Abstract
We investigated the etiology of attentional control (AC) and four different anxiety symptom types (generalized, obsessive-compulsive, separation, and social) in an adolescent sample of over 400 twin pairs. Genetic factors contributed to 55% of the variance in AC and between 43 and 58% of the variance in anxiety. Negative phenotypic associations between AC and anxiety indicated that lower attentional ability is related to increased risk for all 4 anxiety categories. Genetic correlations between AC and anxiety phenotypes ranged from −.36 to −.47, with evidence of nonshared environmental covariance between AC and generalized and separation anxiety. Results suggest that AC is a phenotypic and genetic risk factor for anxiety in early adolescence, with somewhat differing levels of risk depending on symptomatology.
Anxiety is one of the most common psychiatric diagnoses in children and adolescents (Beesdo, Knappe, & Pine, 2009; Canino et al., 2004; Costello, Mustillo, Erkanli, Keeler, & Angold, 2003; Essau, Conradt, & Petermann, 2000; Ford, Goodman, & Meltzer, 2003). A recent epidemiological study commissioned by the Centers for Disease Control found that 3.0% of children in the United States aged 3–17 years had current anxiety diagnoses during the years 2005–2011 (Perou et al., 2013). The National Institute of Mental Health (NIMH) reports that 8% of teenagers aged 13–18 have an anxiety disorder with symptoms typically appearing at approximately age 6 (NIMH, 2013). Lifetime prevalence was reported at 25.1% for these adolescents. Onset of clinically relevant anxiety symptoms often occurs during adolescence, and anxiety-related problems peak during this period (Zahn-Waxler, Shirtcliff, & Marceau, 2007). Anxiety that emerges in childhood and adolescence contributes to the impairment of current and future psychological functioning, with most adults diagnosed with anxiety or mood disorders reporting the presence of anxiety symptoms earlier in childhood (Fox & Pine, 2012; Pine, 2007).
Childhood anxiety is also associated with increased levels of severity and, perhaps obviously, with longevity of the anxiety diagnosis over time (Pérez-Edgar & Fox, 2005). Anxiety disorders that occur in childhood or adolescence may also put young people at risk for comorbid depressive disorders, drug dependence, suicidal behavior, and educational underachievement (Woodward & Fergusson, 2001). Therefore, the identification of developmental risk factors for anxiety can contribute to appropriate prediction and intervention strategies.
Anxiety Disorders: Descriptions, Prevalence, and Genetic Factors
The broad diagnostic category of anxiety includes specific disorders related to obsessive-compulsive, social, separation, and generalized anxiety symptoms, as well as other anxiety disorders. Obsessive-compulsive anxiety is characterized by dysfunctional repetitive obsessions or compulsions (APA, 2000). It often emerges in early childhood or adolescence (prevalence rates of 1%–3% among children and adolescents) and affects males and females at approximately the same rate (Flament et al., 1988; Fontenelle & Hasler, 2008; Valleni-Basile et al., 1994). Social anxiety is typically diagnosed in late adolescence; lifetime prevalence rates range from approximately 7.3 to 12.1%, and females have nearly twice the risk for developing this disorder (Beesdo et al., 2007; Kessler et al., 2005; Wittchen, Stein, & Kessler, 1999). Separation anxiety is typified by extreme distress when separated from one’s primary caregiver (APA, 2000). Normative onset occurs between twelve and eighteen months of age (Beesdo et al., 2009), but separation anxiety is considered atypical later in childhood. Prevalence rates for separation anxiety disorder range from 2.4% to 5.2%, with an average onset of approximately seven years (Bolton et al., 2006; Bowen, Offord, & Boyle, 1990; Kessler et al., 2005). Age of onset for generalized anxiety is typically adolescence to early adulthood with prevalence rates between 3.6 and 8.6% (Beesdo, Pine, Lieb, & Wittchen, 2010; Beesdo et al., 2009; Bowen et al., 1990; De Graaf, Bijl, Spijker, Beekman, & Vollebergh, 2003; Kessler et al., 2005). Each type of anxiety shows somewhat distinct symptomatology, yet there is strong comorbidity in childhood and adolescence based on multiple studies using comparison samples (Essau et al., 2000; Ford et al., 2003).
Although symptoms of these different anxiety disorders indicate some phenotypic heterogeneity, common genetic and biological characteristics have been proposed, partly due to similar responses to pharmacological treatments (Pérez-Edgar & Fox, 2005). Etiologically, both genetic and environmental factors contribute to individual differences in childhood anxiety as well as links between early anxiety and later developing psychiatric disorders (Pine, 2007). Behavioral genetic studies of anxiety generally find moderate heritability estimates (Pérez-Edgar & Fox, 2005). Because these investigations typically focus on broad anxiety phenotypes or one specific anxiety diagnosis, mapping genetic factors onto individual anxiety diagnoses is difficult. One fairly comprehensive meta-analysis of family and twin studies of panic, generalized anxiety, phobic and obsessive-compulsive disorders in adults examined odds ratios and heritabilities of the various anxiety diagnoses (Hettema, Neale, & Kendler, 2001). Odds ratios for first-degree relatives of pro-bands in the family studies ranged from 4 to 6 for all disorders, and heritability estimates for panic and generalized anxiety were .43 and .32, respectively (Hettema et al., 2001). Phobias and obsessive-compulsive disorders were not included in the meta-analysis of heritability due to insufficient large-scale studies. The nonshared environment was significant for both panic and generalized anxiety, and the shared environment was significant for females with generalized anxiety disorder (Hettema et al., 2001). These results suggest that the biometric architectures of different anxiety disorders show some similarities (magnitude of heritability estimates) and some differences (shared environment is only important for women with generalized anxiety). Clearly, further genetic research focusing on multiple aspects of anxiety is needed.
Attentional Control and Anxiety
Individual differences in child temperament dimensions such as attentional control (AC) have been consistently linked to the emergence of anxiety disorders. Temperament is defined as individual differences in human reactivity and regulation, encompassing the broad domains of emotion, motor activity, and attention (Rothbart & Bates, 2006). It is a fairly stable, early-emerging construct that represents the affective, activational, and attentional aspects of adult personality. Extreme levels of temperament may be representative of behavior problems and psychopathology, and temperament may be an important liability factor for child psychopathology (Goldsmith, Lemery, & Essex, 2004). Research examining temperamental risk factors for anxiety indicates that these behavioral dimensions can be assessed as early as the preschool period and may have predictive validity from early childhood through adulthood (Pérez-Edgar & Fox, 2005; Pine, 2007). Temperament dimensions may predict multiple anxiety diagnoses reflecting a general vulnerability, or they may show more specificity by predicting only one type of anxiety diagnosis.
The temperament dimension of attentional control (AC) refers to individual differences in the ability to regulate one’s awareness and concentration (Rothbart & Bates, 2006). Specifically, AC reflects stable individual differences in the monitoring of attention, how it is allocated, and the purposeful ability to focus or disengage attention (Helzer, Connor-Smith, & Reed, 2009). AC is theorized to develop later than emotional reactive control systems such as fear and behavioral inhibition (BI) and is considered self-regulatory in nature (Rothbart & Bates, 2006). In Rothbart’s theory of temperament, AC is viewed as a component of the broader effortful control (EC) factor which focuses on self-regulation and the efficiency of executive attention, and involves the inhibition and activation of appropriate responses, planning, error detection, and sustained attention (Rothbart, Ellis, & Posner, 2004). Effortful control temperament dimensions like AC are posited to emerge in toddlerhood and preschool and continue to develop into middle childhood, reflecting cognitive underpinnings.
AC is considered to be a dimension of temperament; however, other psychological theorists and researchers have conceptualized AC as a cognitive construct or executive function (EF) related to processing efficiency that is often assessed in adolescence and adulthood (Eysenck, Derakshan, Santos, & Calvo, 2007). Although we view AC primarily through the lens of temperament theory, we do not believe that the cognitive/EF perspective on AC is incompatible with ours. We agree with both Zhou, Chen, and Main (2012), and Liew (2012) who argue that the temperament and EF perspectives have considerable overlaps in definition and in empirical research programs and that differences between the two perspectives are largely a matter of research traditions as opposed to true differences in concept. Both Zhou et al. (2012) and Liew (2012) view both perspectives as complementary and propose integration of the EF and temperament perspectives in a more comprehensive theory of child self-regulation.
