Abstract
For advanced non-squamous non-small cell lung cancer (NSCLC), although platinum/pemetrexed is known to result in a longer survival compared with other regimens, the outcome in the adjuvant setting is still unknown. In this study, the difference of the disease-free survival (DFS) between lung adenocarcinoma patients treated with platinum/pemetrexed and with other platinum-based doublets was concerned. A total of 389 radically resected lung adenocarcinoma patients received adjuvant chemotherapy with platinum/pemetrexed chemotherapy (Group A, n = 143) or other third generation platinum-based regimens (Group B, n = 246) were analyzed in terms of DFS. Propensity score matching (PSM) allowed generation of best matched pairs for the two categories. DFS was proved to be considerably better in pemetrexed doublets group (P = 0.0079); and platinum/pemetrexed was found to be associated with lower rates of several hematological and non-hematological adverse events (AEs), when compared with gemcitabine containing chemotherapy (leukopenia: RR 0.514, p = 0.001; neutropenia: RR 0.688, p = 0.002), or taxanes-doublets treatment (leukopenia: RR 0.685, p = 0.019; neutropenia: RR 0.805, p = 0.032). For patients with radically resected pulmonary adenocarcinoma, adjuvant chemotherapy with platinum/pemetrexed results in a better DFS and a less clinical toxicity in comparison with non-pemetrexed based doublets.
Introduction
Lung cancer, and non-small cell lung cancer (NSCLC) in particular, is a common malignancy and the leading cause of cancer-related death worldwide1. Despite optimal surgical resection for localized NSCLC, the 5-year survival rate without additional treatment is 73% for stage IA disease but declines to 25% for stage IIIA disease. According to the results of several large randomized controlled trials, platinum-based adjuvant chemotherapy (AC) has improved the survival of NSCLC patients with curative resection2–5. The Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis reviewed data from 5 large adjuvant trials of cisplatin-based chemotherapy in NSCLC. The results confirmed the significant effect of postoperative cisplatin-based treatment with both a 5.4% benefit of 5-year overall survival (OS) and a 5.8% benefit of DFS6.
Pemetrexed is an antineoplastic agent, which inhibits folate-dependent metabolic processes indispensable to cell replication7. Several trials on advanced NSCLC had found that, with a satisfying safety profile, pemetrexed combined with cisplatin showed a promising efficacy comparable with other platinum-based therapy8, 9. Platinum/pemetrexed now is recommended as the first-line and maintenance therapy for locally advanced or metastatic non-squamous NSCLC and a single agent pemetrexed regimen is indicated as a second-line therapy10. Furthermore, the result of the TREAT study, a phase II trial on early-stage NSCLC, indicated that cisplatin/pemetrexed provokes less toxicity and maintains better dose delivery than cisplatin/vinorelbine; on the other hand, relapse-free survival (RFS) and OS were not influenced by treatment arm11, 12.
To date, there exists no published data comparing pemetrexed with other third-generation cytotoxic agents, including paclitaxel, docetaxel and gemcitabine, with regard to clinical toxicity and survival in the adjuvant treatment setting for early-stage lung adenocarcinoma. To address this important gap, we undertook a retrospective study to assess the association between clinical toxicity and various platinum-based doublets and to evaluate the survival associated with these therapy regimens.
Materials and Methods
Patients
This retrospective study included 389 consecutive patients who underwent curative resection of lung adenocarcinoma in the Cancer Hospital of the Chinese Academy of Medical Sciences (Beijing, China) between January 2003 and December 2013 (Fig. 1). Inclusion criteria were patients who had fully recovered after resection of pathologically confirmed NSCLC stages (according to the TNM Classification of Malignant Tumors (7th Edition)13) IB, IIA, IIB or IIIA and received postoperative AC, and eligible tumor type was adenocarcinoma. Exclusion criteria were based on histologic type other than adenocarcinoma, prior neoadjuvant chemotherapy, stage IB disease without high-risk factors in terms of poorly differentiated tumors, vascular invasion, wedge resection, tumors >4 cm, visceral pleural involvement, and incomplete lymph node sampling [Nx]. Patients with stage IA, IIIB, or IV were also not eligible. Patients were classified on the basis of age at diagnosis, gender, smoking history, tumor differentiation, pathologic stage, type of resection, lymphatic involvement stage, use of adjuvant radiotherapy, Eastern Cooperative Oncology Group (ECOG) performance status, comorbidity score and number of chemotherapy cycles. All patients who underwent pulmonary resection were followed up from the day of surgery.
