independent of BP. The mechanisms responsible continue to be elucidated. Endothelial dysfunction and increased arterial stiffness are predictors of cardiovascular disease and data from Framingham indicate that both are associated with incident hypertension (71). Therefore, reducing excess dietary salt should be considered important for overall vascular health in addition to BP.
Key Points.
Dietary salt has health implications for hypertensive and/or salt sensitive individuals as well as normotensive salt resistant individuals due to salt's blood pressure independent effects.
High dietary salt results in impaired endothelial function and increased arterial stiffness, both predictors of cardiovascular disease, independent of changes in blood pressure
Reducing excess dietary salt should be considered important for overall vascular health in addition to blood pressure.
Acknowledgments
Supported by National Institutes of Health Grant HL104106.
Footnotes
There are no conflicts of interest.
References
- 1.U S Department of Agriculture: Dietary Guidelines for Americans. 7th. Washington, D.C.: U.S. Government Printing Office; 2010. [Google Scholar]
- 2.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. The Cochrane database of systematic reviews. 2011;(11):CD004022. doi: 10.1002/14651858.CD004022.pub3. [DOI] [PubMed] [Google Scholar]
- 3.He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. The Cochrane database of systematic reviews. 2013;4:CD004937. doi: 10.1002/14651858.CD004937.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA : the journal of the American Medical Association. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27(3 Pt 2):481–90. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 7.Luft FC, Miller JZ, Grim CE, Fineberg NS, Christian JC, Daugherty SA, et al. Salt sensitivity and resistance of blood pressure. Age and race as factors in physiological responses. Hypertension. 1991;17(1 Suppl):I102–8. doi: 10.1161/01.hyp.17.1_suppl.i102. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger MH. Salt sensitivity is associated with an increased mortality in both normal and hypertensive humans. Journal of clinical hypertension. 2002;4(4):274–6. doi: 10.1111/j.1524-6175.2002.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension. 1991;18(1):67–71. doi: 10.1161/01.hyp.18.1.67. [DOI] [PubMed] [Google Scholar]
- 10.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) Bmj. 2007;334(7599):885–8. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Wardener HE, MacGregor GA. Harmful effects of dietary salt in addition to hypertension. Journal of human hypertension. 2002;16(4):213–23. doi: 10.1038/sj.jhh.1001374. [DOI] [PubMed] [Google Scholar]
- 12*.Boegehold MA. The effect of high salt intake on endothelial function: reduced vascular nitric oxide in the absence of hypertension. Journal of vascular research. 2013;50(6):458–67. doi: 10.1159/000355270. This is a recent review of the effects of high salt on the endothelium. [DOI] [PubMed] [Google Scholar]
- 13.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 14.Luscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annu Rev Med. 1993;44:395–418. doi: 10.1146/annurev.me.44.020193.002143. [DOI] [PubMed] [Google Scholar]
- 15.Schachinger V, Zeiher AM. Prognostic implications of endothelial dysfunction: does it mean anything? Coron Artery Dis. 2001;12(6):435–43. doi: 10.1097/00019501-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 17.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120(6):502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Reviews in endocrine & metabolic disorders. 2010;11(1):61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clinical science. 2011;120(9):357–75. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kizhakekuttu TJ, Widlansky ME. Natural antioxidants and hypertension: promise and challenges. Cardiovascular therapeutics. 2010;28(4):e20–32. doi: 10.1111/j.1755-5922.2010.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ying WZ, Aaron KJ, Sanders PW. Transforming growth factor-beta regulates endothelial function during high salt intake in rats. Hypertension. 2013;62(5):951–6. doi: 10.1161/HYPERTENSIONAHA.113.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying WZ, Sanders PW. Dietary salt increases endothelial nitric oxide synthase and TGF-beta1 in rat aortic endothelium. The American journal of physiology. 1999;277(4 Pt 2):H1293–8. doi: 10.1152/ajpheart.1999.277.4.H1293. [DOI] [PubMed] [Google Scholar]
- 23.Frohlich ED, Chien Y, Sesoko S, Pegram BL. Relationship between dietary sodium intake, hemodynamics, and cardiac mass in SHR and WKY rats. Am J Physiol. 1993;264(1 Pt 2):R30–4. doi: 10.1152/ajpregu.1993.264.1.R30. [DOI] [PubMed] [Google Scholar]
- 24.Matavelli LC, Zhou X, Varagic J, Susic D, Frohlich ED. Salt loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292(2):H814–9. doi: 10.1152/ajpheart.00671.2006. [DOI] [PubMed] [Google Scholar]
- 25.Yu HC, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, et al. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation. 1998;98(23):2621–8. doi: 10.1161/01.cir.98.23.2621. [DOI] [PubMed] [Google Scholar]