In their attentional control theory, Eysenck et al. (2007; Eysenck & Derakshan, 2011) describe AC within the framework of anxiety and cognitive performance. Individuals tend to experience anxiety when their goals are threatened, resulting in an allocation of attention to the source of threats (Eysenck et al., 2007). Attentional control theory further describes anxious individuals as having reduced AC because they are attending to threats preferentially. Therefore, anxiety may increase attentional focus or bias to threat, yet overall AC performance is reduced. As the attentional control theory was first posited, several investigations have found associations between AC and anxiety behaviors, although research on the underlying neural functioning is somewhat mixed, with different studies evincing increased or decreased activity in frontal areas (Berggren & Derakshan, 2013a).
In one of the earliest behavioral investigations of AC and anxiety, Derryberry and Reed (2002) found that undergraduates with low AC and high trait anxiety had higher rates of biases to a threatening target versus a safe target. They concluded that anxious individuals with better AC may not show strong biases to threat, a theme that is consistent with attentional control theory. In a similar study, Susa, Pitica, Benga, and Miclea (2012) showed that an interaction between AC and attentional bias significantly contributed to anxiety level in children aged 9–14. In addition to attentional biases, studies of other specific cognitive variables have been designed to assess AC in anxious individuals. For example, anxious participants had significantly impaired performance on a task-switching cognitive task in an adult sample (Derakshan, Smyth, & Eysenck, 2009). A recent analysis of adults’ formation of expectations in response to an AC task showed that individuals with higher rates of trait anxiety did not engage in predictions or cues to expectation, while those with low anxiety did (Berggren & Derakshan, 2013b). These findings suggest a number of specific AC impairments in broadly anxious individuals, but few specific anxiety disorder categories have been examined.
A study that included the specific psychopathology outcomes of state anxiety, social anxiety, and depression showed that the association between social anxiety and AC remained robust even when accounting for depression, state anxiety, and other subscales of effortful control (Moriya & Tanno, 2008). A socially anxious sample made more errors on saccade tasks with emotional facial expressions as stimuli versus neutral stimuli; these results suggest an AC deficit in social anxiety (Wieser, Pauli, & Muhlberger, 2009). In a mediational model of AC, social anxiety, and positive affect in an undergraduate sample, Morrison and Heimberg (2013) found that social anxiety was negatively related to AC which was positively associated with positive affect, even after controlling for depression. Therefore, socially anxious individuals with lower AC may be at less risk for negative affectivity. This pattern is somewhat similar to findings from Derryberry and Reed’s (2002) research on threat bias wherein anxious persons with higher AC had lower attentional threat biases. In addition to research on AC and social anxiety, one study examined AC in the context of major components of obsessive-compulsive disorder (OCD) and generalized anxiety disorder (GAD). Results of this investigation showed that OCD and GAD patients had greater deficits in AC than a control group (Armstrong, Zald, & Olatunji, 2011). Aside from these somewhat nonspecific findings related to social anxiety and the single investigation on OCD/GAD, less is known about AC and the broad range of anxiety subtypes and few have compared multiple anxiety symptoms in the same sample. In addition, differences in the ages of these various samples do not allow for a close examination of developmental considerations for adolescence, a critical period for anxiety disorders.
As we have reviewed, several researchers believe higher AC allows individuals to better regulate the interpretation of stimuli as nonthreatening and therefore better control anxiety (e.g., Derryberry & Reed, 2002). There is also evidence suggesting that higher AC and related executive functioning (EF) abilities are linked to higher anxiety symptoms. For example, some temperament research shows that high AC or high inhibitory control can result in increased risk for anxiety (e.g., Brooker et al., 2011; White, McDermott, Degnan, Henderson, & Fox, 2011). In addition, several neuroscientific studies of anxiety have shown a positive relationship between error detection (often described as an EF or an aspect of cognitive performance) and anxiety levels. The detection of errors produces a neural response in the brain, generated in the anterior cingulate cortex, called the error-related negativity (ERN; Gehring, Goss, Coles, Meyer, & Donchin, 1993). The ERN is a neural response following an error occurring within 100 ms of an erroneous response, where a larger negative response reflects greater error detection. It has been repeatedly demonstrated that individuals with anxiety show a larger ERN compared to controls (Gehring, Himle, & Nisenson, 2000; Hajcak, Franklin, Foa, & Simons, 2008; Lahat et al., 2014; Meyer et al., 2013; McDermott et al., 2009; Weinberg, Olvet, & Hajcak, 2010). However, there is considerable debate over what the ERN represents and it at least partially represents an emotional response to errors (Inzlicht & Al-Khindi, 2012). Using the misattribution of arousal paradigm, which selectively manipulates state anxiety without manipulating cognitive performance, Inzlicht & Al-Khindi (2012) demonstrated that the ERN is dissociable from cognitive performance but not negative affect. The amplified ERN response among anxious individuals may thus reflect not only the detection of an error, but also the affective response to the error. At the very least, the ERN phenomenon is likely not a “pure” EF, nor is it consistent with our definition of the temperament dimension of AC.
The Etiology of Anxiety and Attentional Control in Childhood and Adolescence
Widespread consensus holds that genetic factors contribute substantially to individual differences in anxiety phenotypes (Anagnostaras, Craske, & Fanselow, 1999; Schienle, Hettema, Cáceda, & Nemeroff, 2011) although environmental factors also play an important role. Quantitative genetic analyses indicate patterns of heritability that vary depending on age, methods of assessment, and subtypes or categories of anxiety. Broad measures of anxiety sensitivity in children (Eley, Gregory, Clark, & Ehlers, 2007) and adults (Taylor, Jang, Stewart, & Stein, 2008), as well as self-reported anxiety symptoms in childhood (Warren, Schmitz, & Emde, 1999), show significant genetic influences and few shared environmental influences.
Developmental stability of anxiety symptoms in a longitudinal twin study from age 7 to 9 was moderate and largely the result of overlapping genetic factors (Trzaskowski, Zavos, Haworth, Plomin, & Eley, 2012). Measures of anxiety sensitivity across three occasions in adolescence (mean ages for each time point were 15, 17, and 20 years, respectively) also showed longitudinal genetic continuity although new genetic factors did appear during the period under examination (Zavos, Gregory, & Eley, 2012).
When examining subtypes of anxiety, the magnitude of heritability tends to be moderate although estimates may vary (moderate heritability ranges from .30 to .60). For example, heritability estimates from studies in childhood are .54 for obsessive-compulsive behaviors (Eley et al., 2003), range from .60 to .80 for specific phobia (Bolton et al., 2006), from .39 to .73 for separation anxiety (Bolton et al., 2006; Eley et al., 2003), and from .56 to .64 for social anxiety or shyness phenotypes (Eley et al., 2003; Hallett, Ronald, Rijsdijk, & Eley, 2009). Shared environmental variance ranged from .36 to .40 for separation anxiety (Eley et al., 2003; Topolski et al., 1997) and from .07 to .21 for shyness phenotypes in one study (Hallett et al., 2009); otherwise the remaining variances were due to nonshared environmental factors. The findings have generally been similar in adult samples (e.g., Hettema et al., 2001) with heritabilities ranging from .32 to .43 for anxiety types, and no evidence of shared environment except for in females with generalized anxiety disorder.
A smaller number of studies have found significant genetic influences on effortful and attentional control in middle childhood (Lemery-Chalfant, Doelger, & Goldsmith, 2008) and late adolescence and early adulthood (Yamagata et al., 2005). In an adoption study examining the temperament dimension of attention, ratings made by teachers revealed moderate heritability (ranging from .25 to .51) for children at age 9, 10, 11, and 12, although not all estimates were significant (Gagne, Saudino, & Cherny, 2003). The Lemery-Chalfant, Doelger, and Goldsmith (2008) study also focused on child behavior problems and reported genetic contributions to associations between AC and internalizing and externalizing problems across reporters (parents and observers) and age.
The Current Study
Here, we examined the association between anxiety symptoms and AC in an adolescent twin sample. We used a multiinformant approach to assess both anxiety and the temperament dimension of AC, utilizing a combination of self-ratings and mother ratings. We calculated four anxiety symptom scores (obsessive, social, separation, and generalized anxiety). Bivariate genetic analyses explored the extent to which links between AC and anxiety symptoms are genetically and environmentally mediated in adolescence. A few studies have shown genetic influences on AC and effortful control (Lemery-Chalfant et al., 2008; Yamagata et al., 2005), and one found genetic overlap between AC and internalizing behavior problems in childhood (Lemery-Chalfant et al., 2008). Therefore, overlapping genetic factors may contribute to phenotypic associations between AC and anxiety symptoms in adolescence, but this remains an open empirical question. We expected AC to be significantly associated with the multiple anxiety symptoms in our adolescent sample. AC was also hypothesized to share overlapping genetic variance with these anxiety outcomes. Confirming evidence for these hypotheses would support the role of early adolescent AC as an important developmental and genetic risk factor for concurrent and future anxiety disorders.