Figure 1.
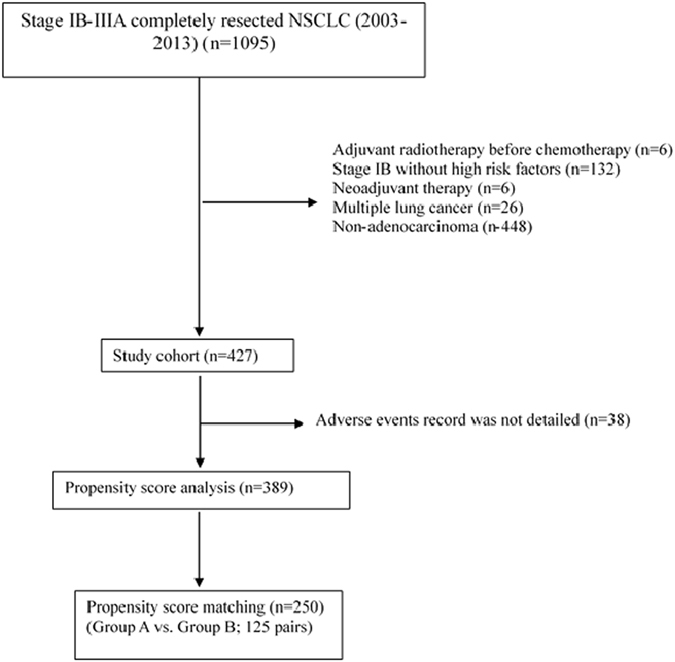
Flow Chart for Selection of Patients.
Comorbidity and outcomes
Based on all non-cancer diagnosis records in the hospital files before surgery, comorbid disorders were assessed by Charlson Comorbidity Index (CCI)14–16. The primary endpoint of the study was DFS, which is defined by the time from surgery to recurrence (local, regional, and/or distant) or death from any cause. Data was censored on the last contact date. Patients who were still alive at the final follow-up (December 20, 2014) were regarded as censored, and the duration between the initial operation and the final follow-up was included in the survival analysis. The secondary endpoint was to evaluate clinical toxicity in all enrolled patients on an intention-to-treat basis. The National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 was used to grade toxicities17. The identified AEs related with the platinum-based regimens were classified as hematological or non-hematological. Both the hematological AEs noted in medical records and obtained from laboratory examination of blood during follow-up were used to decide the extent of chemotherapy-associated hematological AE in order to minimize the risk of losing information. The estimation of non-hematological AEs was only based on medical records.
Statistical analysis
All statistical analysis was performed by using SAS 9.3 software (SAS Institute Inc., Cary, North Carolina, U.S.A.). Baseline characteristics were presented by applying descriptive statistics. A chi-square test was utilized to compare categorical data. Survival curves were generated by using Kaplan-Meier methods. GraphPad Prism 5.0 was used to present the survival curves. Univariate analyses were performed by using the log-rank test and multivariable analysis by using Cox proportional hazard regression model. All statistical tests were two-tailed with p < 0.05 set as significant. To reduce the influence of potential confounding factors and to generate comparable study arms, propensity score matching (PSM) method was applied. Variables were gender, age, smoking history, tumor differentiation, pathological stage, use of adjuvant radiotherapy, ECOG PS, comorbidity score, type of resection and number of chemotherapy cycles. After greedy matching, patients with an equivalent propensity score in the two groups were selected by 1:1 matching without replacement. Subgroup analyses were conducted by Cox regression method to investigate whether any significant difference exists between different ages, gender, smoking history, differentiation, pathological stage, type of resection, PS, comorbidity score, adjuvant radiotherapy and number of chemotherapy cycles.
Results
Patient characteristics
The mean age of the patients was 56 (range 25–76) years, and the proportion of male and female patients was 50.6% vs. 49.4%. Characteristics of the 389 patients are shown in Table 1. The baseline characteristics with significant difference included performance status, use of adjuvant radiotherapy and number of chemotherapy cycles. Patients included in the analysis were then classified into two groups: Group A (n = 143), comprising the patients that received adjuvant chemotherapy with platinum/pemetrexed regimen, and Group B (n = 246), comprising the patients that received non-pemetrexed platinum-based regimens (146 patients received platinum/paclitaxel, 8 patients platinum/docetaxel, 63 patients platinum/gemcitabine, and 29 patients platinum/vinorelbine). Comparing the percentages of cisplatin and carboplatin use, cisplatin predominated in Group A patients (70%), whereas carboplatin was administered with almost equal frequency to patients in Group B (48.8%).