- 26.Lenda DM, Boegehold MA. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. American journal of physiology Heart and circulatory physiology. 2002;282(2):H395–402. doi: 10.1152/ajpheart.0354.2001. [DOI] [PubMed] [Google Scholar]
- 27.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. American journal of physiology Heart and circulatory physiology. 2000;279(1):H7–H14. doi: 10.1152/ajpheart.2000.279.1.H7. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. Journal of vascular research. 2007;44(5):382–90. doi: 10.1159/000102955. [DOI] [PubMed] [Google Scholar]
- 29.Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2007;292(4):R1550–6. doi: 10.1152/ajpregu.00703.2006. [DOI] [PubMed] [Google Scholar]
- 30.Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension. 2008;51(6):1525–30. doi: 10.1161/HYPERTENSIONAHA.108.109868. [DOI] [PubMed] [Google Scholar]
- 31*.DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, et al. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. Journal of hypertension. 2013;31(3):530–6. doi: 10.1097/HJH.0b013e32835c6ca8. This study demonstrated that high salt impairs endothelial function indepednent of blood pressure by studying salt resistant subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55(5):1075–85. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. The Journal of physiology. 2012;590(Pt 21):5519–28. doi: 10.1113/jphysiol.2012.236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kellogg DL, Jr, Zhao JL, Wu Y. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. American journal of physiology Heart and circulatory physiology. 2008;295(1):H123–9. doi: 10.1152/ajpheart.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, et al. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. Journal of applied physiology. 2012;112(12):2019–26. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr. 2009:485–90. doi: 10.3945/ajcn.2008.26856. [DOI] [PubMed] [Google Scholar]
- 37**.Dickinson KM, Clifton PM, Keogh JB. A reduction of 3 g/day from a usual 9 g/day salt diet improves endothelial function and decreases endothelin-1 in a randomised cross-over study in normotensive overweight and obese subjects. Atherosclerosis. 2014;233(1):32–8. doi: 10.1016/j.atherosclerosis.2013.11.078. This study demonstrated that a moderate reduction in dietary salt improved endothelial function in overweight and obese subjects. Importantly, this effect was independent of blood pressure. [DOI] [PubMed] [Google Scholar]
- 38.Jablonski KL, Gates PE, Pierce GL, Seals DR. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Therapeutic advances in cardiovascular disease. 2009;3(5):347–56. doi: 10.1177/1753944709345790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, et al. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. Journal of the American College of Cardiology. 2013;61(3):335–43. doi: 10.1016/j.jacc.2012.09.010. This study demonstrated that sodium restriction improves both condit and resistance vessel endothelial function and that the effect was independent of the decrease in blood pressure. Additionally, sodium resitriction abolished the effect of ascorbic acid or tetrahydrobiopterin on endothelial funcion in middle age/older adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisenach JH, Gullixson LR, Kost SL, Joyner MJ, Turner ST, Nicholson WT. Sex differences in salt sensitivity to nitric oxide dependent vasodilation in healthy young adults. Journal of applied physiology. 2012;112(6):1049–53. doi: 10.1152/japplphysiol.01197.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lennon-Edwards S, Ramick MG, Matthews EL, Brian MS, Farquhar WB, Edwards DG. Salt loading has a more deleterious effect on flow-mediated dilation in salt-resistant men than women. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2014;24(9):990–5. doi: 10.1016/j.numecd.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nurkiewicz TR, Wu G, Li P, Boegehold MA. Decreased arteriolar tetrahydrobiopterin is linked to superoxide generation from nitric oxide synthase in mice fed high salt. Microcirculation. 2010;17(2):147–57. doi: 10.1111/j.1549-8719.2009.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Durand MJ, Lombard JH. Low-dose angiotensin II infusion restores vascular function in cerebral arteries of high salt-fed rats by increasing copper/zinc superoxide dimutase expression. American journal of hypertension. 2013;26(6):739–47. doi: 10.1093/ajh/hpt015. This study provides further support for reduced antioxidant capacity as a result of high salt suppression of angiotensin II. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lenda DM, Boegehold MA. Effect of a high salt diet on microvascular antioxidant enzymes. Journal of vascular research. 2002;39(1):41–50. doi: 10.1159/000048992. [DOI] [PubMed] [Google Scholar]
- 45.Zhu J, Drenjancevic-Peric I, McEwen S, Friesema J, Schulta D, Yu M, et al. Role of superoxide and angiotensin II suppression in salt-induced changes in endothelial Ca2+ signaling and NO production in rat aorta. American journal of physiology Heart and circulatory physiology. 2006;291(2):H929–38. doi: 10.1152/ajpheart.00692.2005. [DOI] [PubMed] [Google Scholar]