METHODS
Overview and Sample
The Wisconsin Twin Project is a population-based study of early emotional development, temperament, psychopathology, and related psychobiological and behavioral phenotypes in children and adolescents across the state of Wisconsin (Goldsmith, Lemery-Chalfant, Schmidt, Arneson, & Schmidt, 2007). The project uses a range of research methods, including diagnostic interviews, child self-report, caregiver report, and in-person cognitive testing. Of the twin pairs that completed the relevant adolescent assessments, 17 families were excluded because of special circumstances (e.g., intellectual disability in one twin). The sample whose data we analyzed comprised 446 twin pairs (892 total participants, 46.4% male), with a mean age of 13.6 years (SD = 1.64 years) and 42.1% categorized as MZ twins. Confirmed contact was obtained from 98% of the initial eligible pool of 503 families for this study cohort, and of this pool, 7.1% declined participation. The ethnicity of the twins in this study was 96.2% non-Hispanic or Latino, and 83.4% were categorized as White (8% Black; 2% Native American; 3.8% more than one race; 2% other: and less than 1% Filipino, Hmong, or Other Pacific Islander categories combined). Mothers had an average education of 14.81 years (SD = 2.33), and fathers had an average education of 14.24 years (SD = 2.44). The median income bracket was $60,001 to $70,000 with 19.2% of the sample having a family income of $40,000 or less and 39.7% of the sample having a family income of $80,000 or more. At the time of assessment, 78.4% of parent participants were married with the vast majority of these (73.9%) married to the biological parent, 7.6% were divorced, and 5.9% were single and never married (3.8% declined to answer and 4.3% answered “other”—most of these individuals said “yes” to the question “are you living with a partner?”).
Anxiety Assessment
Anxiety was assessed with two independent measures employing self-report and mother reports. The National Institute of Health’s computer-assisted Diagnostic Interview Schedule for Children, version IV (C-DISC-IV; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000), was administered to each child and primary caregiver independently. We used the obsessive-compulsive, social anxiety, separation anxiety, and generalized anxiety module symptom counts. The C-DISC-IV is a structured diagnostic instrument based on the DSM-IV designed for nonclinicians for use with 30 childhood and adolescent diagnoses occurring over the past 12 months and the past 4 weeks (Shaffer et al., 2000). Because of the nature of the C-DISC-IV interview format and scoring procedure, we don’t have scale-based data, and it is not possible to provide reliability estimates. However, in a psychometric study of the DISC-IV, test–retest reliability for the anxiety diagnoses ranged from .48 to .86 when the results of parent and youth scores were combined and exceeded that of previous versions of the DISC (Shaffer et al., 2000). Due to the categorical nature of DSM diagnoses, standard estimates of reliability may appear “misleadingly low” as compared to dimensional variables, and in fact, reliability is overall higher when assessed using C-DISC-IV symptom and criterion counts (Shaffer et al., 1996, 2000). Validity data for the C-DISC-IV are not yet available; however, previous versions of the DISC were found to be valid across multiple analyses (see Schwab-Stone et al., 1996).
The overanxious (OA), separation anxiety (SA), and social inhibition (SI) scales from the MacArthur Health and Behavior Questionnaire (HBQ; Essex et al., 2002) were also rated by each caregiver and child (28 items). The HBQ has shown to be a reliable and valid questionnaire assessment of multiple dimensions of health and dysfunction in middle childhood which can be used to screen children who may require more intensive diagnostic assessments (Ablow et al., 1999; Essex et al., 2002; Lemery-Chalfant et al., 2007). Estimates of internal consistency using Cronbach’s alpha ranged from .81 to .85 for caregivers and .79–.85 for children on the HBQ (consistent with published internal consistency estimates for HBQ scales ranging from .56 to .92; Ablow et al., 1999). Raw scores on all anxiety measures were transformed to z-scores and square root transformations were applied to positively skewed variables (C-DISC-IV twin obsessive-compulsive and separation anxiety; C-DISC-IV parent separation, social, and generalized anxiety; HBQ twin separation anxiety; HBQ parent overanxious, separation anxiety, and social inhibition) before forming mean-level scores. The components of the composites were as follows (with mean intercorrelations of the constituent scales in parentheses—all intercorrelations were significant at the p < .01 level):
Generalized anxiety composite: child and parent DISC generalized anxiety symptom scales; child and parent HBQ overanxious scales (.33).
Obsessive-compulsive anxiety composite: child and parent DISC obsessive-compulsive symptom scales (.23).
Separation anxiety composite: child and parent DISC separation anxiety symptom scales; child and parent HBQ separation anxiety scales (.34).
Social anxiety composite: child and parent DISC social anxiety symptom scales; child and parent HBQ social anxiety scales (.34).
Attentional Control
We quantified attentional control (AC) variables using two independent measures. The Early Adolescent Temperament Questionnaire (EATQ; Capaldi & Rothbart, 1992) attention scale and the HBQ inattention scale were both rated by the child and the primary caregiver. The EATQ is a commonly used early adolescent temperament questionnaire that is reliable and valid (Capaldi & Rothbart, 1992; Muris & Meester, 2009). Both the EATQ and the HBQ characterize AC from a temperament perspective rather than from the EF perspective (as many traditional attention tasks do). Estimates of internal consistency using Cronbach’s alpha for the EATQ attention scale were .60 and .80, for the child and parent, respectively. For HBQ inattention, α = .82 for the child-rated version, and α = .89 for the parent version. The attentional control composite consisted of the mean of the following four standardized variables; child and parent EATQ attention scales; and child and parent HBQ inattention scales (reversed). A square root transformation was applied to the HBQ parent inattention scale due to positive skewness. The mean intercorrelation of the AC constituent scales was .47.
Vocabulary Level
All vocabulary testing was administered by experimenters during a family home visit. The Peabody Picture Vocabulary Task (PPVT), a standardized, reliable, and valid measure of expressive vocabulary and word retrieval for Standard American English (Dunn, 1965; Dunn & Dunn, 2007) was used to test vocabulary level. The experimenter read a word aloud and the child was asked to choose one of four pictures that accurately corresponded to the word. We used age-equivalence scores. Although vocabulary is not a primary variable of interest in this study, we residualized all of the primary variables for the effects due to age-equivalent vocabulary scores on the PPVT in our genetic analyses. The goal of this residualization was to eliminate the effect of vocabulary knowledge on relationships between AC and anxiety, as we view AC primarily as a dimension of temperament rather than a purely cognitive construct.
Zygosity Status
The Zygosity Questionnaire for Young Twins (Goldsmith, 1991) was initially administered to all caregivers to classify zygosity. Ambiguous zygosity was resolved with a review of hospital placenta(e) pathology reports, evaluations of physical similarity using video footage, and, in some cases, DNA-based zygosity tests. There were no cases of unresolved zygosity.
Statistical Approach
Descriptive statistics, tests of mean sex differences, and phenotypic correlational analyses were computed for all variables. To account for the nested nature of twin data, we used generalized estimating equation models to test for mean gender differences (Liang & Zeger, 1986; Zeger & Liang, 1986). A Bonferroni correction was applied to all phenotypic correlations to conservatively estimate significance levels corrected for alpha inflation. Twin covariances can be inflated by the variance due to gender. Therefore, scores for all variables in the genetic analyses were residualized for sex effects (McGue & Bouchard, 1984). In addition, we residualized all variables for effects due to vocabulary scores on the PPVT. Twin intraclass correlations were computed for each trait using a double-entry procedure to provide an index of twin similarity. When MZ twin correlations exceed DZ twin correlations for a trait, genetic factors are implicated as a contributor to individual differences for that trait. Although the focus of our genetic analyses was on the covariance between AC and anxiety, we conducted basic univariate ACE model-fitting before bivariate analyses.