Table 1.
Patients demographic characteristics and propensity score-matched characteristics between two groups.
| Characteristic | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| Pemetrexed (n = 143) | Non- Pemetrexed (n = 246) | P value | Pemetrexed (n = 125) | Non- Pemetrexed (n = 125) | P value | |
| Age | ||||||
| ≤65 | 124 | 206 | 0.467 | 109 | 107 | 0.854 |
| >65 | 19 | 40 | 16 | 18 | ||
| Gender | ||||||
| Male | 73 | 124 | 0.917 | 63 | 64 | 1.0 |
| Female | 70 | 122 | 62 | 61 | ||
| Smoking history | ||||||
| No | 87 | 153 | 0.829 | 73 | 77 | 0.699 |
| Yes | 56 | 93 | 52 | 48 | ||
| Differentiation | ||||||
| Poorly | 28 | 55 | 0.475 | 26 | 27 | 0.750 |
| Moderately | 102 | 171 | 93 | 88 | ||
| Well | 9 | 19 | 5 | 7 | ||
| Unknown | 4 | 1 | 1 | 3 | ||
| Pathologic Stage | ||||||
| IB | 21 | 37 | 0.178 | 21 | 20 | 0.536 |
| IIA | 27 | 69 | 33 | 26 | ||
| IIB | 9 | 10 | 5 | 9 | ||
| IIIA | 86 | 130 | 66 | 70 | ||
| Type of Resection | ||||||
| Lobectomy | 138 | 235 | 0.894 | 123 | 120 | 0.399 |
| Pneumonectomy | 4 | 9 | 1 | 4 | ||
| Wedge resection | 1 | 2 | 1 | 1 | ||
| Performance status | ||||||
| 0 | 48 | 43 | <0.001 | 31 | 32 | 1.0 |
| 1 | 95 | 202 | 94 | 93 | ||
| 2 | 0 | 1 | 0 | 0 | ||
| Charlson | ||||||
| 0 | 103 | 172 | 0.349 | 89 | 90 | 0.932 |
| 1 | 25 | 57 | 27 | 21 | ||
| 2 | 12 | 15 | 8 | 11 | ||
| 3 | 3 | 2 | 1 | 3 | ||
| Adjuvant radiotherapy | ||||||
| No | 103 | 218 | <0.001 | 101 | 101 | 1.0 |
| Yes | 40 | 28 | 24 | 24 | ||
| Cycle | ||||||
| <4 | 4 | 24 | 0.027 | 5 | 3 | 0.732 |
| =4 | 134 | 210 | 115 | 118 | ||
| >4 | 5 | 12 | 5 | 4 | ||
After adjustment of propensity score matching and variables of gender, age, histologic subtype, smoking history, tumor differentiation, pathological stage, use of adjuvant radiotherapy, performance status and comorbidity score, type of resection and number of chemotherapy cycles, the two groups were well-matched (125 patients each) without significant differences in baseline characteristics (Table 1). The summary shows that the most often used platinum combined with pemetrexed was cisplatin (69.6%) and in the other group it was carboplatin (56.8%).
Survival Outcome
The results of analysis with Cox proportional hazard regression model before matching are shown in Table 2. Multivariate analysis showed that the tumor histology, pathologic stage, PS, use of adjuvant radiotherapy and number of chemotherapy cycles had significant impact on patient survival, while the use of pemetrexed did not turn out to be an independent prognostic factor of DFS (HR = 0.759, 95%CI: 0.563–1.023, p = 0.07). DFS was not significantly different between the two groups before propensity score-matched (P = 0.16) (Fig. 2A). However, after PSM, patients who received adjuvant chemotherapy with platinum/pemetrexed regimen show better DFS than those who did not (P = 0.0079) (Fig. 2B).
Table 2.