- 46.McEwen ST, Schmidt JR, Somberg L, Cruz Lde L, Lombard JH. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high-salt diet. Microcirculation. 2009;16(3):220–34. doi: 10.1080/10739680802544177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(41):16281–6. doi: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korte S, Wiesinger A, Straeter AS, Peters W, Oberleithner H, Kusche-Vihrog K. Firewall function of the endothelial glycocalyx in the regulation of sodium homeostasis. Pflugers Archiv : European journal of physiology. 2012;463(2):269–78. doi: 10.1007/s00424-011-1038-y. [DOI] [PubMed] [Google Scholar]
- 49*.Kusche-Vihrog K, Jeggle P, Oberleithner H. The role of ENaC in vascular endothelium. Pflugers Archiv : European journal of physiology. 2014;466(5):851–9. doi: 10.1007/s00424-013-1356-3. This is a comprehensive review of epithelial sodium channels in the endothelium. [DOI] [PubMed] [Google Scholar]
- 50.Oberleithner H, Peters W, Kusche-Vihrog K, Korte S, Schillers H, Kliche K, et al. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Archiv : European journal of physiology. 2011;462(4):519–28. doi: 10.1007/s00424-011-0999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51*.Jeggle P, Callies C, Tarjus A, Fassot C, Fels J, Oberleithner H, et al. Epithelial sodium channel stiffens the vascular endothelium in vitro and in Liddle mice. Hypertension. 2013;61(5):1053–9. doi: 10.1161/HYPERTENSIONAHA.111.199455. This study utilized a rodent model of reduced epithelial sodium channels on the endothelium and demonstrated their importance in mediating endothelial cell stiffness. [DOI] [PubMed] [Google Scholar]
- 52.Paar M, Pavenstadt H, Kusche-Vihrog K, Druppel V, Oberleithner H, Kliche K. Endothelial sodium channels trigger endothelial salt sensitivity with aging. Hypertension. 2014;64(2):391–6. doi: 10.1161/HYPERTENSIONAHA.114.03348. [DOI] [PubMed] [Google Scholar]
- 53.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(5):932–43. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 54.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 55.Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke; a journal of cerebral circulation. 2003;34(5):1203–6. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 56.Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney international. 2003;63(5):1852–60. doi: 10.1046/j.1523-1755.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- 57.Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension. 1998;32(3):570–4. doi: 10.1161/01.hyp.32.3.570. [DOI] [PubMed] [Google Scholar]
- 58.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113(5):657–63. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 59.Safar ME, Thuilliez C, Richard V, Benetos A. Pressure-independent contribution of sodium to large artery structure and function in hypertension. Cardiovascular research. 2000;46(2):269–76. doi: 10.1016/s0008-6363(99)00426-5. [DOI] [PubMed] [Google Scholar]
- 60.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44(1):35–41. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]
- 61.Todd AS, Macginley RJ, Schollum JB, Johnson RJ, Williams SM, Sutherland WH, et al. Dietary salt loading impairs arterial vascular reactivity. The American journal of clinical nutrition. 2010;91(3):557–64. doi: 10.3945/ajcn.2009.28645. [DOI] [PubMed] [Google Scholar]
- 62.Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71(2):202–10. doi: 10.1161/01.cir.71.2.202. [DOI] [PubMed] [Google Scholar]
- 63.Avolio AP, Clyde KM, Beard TC, Cooke HM, Ho KK, O'Rourke MF. Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis. 1986;6(2):166–9. doi: 10.1161/01.atv.6.2.166. [DOI] [PubMed] [Google Scholar]
- 64.Sanders PW. Vascular consequences of dietary salt intake. American journal of physiology Renal physiology. 2009;297(2):F237–43. doi: 10.1152/ajprenal.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanbay M, Chen Y, Solak Y, Sanders PW. Mechanisms and consequences of salt sensitivity and dietary salt intake. Current opinion in nephrology and hypertension. 2011;20(1):37–43. doi: 10.1097/MNH.0b013e32834122f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA : the journal of the American Medical Association. 2011;306(20):2229–38. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 67.Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, et al. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Archives of internal medicine. 2009;169(1):32–40. doi: 10.1001/archinternmed.2008.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA : the journal of the American Medical Association. 1997;277(20):1624–32. doi: 10.1001/jama.1997.03540440058033. [DOI] [PubMed] [Google Scholar]
- 69.Oberleithner H, Callies C, Kusche-Vihrog K, Schillers H, Shahin V, Riethmuller C, et al. Potassium softens vascular endothelium and increases nitric oxide release. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(8):2829–34. doi: 10.1073/pnas.0813069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ying WZ, Aaron K, Wang PX, Sanders PW. Potassium inhibits dietary salt-induced transforming growth factor-beta production. Hypertension. 2009;54(5):1159–63. doi: 10.1161/HYPERTENSIONAHA.109.138255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA : the journal of the American Medical Association. 2012;308(9):875–81. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]