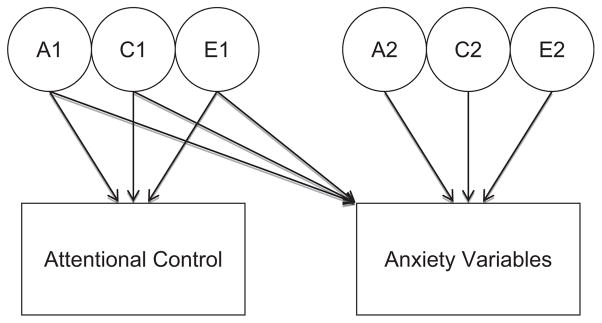
To estimate genetic and environmental variances on individual AC and anxiety variables and to explore sources of covariance between AC and anxiety, bivariate Cholesky decomposition models were fit to raw twin data using Mx maximum-likelihood model-fitting procedures (Neale, Boker, Xie, & Maes, 2001). A path diagram of this model is depicted in Figure 1. The measured variables are represented by the rectangles in the model. The circles signify latent genetic and environmental variables. “A” refers to additive genetic variance, or the effect of independent alleles or loci that “add up” to contribute to twin similarity; “C” represents shared environmental variance, environmental factors that contribute to twin similarity; and “E” signifies nonshared environmental variance which does not contribute to twin similarity (Plomin, DeFries, McClearn, & Rutter, 1997). The direct paths (arrows) estimated in these models from A1, C1, and E1 to AC and A2, C2 and E2 to anxiety yield genetic and environmental variance components for all variables. The genetic and environmental covariance between AC and anxiety is represented by the paths between A1, C1, and E1 and anxiety. Genetic and environmental correlations (i.e., rg, rc, re) between AC and the various anxiety variables can be derived using this model. These genetic and environmental correlations index the degree to which genetic or environmental factors for AC overlap with the anxiety variables, independent of their heritabilities. In addition, the genetic and environmental contributions to phenotypic correlations between AC and anxiety can also be estimated.
FIGURE 1.
Bivariate Cholesky Model.
Note. A = additive genetic variance; C = shared environmental variance; E = nonshared environmental variance. Direct paths (arrows) estimated in these models from A1, C1, and E1 to AC and A2, C2 and E2 to anxiety yield genetic and environmental variance components for all variables. Genetic and environmental covariance between AC and anxiety is represented by the paths between A1, C1, and E1 and anxiety.
The full bivariate model was fit separately to AC and each of the 4 measures of anxiety (generalized, obsessive, separation, and social). The overall fit of each ACE model was assessed by calculating twice the difference between the negative log-likelihood (−2LL) of the model and that of a saturated model (i.e., a model in which the variance/covariance structure is not estimated and all variances and covariances for MZ and DZ twins are estimated). The difference in −2LL is asymptotically distributed as χ2 with degrees of freedom equal to the difference in the number of parameters in the full model and that in the saturated model. Alternate models were then tested and compared to the full models using the χ2 difference test. Specifically, the additive genetic and shared environmental variances for AC and each anxiety variable, and additive genetic, shared environmental, and nonshared environmental covariances between variables were dropped from the models to determine whether genetic and/or environmental variances and covariances were significant. If a significant difference in chi-square between a reduced model and the full model occurs, a poorer fit is indicated, and the eliminated parameter is considered significant. Heritability estimates, environmental variances, genetic and environmental correlations, and their 95% confidence intervals were estimated from the best-fitting model (i.e., the most parsimonious model with no significant difference in chi-square from the full model).
RESULTS
Descriptive Statistics, Gender Differences, and Phenotypic Correlations
Table 1 lists the means and standard deviations by gender and the effect sizes for gender differences for AC and the anxiety variables. Overall, males had lower levels of anxiety than females, and all differences were significant. Effect sizes as measured by Cohen’s d indicated that the means for males were approximately 14%–28% of a standard deviation lower than females for the anxiety scores. The gender difference for AC was significant, with males scoring approximately 34% of a standard deviation lower than females. Table 2 lists phenotypic correlations for all variables. The anxiety variables were moderately intercorrelated, and all four anxiety variables were negatively correlated with AC. When examining phenotypic correlations between AC and anxiety in males and females separately, all associations were significant and negative regardless of gender. However, the magnitude of all correlations was slightly higher for males (ranging from −.27 to −.44) than for females (−.17 to −.35).
TABLE 1.
Means (and Standard Deviations) for the Full Sample, Males, and Females, and Effect Sizes of Gender Differences for All Variables
| Overall | Males | Females | Effect Size (Cohen’s d) | |
|---|---|---|---|---|
| Attentional control | .00 (.82) | −.15 (.79) | .12 (.81) | −.34** |
| Generalized anxiety | .00 (.72) | −.11 (.67) | .09 (.75) | −.28** |
| Obsessive-compulsive anxiety | −.04 (.74) | −.09 (.73) | .01 (.74) | −.14* |
| Separation anxiety | −.01 (.73) | −.11 (.69) | .08 (.75) | −.26** |
| Social anxiety | .01 (.72) | −.08 (.72) | .08 (.71) | −.22** |
Note. N = 892; male n = 414; female n = 478 (except obsessive-compulsive anxiety with N = 864; male n = 397; female n = 467). Effect size estimated as Cohen’s d express group differences in standard deviation units.
Difference is significant at the p < .05 level (2-tailed);
Difference is significant at the p < .01 level (2-tailed).
TABLE 2.
Phenotypic Correlations for All Variables
| Generalized Anxiety | Obsessive-Compulsive Anxiety | Separation Anxiety | Social Anxiety | |
|---|---|---|---|---|
| Attentional control | −.36 | −.22 | −.35 | −.26 |
| Generalized anxiety | .44 | .62 | .60 | |
| Obsessive-compulsive anxiety | .41 | .30 | ||
| Separation anxiety | .42 | |||
| Phenotypic correlations between attentional control and anxiety by gender | ||||
| Attentional control (Females) | −.43 | −.27 | −.44 | −.37 |
| Attentional control (Males) | −.35 | −.17 | −.29 | −.18 |
Note. All correlations are significant at p < .01 after Bonferroni corrections.
Twin Correlations and Bivariate Model-Fitting
For all variables except obsessive-compulsive anxiety, MZ intraclass twin correlations exceeded DZ correlations, indicating genetic influences (Table 3). Intraclass twin correlations were also calculated separately by gender, but in general patterns were consistent across gender. The two exceptions include obsessive-compulsive anxiety, where females have a higher DZ correlation than MZ (.45 vs. .28), and separation anxiety, where males have a slightly higher DZ correlation than MZ (.53 vs. .48). Because we are underpowered to conduct sex-limitation model-fitting analyses, the remaining analyses refer to results pooled across gender alone. Therefore, all genetic model-fitting included both males and females. Univariate ACE model-fitting results (Table 3) showed significant genetic variances for all variables and small estimates of shared environmental variance for generalized and separation anxiety only (the remaining variance was due to nonshared environmental variance). The fit statistics for all bivariate Cholesky decomposition models are presented in Table 4. Table 5 presents estimates of genetic and environmental variances for AC and each anxiety variable, as well as significant covariances between AC and the anxiety variables for the best-fitting models. We also calculated the genetic and environmental variance shared (“overlap”) between AC and each anxiety variable when covariances were present. For all sets of bivariate models except AC and separation anxiety, we were able to drop all shared environmental paths with no significant decrement in fit. With both obsessive-compulsive and social anxiety, we were also able to drop nonshared environmental covariance between AC and those variables. Shared environmental influences were significant for separation anxiety, but there was no shared environmental covariance between AC and separation anxiety.
TABLE 3.
Twin Intraclass Correlations and Univariate ACE Model Estimates for All Variables
| Attentional Control
|
Generalized Anxiety
|
Obsessive-Compulsive Anxiety
|
Separation Anxiety
|
Social Anxiety
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | |
| All | .59 | .15 | .53 | .35 | .33 | .35 | .55 | .45 | .57 | .26 |
| Females | .61 | .13* | .55 | .33 | .28 | .45 | .62 | .38 | .64 | .24 |
| Males | .57 | .17 | .52 | .38 | .36 | .20 | .48 | .53 | .51 | .28 |
|
| ||||||||||
| Additive genetic, shared environmental and nonshared environmental parameter estimates from univariate ACE models
|
||||||||||
| Additive genetic | .73 | .53 | .39 | .52 | .77 | |||||
| Shared env. | – | .05 | – | .07 | – | |||||
| Nonshared env. | .27 | .42 | .61 | .41 | .23 | |||||
Note.
All correlations are significant at p < .01, except the female DZ twin correlation for AC which is significant at p < .05. MZ = monozygotic twins; DZ = dizygotic twins.
TABLE 4.