Multivariate Cox proportional hazards model of disease-free survival.
| Variable | HR | 95% CI | P | |
|---|---|---|---|---|
| Pathologic stage | IIA vs IB | 2.718 | 1.498–4.930 | 0.001 |
| IIB vs IB | 1.976 | 0.823–4.744 | 0.128 | |
| IIIA vs IB | 6.109 | 3.480–10.723 | <0.001 | |
| Performance status | ≥1 vs 0 | 0.62 | 0.445–0.864 | 0.005 |
| Adjuvant radiotherapy | Yes vs No | 0.684 | 0.481–0.972 | 0.034 |
| Cycle | =4 vs < 4 | 1.337 | 0.799–2.238 | 0.268 |
| >4 vs < 4 | 2.477 | 1.208–5.082 | 0.013 | |
| Platinum/pemetrexed | Pem vs Other | 0.759 | 0.563–1.023 | 0.07 |
Figure 2.
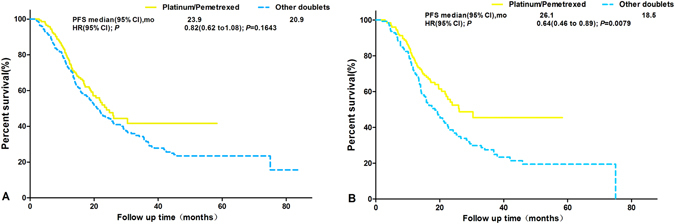
Kaplan-Meier Curves of DFS before (A) and after (B) PSM.
Exploratory subgroup analysis
Exploratory subgroup analysis was performed to see whether the use of pemetrexed had any significant impact on survival independent of age, gender, smoking history, histology subtype, tumor differentiation, lymphatic involvement stage, pathological stage, use of adjuvant radiotherapy, performance status, comorbidity score, type of resection or number of chemotherapy cycles. From the analysis, pemetrexed benefit is consistent across different subgroups, and especially age >65 years was associated with the decision to use platinum/pemetrexed (HR = 0.25, 95%CI 0.09–0.73, P = 0.011) (Fig. 3).
Figure 3.
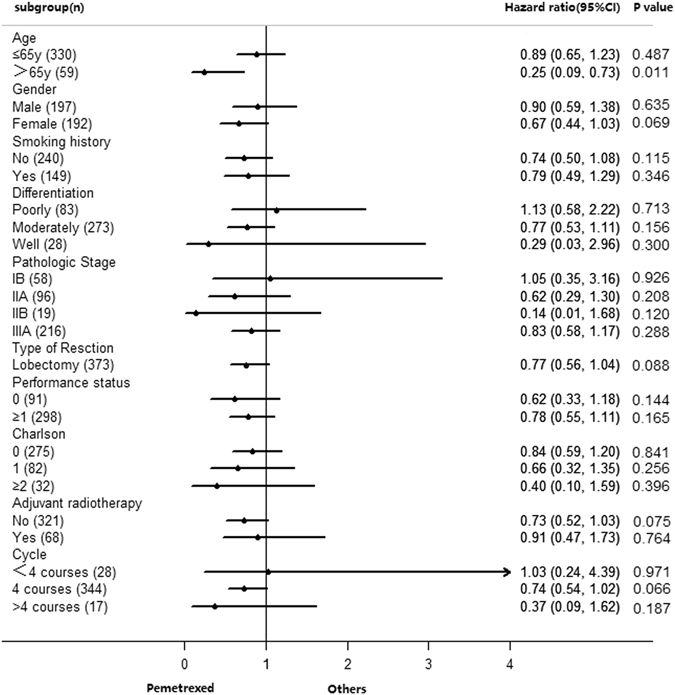
Subgroup Analysis.
Clinical toxicity
Propensity score methods created 107 best-matched pairs for platinum/pemetrexed versus platinum/paclitaxel and platinum/docetaxel (due to the small case number of docetaxel, we combined the 2 regimen), 56 best-matched pairs for platinum/pemetrexed versus platinum/gemcitabine, and 24 best-matched pairs for platinum/pemetrexed versus platinum/vinorelbine for comparisons of clinical toxicity. With adjusting bias after propensity score analysis, platinum/pemetrexed was confirmed to have a significantly lower hematological toxicity than gemcitabine (leukopenia: RR 0.514, p = 0.001; neutropenia: RR 0.688, p = 0.002) as well as paclitaxel- and docetaxel-based chemotherapy (leukopenia: RR 0.685, p = 0.019; neutropenia: RR 0.805, p = 0.032). Comparisons of non-hematological AEs between PSM groups indicated that platinum/pemetrexed treatment was associated with less alopecia than the combination of platinum with docetaxel or paclitaxel (10.28% vs. 20.56%, RR 0.500, p = 0.037). Platinum/pemetrexed was also associated in fewer patients with constipation than the platinum/gemcitabine doublet (0% vs. 25%, p = 0.022) (Table 3). Moreover, both grade 3/4 hematologic toxicity and non-hematologic toxicity were significantly lower for platinum/pemetrexed compared with other platinum-based doublets (Table 4). Otherwise, platinum/pemetrexed was associated with more vomiting when compared with paclitaxel- and docetaxel-based doublet (52.34% vs. 22.43%, RR 2.333, p < 0.001). All other toxicities were not significantly different. (Table 3), and a similar result was found in the 125 well-matched pairs (Table 5).