Fit Statistics for Bivariate Cholesky Models of Attentional Control and Anxiety Variables
| Variables and Model | Overall Fit of Model
|
Relative Fit of Model
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| −2LL | df | χ2 | df | p | AIC | Δχ2 | df | p | |
| Attentional control and generalized anxiety | |||||||||
| Saturated | 3640.14 | 1713 | |||||||
| 1. ACE | 3668.12 | 1724 | 27.98 | 11 | .00 | 5.98 | |||
| 2. CE | 3700.67 | 1727 | 60.53 | 14 | .00 | 32.53 | 32.55 | 3 | .00 |
| 3. AE | 3669.63 | 1727 | 29.49 | 14 | .01 | 1.49 | 1.51 | 3 | .68 |
| 4. E | 3800.16 | 1730 | 160.02 | 17 | .00 | 126.02 | 132.04 | 6 | .00 |
| Attentional control and obsessive-compulsive anxiety | |||||||||
| Saturated | 4056.85 | 1718 | |||||||
| 1. ACE | 4090.83 | 1729 | 33.99 | 11 | .00 | 11.99 | |||
| 2. CE | 4116.27 | 1732 | 59.43 | 14 | .00 | 31.43 | 25.44 | 3 | .00 |
| 3. AE | 4092.90 | 1732 | 36.06 | 14 | .00 | 8.06 | 2.07 | 3 | .56 |
| 4. E | 4199.46 | 1735 | 142.61 | 17 | .00 | 108.61 | 108.62 | 6 | .00 |
| 5. AE drop e covariance | 4092.91 | 1733 | 36.07 | 15 | .00 | 6.07 | 2.08 | 4 | .72 |
| Attentional control and separation anxiety | |||||||||
| Saturated | 3609.98 | 1717 | |||||||
| 1. ACE | 3644.39 | 1728 | 34.42 | 11 | .00 | 12.42 | |||
| 2. CE | 3674.68 | 1731 | 64.69 | 14 | .00 | 36.69 | 30.28 | 3 | .00 |
| 3. AE | 3652.41 | 1731 | 42.43 | 14 | .00 | 14.43 | 8.02 | 3 | .05 |
| 4. E | 3827.85 | 1734 | 217.87 | 17 | .00 | 183.87 | 183.45 | 6 | .00 |
| 5. AE drop c covariance | 3647.00 | 1729 | 37.03 | 12 | .00 | 13.03 | 2.61 | 1 | .11 |
| Attentional control and social anxiety | |||||||||
| Saturated | 3776.88 | 1717 | |||||||
| 1. ACE | 3804.90 | 1728 | 28.03 | 11 | .00 | 6.03 | |||
| 2. CE | 3843.40 | 1731 | 66.53 | 14 | .00 | 38.53 | 38.50 | 3 | .00 |
| 3. AE | 3804.91 | 1731 | 28.03 | 14 | .01 | 0.03 | .00 | 3 | 1.0 |
| 4. E | 3926.22 | 1734 | 149.34 | 17 | .00 | 115.34 | 121.32 | 6 | .00 |
| 5. AE drop e covariance | 3808.14 | 1732 | 31.26 | 15 | .01 | 1.26 | 3.24 | 4 | .52 |
Note. −2LL = likelihood statistic; df = degrees of freedom; AIC = Akaike’s information criterion; χ2diff = Chi-square difference between full ACE/ACE model and reduced model; dfdiff = df difference between full ACE/ACE model and reduced model; A = additive genetic effects; C = shared environmental effects; E = nonshared environmental effects. Boldface denotes best-fitting model.
TABLE 5.
Bivariate Estimates of Genetic and Environmental Variance, and Genetic and Environmental Correlations (and 95% Confidence Intervals) for Attentional Control and Anxiety Variables
| Variance Components
|
Genetic and Environmental Correlations
|
|||||
|---|---|---|---|---|---|---|
| a2 | c2 | e2 | rg | re | ||
| 1. Attentional control and generalized anxiety | ||||||
| Attentional control | .55 (.42; .65) | – | .45 (.35; .58) | Attentional control–generalized anxiety | −.41 (−.56; −.26) | −.31 (−.44; −.16) |
| Overlap | .10 (.04; .19) | – | .04 (.01; .09) | |||
| Generalized anxiety | .48 (.38; .57) | – | .38 (.30; .47) | |||
| 2. Attentional control and obsessive-compulsive anxiety | ||||||
| Attentional control | .55 (.43; .65) | – | .45 (.35; .57) | Attentional control–obsessive-compulsive anxiety | −.36 (−.50; −.23) | – |
| Overlap | .06 (.03; .12) | – | – | |||
| Obsessive-compulsive anxiety | .42 (.30; .52) | – | .52 (.42; .64) | |||
| 3. Attentional control and separation anxiety | ||||||
| Attentional control | .54 (.41; .64) | – | .46 (.36; .59) | Attentional control–separation anxiety | −.47 (−.77; −.28) | −.24 (−.38; −.09) |
| Overlap | .10 (.03; .19) | – | .02 (.35; .58) | |||
| Separation anxiety | .33 (.09; .55) | .21 (.03;.38) | .34 (.26; .44) | |||
| 4. Attentional control and social anxiety | ||||||
| Attentional control | .55 (.43; .65) | – | .45 (.35; .57) | Attentional control–social anxiety | −.42 (−.54; −.29) | – |
| Overlap | .10 (.05; .17) | – | – | |||
| Social anxiety | .47 (.35; .57) | – | .43 (.34; .54) | |||
Note. a2 = genetic variance; c2 = shared environmental variance; e2 = nonshared environmental variance; rg = genetic correlation; re = nonshared environmental correlation. Overlap refers to genetic and environmental variance that is shared by attentional control and the anxiety variables.
Genetic factors accounted for between 54% and 55% of the variance in attentional control depending on the specific model and between 43% and 58% of the variance in our anxiety variables. For all variables (excepting separation anxiety), the remaining variance was due to nonshared environmental influences. Shared environmental influences accounted for 21% of the variance in separation anxiety. Genetic correlations between AC and the four anxiety variables ranged from −.47 to −.36, which indicates that overlapping genetic effects contribute to associations between attentional control and anxiety. There were also significant nonshared environmental correlations between AC and generalized anxiety and AC and separation anxiety (re = −.31 and −.24, respectively). The negative values reflect that low attentional control was associated with increased levels of anxiety. Variance components for the anxiety variables (i.e., a2, c2, and e2) are presented as values that contribute to overlap between AC and anxiety, or to anxiety independent of AC. For example, the total genetic variance for generalized anxiety was .10 (overlap) plus .48 (unique to generalized anxiety) = .58. We saw similar patterns for genetic factors on all other anxiety variables and for nonshared environmental influences on generalized and separation anxiety. The percentages of overlap between genetic influences on AC and the anxiety composites were 17% (.10/.58 = .17), 13% (.06/.48 = .13), 23% (.10/.43 = .23), and 18% (.10/.57 = .18) for generalized, obsessive-compulsive, separation, and social anxiety, respectively.
DISCUSSION
We examined genetic and environmental influences on individual differences in AC and several anxiety phenotypes in adolescent twins with a primary focus on the phenotypic and etiological association between AC and various aspects of anxiety. Previous behavior genetic research on this topic has either focused on broad anxiety phenotypes or one specific anxiety diagnosis, and few studies have assessed early adolescents. This project provides the first twin study–based examination of genetic and environmental factors that contribute to both AC and several distinct aspects of anxiety in adolescence. Utilizing generalized, obsessive-compulsive, separation, and social anxiety phenotypes provides important information about differential etiology, as well as the specificity of phenotypic and etiological associations with AC. All variables (AC and the four anxiety variables) were significantly heritable, as expected from previous research. However, patterns of genetic and environmental variance for anxiety as well as relations with AC differed somewhat depending on the type of anxiety. We conclude that AC is a risk factor for anxiety in early adolescence, but that it may confer somewhat different levels of risk for different anxiety disorders.
The four aspects of anxiety were moderately correlated, suggesting that although there is some overlap between the four categories, there is also partial independence. This partial overlap lends support to our rationale for examining generalized, obsessive-compulsive, separation, and social anxiety phenotypes separately. If phenotypic covariance across the four anxiety diagnoses was to be much higher, the argument for examining different aspects would be diminished. Most importantly, moderate negative phenotypic associations were observed for AC with all 4 anxiety variables (correlations ranged from −.22 to −.36). These findings show that adolescents with low levels of AC are more likely to exhibit anxiety symptoms, as posited by attentional control theory (Eysenck & Derakshan, 2011; Eysenck et al., 2007). Relationships between AC and generalized and separation anxiety were strongest, whereas those between AC and obsessive-compulsive and social anxiety were slightly less robust. These phenotypic associations are similar to the many findings in the literature that focus on AC and broad anxiety traits across age (e.g., Berggren & Derakshan, 2013b; Derakshan et al., 2009; Derryberry & Reed, 2002; Susa et al., 2012), as well as those that focus on AC and specific anxiety categories (e.g., Armstrong et al., 2011; Moriya & Tanno, 2008; Wieser et al., 2009).
As expected, genetic and nonshared environmental influences were significant for AC and all anxiety variables. Shared environmental variance was present for the separation anxiety variable only. Phenotypic relationships between AC and the anxiety variables were largely due to genetic covariance. In the case of phenotypic associations between AC and generalized and separation anxiety, nonshared environmental covariance was also significant. Thirteen percent to 23% of genetic factors were shared by AC and anxiety disorders, meaning that 77%–87% of the genetic variance in our anxiety phenotypes was unique. Therefore, although there is significant genetic overlap between AC and anxiety in early adolescence, the remainder of the genetic variance is specific to the anxiety phenotypes. Only 4% of the nonshared environmental variance was shared by AC and generalized anxiety, and 2% was shared by AC and separation anxiety. Thus, almost all of the nonshared environmental variance in our anxiety phenotypes was unique.
Although not present for most variables, significant shared environmental effects emerged for separation anxiety, a finding anticipated by at least one other study in childhood (Topolski et al., 1997). Possible sources of shared environmental variance that could influence separation anxiety in adolescence include experiences living in the same home and similarities in school routine and dress, consistency in parent behavior across the twins, and measurement effects related to the same parent rating both twins. However, most of these potential shared environmental factors would be relevant for other forms of anxiety. It may be that one parent expresses a particular form of neuroticism or clinginess that is modeled by both twins and is reflected as shared environmental variance for separation anxiety only. Unfortunately, we cannot empirically pinpoint specific sources of shared environmental variance in this study.
As is typical for most psychological traits in adolescence and all previous studies of AC and anxiety, nonshared environmental variance was present for all variables, and in two instances contributed to covariance between AC and anxiety variables (generalized and separation anxiety). Nonshared environmental variance includes measurement error, and because some of our measures were less reliable than others, it is possible that measurement error is responsible for some of these effects. However, there were no appreciable differences between nonshared environmental estimates for AC (which was made up of component variables with the lowest levels of reliability) and our anxiety variables. Additionally, the use of composite variables reduces overall unreliability. Therefore, it is unlikely that all nonshared environmental factors are due entirely to error. Other possible sources of nonshared environment may include differential treatment by parents, different peer groups and school activities, and other experiences that are specific to the child such as injuries and accidents (Plomin et al., 1997). For example, only one twin could be mistreated by a parent, or only one twin might participate in an after-school club or sport. These sources of nonshared environment tend to increase throughout middle childhood as children are exposed to increasingly unique environments as they age.
Findings of significant heritability for AC in early adolescence were consistent with previous twin studies of AC in middle childhood (Lemery-Chalfant et al., 2008) and late adolescence and early adulthood (Yamagata et al., 2005), as well as modest heritability from ages 9 to 12 in an adoption study of attention (Gagne et al., 2003). Relatedly, patterns of heritability for our anxiety phenotypes were similar to those for previous behavior genetic studies of broad anxiety variables across age (e.g., Eley et al., 2007; Taylor et al., 2008; Trzaskowski et al., 2012; Warren et al., 1999; Zavos et al., 2012). When focusing on the specific types of anxiety, heritabilities are also similar to those from previous studies. Genetic variances for generalized, obsessive-compulsive, and social anxiety ranged from .48 to .58 overlapping with findings from other children (Eley et al., 2003; Hallett et al., 2009) and adult twin studies (Hettema et al., 2001). The heritability estimate for separation anxiety in our sample was slightly lower than our other anxiety variables (.43), probably due to the presence of shared environmental effects for this trait. This finding is consistent with the low end of the range of heritability for separation anxiety from previous investigations (.39–.73; Bolton et al., 2006; Eley et al., 2003).
Phenotypic associations between AC and all anxiety variables were primarily due to genetic covariance and, to a smaller extent, nonshared environmental covariance for generalized and separation anxiety. Nonshared environmental effects operating on obsessive-compulsive and social anxiety were entirely unique to those anxiety traits. Therefore, although nonshared environmental influences are significant for both AC and anxiety in our study, most nonshared environmental factors are independent of the two. Sources of nonshared environmental variance such as measurement error, differential parental treatment, different peer groups, and other experiences that are specific to the child (Plomin et al., 1997) affect AC and anxiety in largely different ways. Similarly, although some of the genetic factors were shared across AC and anxiety phenotypes, most are unique to each individually. For many of our anxiety phenotypes, approximately 20% of the variance due to genetic factors is shared by both AC and anxiety. Interestingly, the genetic covariance between AC and obsessive-compulsive anxiety is slightly lower at 13%. This pattern of results suggests that AC can be considered a modest genetic risk factor for generalized, separation, and social anxiety, but slightly less so for obsessive-compulsive anxiety. There is little environmental overlap between AC and the anxiety traits studied. Examining the etiological overlap allows us to move beyond basic phenotypic covariance and determine whether genetic or environmental factors contribute to the association between AC and our anxiety variables.
Although the issue of gender in the development and etiology of both AC and anxiety disorders is an important one, it was not the primary emphasis of our study. Nevertheless, in light of previous findings in the literature showing gender differences for AC and anxiety phenotypes and different patterns of etiology for males versus females for certain anxiety symptoms, we examined gender in multiple analyses. Consistent with the literature, females were more anxious and showed greater attentional control than males. With regard to phenotypic associations between AC and our anxiety symptom variables, the magnitude was greater for males than females (although both male and female correlations were significant and in the same direction). This finding indicates that low AC is a slightly greater phenotypic risk factor for males than females. When calculating twin correlations, the patterns of MZ and DZ correlations were fairly consistent across gender. The two exceptions were that females have a higher DZ correlation than MZ (.45 vs. .28) for OCD, and males have a slightly higher DZ correlation than MZ (.53 vs. .48) for separation anxiety. This pattern of findings suggests low heritability and shared environmental influences. Indeed, these anxiety variables showed the lowest levels of genetic variance in our bivariate models with both genders included, and OCD showed significant shared environmental variance. Interestingly, we did not find shared environment variance for generalized anxiety disorder with our female subsample, as the previous meta-analysis had (Hettema et al., 2001). Patterns of phenotypic and twin correlations indicate that it is possible that genetic covariances between AC and certain types of anxiety may be higher for males, but that is an empirical question that we are unable to answer with our data. Because we only have between 55 and 60 pairs of female MZ twins depending on the variable, we do not have the statistical power to conduct univariate or bivariate Cholesky models by gender. Therefore, we did not pursue model-fitting analyses separately for males and females. However, gender is an important issue to consider in the context of the etiology of AC and anxiety in adolescence, and future investigations with sufficient power should conduct more comprehensive gender-related analyses.
One limitation of the current study is the relatively modest sample size, which is underpowered for examining gender using sex-limitation model-fitting biometric approaches (and to conduct bivariate analyses by gender). However, twin studies of temperament with substantially larger sample sizes tend to focus on one form of temperament assessment, usually parent report. In this case, we have a multimethod approach that includes both parent and twin-reported assessments of AC on multiple indices, which allows us to draw more broadly based conclusions about the shared etiology of AC and different anxiety disorders. Additionally, many large-sample investigations of the etiology of AC and anxiety outcomes in early adolescence focus on broad or limited conceptions of anxiety without the benefit of multimethod assessment. We have four discrete anxiety symptom categories, which allows for more specificity in interpreting our results. Unfortunately, we do not have laboratory-based measures of AC or anxiety in this study, which would further bolster our multimethod approach. Future investigations of these issues would be enhanced by including a behavioral assessment of AC in addition to parent and child ratings.
This study confirms several important previous findings regarding associations between AC and anxiety phenotypes, and strengthens conclusions by incorporating multiple anxiety outcomes and including genetic analyses. Our findings are consistent with the literature in that AC is significantly associated with all anxiety variables, and both AC and anxiety are genetically influenced in our sample. In addition, overlapping genetic covariance contributes to the phenotypic associations between AC and anxiety symptoms in adolescence. Therefore, early adolescent AC can be considered a developmental and genetic risk factor for anxiety disorders. The magnitude of the phenotypic and genetic relationships between AC and anxiety varied to some extent by type of anxiety symptom.
Our results also suggest areas for future study. As mentioned, adding laboratory-based or behavioral assessments of AC would bolster multimethod findings, as would incorporating additional genetic analyses. In particular, molecular approaches would be useful. Although molecular genetic investigations of anxiety phenotypes have been ongoing (for a current review see Perez, Otowa, Roberson-Nay, & Hettema, 2013), we know less about potential genetic risk factors for AC. Adolescence is clearly an important period for AC and anxiety, but extending these results longitudinally in both directions is also necessary for a more comprehensive developmental perspective. Research on these variables has public health relevance and could potentially inform interventions that target attentional abilities in the treatment of anxiety disorders. The assessment of AC could facilitate the identification of children at risk for the development of these disorders and could also inform molecular genetic studies in this area. However, until the developmental and etiological implications of the relationship between AC and anxiety are better explicated, it is unclear what interventions will be appropriate.