Table 3.
Comparing incidences of hematological and non-hematological AEs between the propensity score matched treatment group.
| Treatment Comparison | Platinum/Pemetrexed vs. Platinum/Gemcitabine | RR | P * | Platinum/Pemetrexed vs. Platinum/Vinorelbine | RR | P * | Platinum/Pemetrexed vs. Platinum/Paclitaxel+Docetaxel | RR | P* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matched pairs | 56 pairs | 24 pairs | 107 pairs | |||||||||||||||
| All grades AE | N | % | N | % | N | % | N | % | N | % | N | % | ||||||
| Non-hematological | ||||||||||||||||||
| Fatigue | 10 | 17.86% | 11 | 19.64% | 0.909 | 0.809 | 6 | 25.00% | 8 | 33.33% | 0.750 | 0.525 | 17 | 15.89% | 19 | 17.76% | 0.895 | 0.715 |
| Fever | 0 | 0.00% | 2 | 3.57% | — | 0.495 | 0 | 0.00% | 1 | 4.17% | — | 1.000 | 0 | 0.00% | 3 | 2.80% | 0.000 | 0.246 |
| Nausea | 47 | 83.93% | 52 | 92.86% | 0.904 | 0.140 | 21 | 87.50% | 22 | 91.67% | 0.955 | 0.637 | 90 | 84.11% | 82 | 76.64% | 1.098 | 0.169 |
| Vomiting | 30 | 53.57% | 28 | 50.00% | 1.071 | 0.705 | 13 | 54.17% | 10 | 41.67% | 1.300 | 0.386 | 56 | 52.34% | 24 | 22.43% | 2.333 | <0.001 |
| Mucositis | 1 | 1.79% | 2 | 3.57% | 0.500 | 1.000 | 1 | 4.17% | 1 | 4.17% | 1.000 | 1.000 | 1 | 0.93% | 2 | 1.87% | 0.500 | 1.000 |
| Constipation | 2 | 3.57% | 4 | 7.14% | 0.500 | 0.679 | 0 | 0.00% | 6 | 25.00% | — | 0.022 | 3 | 2.80% | 2 | 1.87% | 1.500 | 1.000 |
| Rash | 1 | 1.79% | 1 | 1.79% | 1.000 | 1.000 | 1 | 4.17% | 0 | 0.00% | — | 1.000 | 4 | 3.74% | 0 | 0.00% | — | 0.121 |
| Alopecia | 9 | 16.07% | 5 | 8.93% | 1.800 | 0.253 | 1 | 4.17% | 4 | 16.67% | 0.250 | 0.348 | 11 | 10.28% | 22 | 20.56% | 0.500 | 0.037 |
| Hematological | ||||||||||||||||||
| Leukopenia | 18 | 32.14% | 35 | 62.50% | 0.514 | 0.001 | 8 | 33.33% | 11 | 45.83% | 0.727 | 0.376 | 37 | 34.58% | 54 | 50.47% | 0.685 | 0.019 |
| Neutropenia | 33 | 58.93% | 48 | 85.71% | 0.688 | 0.002 | 15 | 62.50% | 19 | 79.17% | 0.789 | 0.204 | 62 | 57.94% | 77 | 71.96% | 0.805 | 0.032 |
| Anemia | 22 | 39.29% | 28 | 50.00% | 0.786 | 0.254 | 10 | 41.67% | 11 | 45.83% | 0.909 | 0.771 | 40 | 37.38% | 49 | 45.79% | 0.816 | 0.212 |
| Thrombocytopenia | 14 | 25.00% | 15 | 26.79% | 0.933 | 0.829 | 4 | 16.67% | 7 | 29.17% | 0.571 | 0.303 | 21 | 19.63% | 20 | 18.69% | 1.050 | 0.862 |
AE, adverse event; RR, rate ratio. *P values less than 0.05 were in bold to indicate significant differences.