Acknowledgments
This work was supported by research grants from the National Institute of Mental Health including R01 MH59785, RO1 MH101504, and R37 MH50560; and a Conte Neuroscience Center grant (P50 MH084051, Center Director, R. J. Davidson). The first author was supported by two training grants (T32-MH18931, Director, R. J. Davidson, and T32-MH75880, Director, M. A. Gernsbacher). Infrastructure support was provided by the Waisman Center via a core grant from the National Institute of Child Health and Human Development (P30 HD03352). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Jeffrey R. Gagne, University of Texas at Arlington
Deirdre L. O’Sullivan, University of Wisconsin–Madison
Nicole L. Schmidt, University of Wisconsin–Madison
Catherine A. Spann, University of Texas at Arlington
H. Hill Goldsmith, University of Wisconsin–Madison.
References
- Ablow JC, Measelle JF, Kraemer HC, Harrington R, Luby J, Smider N, … Kupfer DJ. The MacArthur Three-City Outcome Study: Evaluating multi-informant measures of young children’s symptomatology. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1580–1590. doi: 10.1097/00004583-199912000-00020. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. [Google Scholar]
- Anagnostaras SG, Craske MG, Fanselow MS. Anxiety: At the intersection of genes and experience. Nature Neuroscience. 1999;2:780–782. doi: 10.1038/12146. [DOI] [PubMed] [Google Scholar]
- Armstrong T, Zald DH, Olatunji O. Attentional control in OCD and GAD: Specificity and associations with core cognitive symptoms. Behavior Research and Therapy. 2011;49:756–762. doi: 10.1016/j.brat.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Hofler M, Lieb R, Wittchen H. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry. 2007;64:903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. Psychiatric Clinics of North America. 2009;32:483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Pine DS, Lieb R, Wittchen H. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Archives of General Psychiatry. 2010;67:47–57. doi: 10.1001/archgenpsychiatry.2009.177. [DOI] [PubMed] [Google Scholar]
- Berggren N, Derakshan N. Attentional control deficits in trait anxiety: Why you see them and why you don’t. Biological Psychology. 2013a;92:440–446. doi: 10.1016/j.biopsycho.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Berggren N, Derakshan N. Trait anxiety reduces implicit expectancy during target spatial probability cueing. Emotion. 2013b;13:345–349. doi: 10.1037/a0029981. [DOI] [PubMed] [Google Scholar]
- Bolton D, Eley TC, O’Connor TG, Perrin S, Rabe-Hesketh S, Rijsdijk F, Smith P. Prevalence and genetic and environmental influences on anxiety disorders in 6-year-old twins. Psychological Medicine. 2006;36:335–344. doi: 10.1017/S0033291705006537. [DOI] [PubMed] [Google Scholar]
- Bowen RC, Offord DR, Boyle MH. The prevalence of overanxious disorder and separation anxiety disorder: Results from the Ontario Child Health Study. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;29:753–758. doi: 10.1097/00004583-199009000-00013. [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Neiderhiser JM, Kiel EJ, Leve LD, Shaw DS, Reiss D. The association between infants’ attention control and social inhibition is moderated by genetic and environmental risk for anxiety. Infancy. 2011;16:490–507. doi: 10.1111/j.1532-7078.2011.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canino G, Shrout PE, Rubio-Stripec M, Bird HR, Bravo M, Ramirez R, et al. The DSM-IV rates of child and adolescent disorders in Puerto Rico. Archives of General Psychiatry. 2004;61:85–93. doi: 10.1001/archpsyc.61.1.85. [DOI] [PubMed] [Google Scholar]
- Capaldi DM, Rothbart MK. Development and validation of an early adolescent temperament measure. Journal of Early Adolescence. 1992;12:153–173. doi: 10.1177/0272431692012002002. [DOI] [Google Scholar]
- Costello JE, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- De Graaf R, Bijl RV, Spijker J, Beekman ATF, Vollebergh WAM. Temporal sequencing of lifetime mood disorders in relation to comorbid anxiety and substance use disorders: Findings from the Netherlands Mental Health Survey and Incidence Study. Social Psychiatry and Psychiatric Epidemiology. 2003;38:1–11. doi: 10.1007/s00127-003-0597-4. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Smyth S, Eysenck MW. Effects of state anxiety on performance using a task-switching paradigm: An investigation of attentional control theory. Psychonomic Bulletin and Review. 2009;16:1112–1117. doi: 10.3758/PBR.16.6.1112. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037/0021-843X.111.2.225. [DOI] [PubMed] [Google Scholar]
- Dunn LM. Peabody Picture Vocabulary Test: Manual of directions and norms. Minneapolis, MN: American Guidance Service; 1965. [Google Scholar]
- Dunn M, Dunn LM. Peabody Picture Vocabulary Test—4. Circle Pines, MN: American Guidance Service; 2007. [Google Scholar]
- Eley TC, Bolton D, O’Connor TG, Perrin S, Smith P, Plomin R. A twin study of anxiety-related behaviours in pre-school children. Journal of Child Psychology and Psychiatry. 2003;44:945–960. doi: 10.1111/1469-7610.00179. [DOI] [PubMed] [Google Scholar]
- Eley TC, Gregory AM, Clark DM, Ehlers A. Feeling anxious: A twin study of panic/somatic ratings, anxiety sensitivity and heartbeat perception in children. Journal of Child Psychology and Psychiatry. 2007;48:1184–1191. doi: 10.1111/j.1469-7610.2007.01838.x. [DOI] [PubMed] [Google Scholar]
- Essau CA, Conradt J, Petermann F. Frequency, comorbidity, and psychosocial impairment of anxiety disorders in German adolescents. Journal of Anxiety Disorders. 2000;14:263–279. doi: 10.1016/S0887-6185(99)00039-0. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Boyce WT, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ The MacArthur Assessment Battery Working Group. The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the MacArthur Health and Behavior Questionnaire. The Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N. New perspectives in attentional control theory. Personality and Individual Differences. 2011;50:955–960. doi: 10.1016/j.paid.2010.08.019. [DOI] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Flament MF, Whitaker A, Rapoport JL, Davies M, Berg CZ, Kalikow K, Sceery W, Shaffer D. Obsessive compulsive disorder in adolescence: An epidemiological study. Journal of the American Academy of Child and Adolescent Psychiatry. 1988;27:764–771. doi: 10.1097/00004583-198811000-00018. [DOI] [PubMed] [Google Scholar]
- Fontenelle LF, Hasler G. The analytical epidemiology of obsessive-compulsive disorder: Risk factors and correlates. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32:1–15. doi: 10.1016/j.pnpbp.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Ford T, Goodman R, Meltzer H. The British Child and Adolescent Mental Health Survey 1999: The prevalence of DSM-IV disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:1203–1211. doi: 10.1016/j.pnpbp.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Fox NA, Pine DS. Temperament and the emergence of anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;52:125–128. doi: 10.1016/j.jaac.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JR, Saudino KJ, Cherny SS. Genetic influences on temperament in early adolescence: A multimethod perspective. In: Petrill SA, Plomin R, DeFries JC, Hewitt JK, editors. Nature, nurture, and the transition to early adolescence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH. A zygosity questionnaire for young twins: A research note. Behavior Genetics. 1991;21:257–269. doi: 10.1007/BF01065819. 0001-8244/91/0500-0257. [DOI] [PubMed] [Google Scholar]
- Goldsmith H, Lemery K, Essex M. Temperament as a liability factor for childhood behavioral disorders: The concept of liability. In: DiLalla LF, editor. Behavior genetics principles: Perspectives in development, personality, and psychopathology. Washington, DC: American Psychological Association; 2004. pp. 19–39. [Google Scholar]
- Goldsmith HH, Lemery-Chalfant K, Schmidt NL, Arneson CL, Schmidt CK. Longitudinal analyses of affect, temperament, and childhood psychopathology. Twin Research and Human Genetics. 2007;10:118–126. doi: 10.1375/twin.10.1.118. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Franklin M, Foa E, Simons R. Increased error-related brain activity in pediatric obsessive-compulsive disorder before and after treatment. American Journal of Psychiatry. 2008;165:116–123. doi: 10.1176/appi.ajp.2007.07010143. [DOI] [PubMed] [Google Scholar]
- Hallett V, Ronald A, Rijsdijk F, Eley TC. The phenotypic and genetic differentiation of anxiety-related-behaviors in middle childhood. Depression and Anxiety. 2009;26:316–324. doi: 10.1002/da.20539. [DOI] [PubMed] [Google Scholar]
- Helzer EG, Connor-Smith JK, Reed MA. Traits, states, and attentional gates: Temperament and threat relevance as predictors of attentional bias to social threat. Anxiety, Stress and Coping. 2009;22:57–76. doi: 10.1080/10615800802272244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Al-Khindi T. ERN and the placebo: A misattribution approach to studying the arousal properties of the error-related negativity. Journal of Experimental Psychology: General. 2012;141:799–807. doi: 10.1037/a0027586. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA, Fox NA. Early behavioral inhibition and increased response monitoring predict later social phobia symptoms in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:447–455. doi: 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemery-Chalfant K, Doelger L, Goldsmith HH. Genetic relations between effortful and attentional control and symptoms of psychopathology in middle childhood. Infant and Child Development. 2008;17:365–385. doi: 10.1002/icd.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemery-Chalfant K, Schreiber JS, Schmidt NL, Van Hulle CA, Essex MJ, Goldsmith HH. Assessing internalizing, externalizing and attention problems in young children: Validation of the MacArthur HBQ. The Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1315–1323. doi: 10.1097/chi.0b013e3180f616c6. [DOI] [PubMed] [Google Scholar]
- Liang K, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- Liew J. Effortful control, executive functions, and education: Bringing self-regulatory and social-emotional competencies to the table. Child Development Perspectives. 2012;6:105–111. doi: 10.1111/j.1750-8606.2011.00196.x. [DOI] [Google Scholar]
- McDermott JM, Pérez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ. Adjustment of twin data for the effects of age and sex. Behavior Genetics. 1984;14:325–343. doi: 10.1007/BF01080045. 0001-8244/84/0700-032. [DOI] [PubMed] [Google Scholar]
- Meyer A, Hajcak G, Torpey DC, Kujawa A, Kim J, Bufferd S, Carlson G, Klein DN. Increased error-related brain activity in six-year-old children with clinical anxiety. Journal of Abnormal Child Psychology. 2013;41:1257–1266. doi: 10.1007/s10802-013-9762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya J, Tanno Y. Relationships between negative emotionality and attentional control in effortful control. Personality and Individual Differences. 2008;44:1348–1355. doi: 10.1016/j.paid.2007.12.003. [DOI] [Google Scholar]
- Morrison AS, Heimberg RG. Attentional control mediates the effect of social anxiety on positive affect. Journal of Anxiety Disorders. 2013;27:56–67. doi: 10.1016/j.janxdis.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Meester C. Reactive and regulative temperament in youths: Psychometric evaluation of the Early Adolescent Temperament Questionnaire–Revised. Journal of Psychopathological and Behavioral Assessment. 2009;31:7–19. doi: 10.1007/s10862-008-9089-x. [DOI] [Google Scholar]
- National Institute of Mental Health (NIMH) 2013 Retrieved from http://www.nimh.nih.gov/health/publications/anxiety-disorders-in-children-and-adolescents/index.shtml.
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 5. Richmond, VA: Virginia Institute for Psychiatric and Behavioral Genetics, Department of Psychiatry, Virginia Commonwealth University; 2001. [Google Scholar]
- Perez JA, Otowa T, Roberson-Nay R, Hettema JM. Genetics of anxiety disorders. In: Charney DS, Buxbaum JD, Sklar P, Nestler EJ, editors. Neurobiology of mental illness. Vol. 4. New York, NY: Oxford University Press; 2013. pp. 537–548. [Google Scholar]
- Pérez-Edgar K, Fox NA. Temperament and anxiety disorders. Child and Adolescent Psychiatric Clinics of North America. 2005;14:681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Perou R, Bitsko RH, Blumberg SJ, Pastor P, Ghandour RM, Gfroerer JC, … Huang LN. Mental health surveillance among children – United Sates, 2005–2011. CDC Supplements. 2013;62:1–35. [PubMed] [Google Scholar]
- Pine DS. Research review: A neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology and Psychiatry. 2007;48:631–638. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, Rutter M. Behavioral Genetics. Vol. 3. New York, NY: W. H. Freeman; 1997. [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Eisenberg N, Damon W, Lerner RM, editors. Handbook of child psychology: Vol. 3, Social, emotional, and personality development. 6. Hoboken, NJ: John Wiley; 2006. pp. 99–166. [Google Scholar]
- Rothbart MK, Ellis LK, Posner MI. Temperament and self-regulation. In: Baumeister RF, Vohs KD, editors. Handbook of self-regulation: Research, theory, and applications. New York, NY: Guilford Press; 2004. pp. 357–370. [Google Scholar]
- Schienle A, Hettema JM, Cáceda R, Nemeroff CB. Neurobiology and genetics of generalized anxiety disorder. Psychiatric Annals. 2011;41:113–123. doi: 10.3928/00485713-20110203-10. [DOI] [Google Scholar]
- Schwab-Stone ME, Shaffer D, Dulcan MK, Jensen PS, Fisher P, Bird HR, … Rae DS. Criterion validity of the NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3) Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:865–877. doi: 10.1097/00004583-199607000-00013. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Dulcan MK, Davies M, Piacentini J, Schwab-Stone ME, … Regier DA. The NIMH Diagnostic Interview Schedule for Children Version 2.3 (DISC-2.3): Description, acceptability, prevalence rates, and performance in the MECA Study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:878–888. doi: 10.1097/00004583-199607000-00012. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Susa G, Pitica I, Benga O, Miclea M. Anxiety-related attention biases in preschoolers: An investigation using the pictorial dot-probe task. Procedia–Social and Behavioural Sciences. 2012;33:637–641. doi: 10.1016/j.sbspro.2012.01.199. [DOI] [Google Scholar]
- Taylor S, Jang KL, Stewart SH, Stein MB. Etiology of the dimensions of anxiety sensitivity: A behavioral-genetic analysis. Journal of Anxiety Disorders. 2008;22:899–914. doi: 10.1016/j.janxdis.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Topolski TD, Hewitt JK, Eaves LJ, Silberg JL, Meyer JM, Rutter M, Pickles A, Simonoff E. Genetic and environmental influences on child reports of manifest anxiety and symptoms of separation anxiety and overanxious disorders: A community-based twin study. Behavior Genetics. 1997;27:15–28. doi: 10.1023/A:1025607107566. [DOI] [PubMed] [Google Scholar]
- Trzaskowski M, Zavos HMS, Haworth CMA, Plomin R, Eley TC. Stable genetic influence on anxiety-related behaviors across middle childhood. Journal of Abnormal Child Psychology. 2012;40:85–94. doi: 10.1007/s10802-011-9545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valleni-Basile LA, Garrison CZ, Jackson KL, Waller JL, McKeown RE, Addy CL, Cuffe SP. Frequency of obsessive-compulsive disorder in a community sample of young adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:782–791. doi: 10.1097/00004583-199407000-00002. [DOI] [PubMed] [Google Scholar]
- Warren SL, Schmitz S, Emde RN. Behavioral genetic analyses of self-reported anxiety at seven years of age. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1403–1408. doi: 10.1097/00004583-199911000-00015. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Olvet D, Hajcak G. Increased error-related brain activity in generalized anxiety disorder. Biological Psychology. 2010;85:472–480. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- White LK, McDermott JM, Degnan KA, Henderson HA, Fox NA. Behavioral inhibition and anxiety: The moderating roles of inhibitory control and attention shifting. Journal of Abnormal Child Psychology. 2011;39:735–747. doi: 10.1007/s10802-011-9490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, Pauli P, Muhlberger A. Probing the attentional control theory in social anxiety: An emotional saccade task. Cognitive, Affective, and Behavioral Neuroscience. 2009;9:314–322. doi: 10.3758/CABN.9.3.314. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Stein MB, Kessler RC. Social fears and social phobia in a community sample of adolescents and young adults: Prevalence, risk factors, and co-morbidity. Psychological Medicine. 1999;29:309–323. doi: 10.1017/S0033291798008174. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Fergusson DM. Life course outcomes of young people with anxiety disorders in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1086–1093. doi: 10.1097/00004583-200109000-00018. [DOI] [PubMed] [Google Scholar]
- Yamagata S, Takahashi Y, Kijima N, Maekawa H, Ono Y, Ando J. Genetic and environmental etiology of effortful control. Twin Research and Human Genetics. 2005;8:300–306. doi: 10.1375/twin.8.4.300. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: Gender and psychopathology. Annual Review of Clinical Psychology. 2007;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- Zavos HMS, Gregory AM, Eley TC. Longitudinal genetic analysis of anxiety sensitivity. Developmental Psychology. 2012;48:204–212. doi: 10.1037/a0024996. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang K. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. doi: 10.2307/2531248. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Chen SH, Main A. Commonalities and differences in the research on children’s effortful control and executive function: A call for an integrated model of self-regulation. Child Development Perspectives. 2012;6:112–121. doi: 10.1111/j.1750-8606.2011.00176.x. [DOI] [Google Scholar]