Table 4.
Toxic effects.
| Toxicity | Platinum/Pemetrexed | Platinum/Gemcitabine | Platinum/Pemetrexed | Platinum/Vinorelbine | Platinum/Pemetrexed | Platinum/Paclitaxel or Docetaxel |
|---|---|---|---|---|---|---|
| N = 56 | N = 24 | N = 107 | ||||
| Hematologic toxicity G3/4 (%) | 32.1 | 73.2 | 50 | 58.3 | 51.4 | 72.9 |
| WBC | 8.9 | 19.6 | 20.8 | 20.8 | 25.2 | 29.0 |
| Neutropenia | 23.2 | 44.6 | 29.2 | 33.3 | 26.2 | 40.2 |
| Anemia | 0 | 3.6 | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 0 | 5.4 | 0 | 4.2 | 0.9 | 3.7 |
| Non-hematologic toxicity G3/4 (%) | 0 | 19.6 | 0 | 16.7 | 2.7 | 8.4 |
| Fatigue | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea | 0 | 10.7 | 0 | 8.3 | 0.9 | 4.7 |
| Vomiting | 0 | 8.9 | 0 | 4.2 | 0.9 | 0.9 |
| Mucositis | 0 | 0 | 0 | 0 | 0 | 0 |
| Constipation | 0 | 0 | 0 | 0 | 0 | 0 |
| Rash | 0 | 0 | 0 | 0 | 0 | 0 |
| Alopecia | 0 | 0 | 0 | 4.2 | 0.9 | 2.8 |
Table 5.
Comparing incidences of toxic effects between the propensity score matched treatment group.
| Pemetrexed | Non- Pemetrexed | P | ||||
|---|---|---|---|---|---|---|
| 125 pairs | ||||||
| Adverse events | N | % | N | % | ||
| Fatigue | AE | 18 | 14.29% | 26 | 20.63% | 0.184 |
| SAE | 0 | 0 | 0 | 0 | — | |
| Fever | AE | 1 | 0.79% | 3 | 2.38% | 0.622 |
| SAE | 0 | 0 | 0 | 0 | — | |
| Nausea | AE | 102 | 81.60% | 99 | 79.20% | 0.633 |
| SAE | 1 | 0.79% | 6 | 4.76% | 0.12 | |
| Vomiting | AE | 64 | 50.79% | 34 | 26.98% | <0.001 |
| SAE | 0 | 0 | 4 | 3.17% | 0.122 | |
| Mucositis | AE | 2 | 1.59% | 4 | 3.17% | 0.684 |
| SAE | 0 | 0 | 0 | 0 | — | |
| Constipation | AE | 8 | 6.35% | 5 | 3.97% | 0.393 |
| SAE | 0 | 0 | 0 | 0 | — | |
| Rash | AE | 4 | 3.17% | 0 | 0.00% | 0.122 |
| SAE | 0 | 0 | 0 | 0 | — | |
| Alopecia | AE | 7 | 5.56% | 29 | 23.20% | <0.001 |
| SAE | 0 | 0 | 3 | 2.38% | 0.247 | |
| WBC | AE | 57 | 45.60% | 98 | 78.40% | <0.001 |
| SAE | 4 | 3.17% | 28 | 22.22% | <0.001 | |
| Neutropenia | AE | 69 | 55.20% | 95 | 76.00% | 0.001 |
| SAE | 14 | 11.11% | 56 | 44.44% | <0.001 | |
| Anemia | AE | 44 | 35.20% | 57 | 45.24% | 0.124 |
| SAE | 0 | 0 | 0 | 0 | — | |
| Thrombocytopenia | AE | 21 | 16.67% | 24 | 19.05% | 0.622 |
| SAE | 3 | 2.38% | 3 | 2.38% | 1 | |
AE, adverse event SAE, sever adverse event.
Discussion
Patients recruited for clinical trials according to strict inclusion criteria may not inevitably match with unselected patient populations. Compared with general populations, they are usually younger and present with better general conditions and fewer co-existing diseases, thus not representing typical characteristics of patients from daily clinical routine. Well-designed retrospective studies, therefore, can provide important ancillary information for the “real world situation”18.
To the best of our knowledge, our analysis is the first “real-world” study comparing survival results and clinical toxicity associated with pemetrexed/platinum-based doublets and other third-generation doublets routinely used in the adjuvant chemotherapy for completely resected lung adenocarcinoma. Pemetrexed/platinum doublets were shown to be less hematotoxic, particularly regarding leukopenia and neutropenia, in this way increasing the safety of adjuvant chemotherapy for NSCLC. Though we noticed that pemetrexed/platinum treatment was associated with more vomiting than the other combinations. This observation most probably finds its explanation in the higher proportion of cisplatin used instead of carboplatin as combination partner for pemetrexed compared with the other regimens, because cisplatin is known to be one of the most emetogenic cytostatic. The risk of vomiting associated with cisplatin is greater than with non-cisplatin-containing regimens19, 20.
In our study, we utilized propensity score matched methods (PSM) to compare survival data and clinical toxicity. PSM analysis allowed us to compare survival between patients with similar background characteristics21. When potentially confounding variables (gender, age, smoking history, tumor differentiation, pathological stage, use of adjuvant radiotherapy, ECOG PS, comorbidity score, type of resection and number of chemotherapy cycles) were adjusted, patients who received a doublet of platinum/pemetrexed had significantly better DFS than those treated with other third-generation platinum-based doublets. This strongly indicates that adjuvant therapy with platinum/pemetrexed doublets improves survival of patients with completely resected lung adenocarcinoma. The indication generated in our study should have profound significance for clinical research and future studies.
Although a phase II trial showed that adjuvant chemotherapy with cisplatin/pemetrexed yielded less toxicity and better dose delivery than vinorelbine combined with cisplatin, DFS was not influenced by chemotherapy type11, 12, 22. The superior survival data related with pemetrexed observed in our study may be the result of a potential effect of tumor histology. The primary mechanism of action of pemetrexed is the inhibition of the enzyme thymidylate synthase (TS). Docetaxel, paclitaxel, as well as vinorelbine can destroy the mitotic activity of tumor cells23, and gemcitabine is a nucleoside analog24. The anti-cancer activities of the last four cytotoxic drugs, directed to cell replication, are less sensitive to tumor histology25. As a biomarker, TS expression is potentially associated with the response to pemetrexed-based chemotherapy in NSCLC patients26, 27 and had become an important determinant of survival for stage I NSCLC28. Moreover, the differential efficacy of pemetrexed in advanced NSCLC on the basis of tumor histology has been reported in a phase III study8. The better survival and clinical efficacy achieved with pemetrexed/platinum doublets in this study might be explained by the restriction to adenocarcinoma histology, because adenocarcinomas express TS, a key enzymes inhibited by pemetrexed29. We also found that the survival associated with platinum/pemetrexed in our study was better than that reported in the randomized phase II TREAT study11, 12. Reported relapse-free survival did not differ between cisplatin/pemetrexed and cisplatin/vinorelbine in the TREAT study. The different outcome between our study and TREAT study is probably caused by a disparity in patient pathological types. The tumor histology in our study was uniformly adenocarcinoma, which shows lower TS expression, a confirmed predictive marker for better response to pemetrexed-based chemotherapy27, 30. Eventually, the better survival and reduced clinical toxicity observed in our study with pemetrexed-based adjuvant therapy need further evaluation with a better research design to confirm a potential effect on overall survival in lung adenocarcinoma.
The LACE meta-analysis and a retrospective analysis of the JBR.10 trial consistently reported that adjuvant chemotherapy significantly improved survival, and that treatment-related mortality did not differ by age31, 32. Thus, the role of adjuvant chemotherapy should not be underestimated in elderly patients. In this study, in patients older than 65 years, the use of platinum/pemetrexed resulted in better DFS than other doublets. By contrast, there is no overall difference in DFS between patients who received platinum/pemetrexed and other third-generation doublets. In the subgroups stratified by gender, smoking history, tumor differentiation, pathological stage, use of adjuvant radiotherapy, ECOG PS, comorbidity score, type of resection and number of chemotherapy cycles, we also could not identify patients who benefited more from pemetrexed. Thus, patients’ age is a prognostic factor for DFS in patients with completely resected NSCLC.
This is the first study to compare the impact on survival of different regimens for completely resected NSCLC; however, it also has some limitations. First, our study was limited by retrospective nature of the analysis. Although we performed multivariate analysis and used PSM method to eliminate the selection bias as much as possible, some disparities of both known and unknown prognostic factors, such as total dose of chemotherapy, may affect the results. Second, the exploration of OS in this study was limited. As we know, there are different therapies for regional recurrences or distant metastases, governed by different pathological or mutational types. Several studies33, 34 indicated that in advanced NSCLC patients with EGFR mutations, a first-line therapy with gefitinib resulted in encouraging clinical therapeutic outcomes. Furthermore, the use of pemetrexed plus cisplatin showed significant benefits with regard to survival in advanced-stage NSCLC patients with adenocarcinoma and large-cell carcinoma8. Knowing that several factors can affect the OS outcome, we set DFS as the primary end point of this study. Third, because of the relatively small sample size of other platinum-based doublets, this study could not compare the effect on survival among each chemotherapy regimens. In addition, because the histological type of NSCLC of all patients in our study is adenocarcinoma, it is still unclear whether there is a correlation of histology with outcome of adjuvant chemotherapy. The predictive efficacy of pemetrexed activity according to the tumor histology was reported, in a phase III trial, only in patients with advanced NSCLC8. However, so far no prospective trial or meta-analysis in early-stage NSCLC has reported the relation between histology and outcomes. In JBR.10, squamous histologic features (P = 0.002) were associated with significantly prolonged recurrence-free survival. Though cisplatin/vinorelbine had a positive impact in the ANITA trial, a poor outcome in adenocarcinoma was also reported3, 6, 35–38. Likewise, histological type had no impact on adjuvant treatment in LACE study subgroup analysis of vinorelbine39. Accordingly, in adjuvant therapy of early-stage NSCLC, the predictive effect of tumor histology still need further evaluation.
Declaration
Ethics approval and consent to participate
The study protocol was approved by the institutional review board at the Cancer Hospital, Chinese Academy of Medical Sciences, and PUMC in accordance with the Declaration of Helsinki. Patients provide informed consent authorizing the use of their personal information for research purposes.
Author Contributions
Z.W. and Y.L. designed the research. Q.Z. analyzed the data. X.Z. collected the medical record data. L.Y., Y.Z. and J.L. provided the safety data. X.Z., Q.Z., Z.W. and Y.L. wrote the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Xiaoyu Zhai and Qiwen Zheng contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yutao Liu, Email: 13911901165@139.com.
Ziping Wang, Email: wangzp2007@126.com.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Winton T, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. The New England journal of medicine. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. The Lancet. Oncology. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 4.Strauss GM, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:5043–5051. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Liu F, Huang DX, Jiang B. Post-operative treatment with cisplatin and vinorelbine in Chinese patients with non-small cell lung cancer: a clinical prospective analysis of 451 patients. Asian Pacific journal of cancer prevention: APJCP. 2012;13:4505–4510. doi: 10.7314/APJCP.2012.13.9.4505. [DOI] [PubMed] [Google Scholar]
- 6.Pignon JP, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 7.Joerger M, Omlin A, Cerny T, Fruh M. The role of pemetrexed in advanced non small-cell lung cancer: special focus on pharmacology and mechanism of action. Current drug targets. 2010;11:37–47. doi: 10.2174/138945010790030974. [DOI] [PubMed] [Google Scholar]
- 8.Scagliotti GV, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 9.Scagliotti GV, et al. Pemetrexed combined with oxaliplatin or carboplatin as first-line treatment in advanced non-small cell lung cancer: a multicenter, randomized, phase II trial. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11:690–696. doi: 10.1158/1078-0432.CCR-05-9009. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger DS, et al. Non-Small Cell Lung Cancer, Version 6.2015. Journal of the National Comprehensive Cancer Network: JNCCN. 2015;13:515–524. doi: 10.6004/jnccn.2015.0071. [DOI] [PubMed] [Google Scholar]
- 11.Kreuter M, et al. Randomized phase 2 trial on refinement of early-stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine: the TREAT study. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2013;24:986–992. doi: 10.1093/annonc/mds578. [DOI] [PubMed] [Google Scholar]
- 12.Kreuter M, et al. Three-Year Follow-Up of a Randomized Phase II Trial on Refinement of Early-Stage NSCLC Adjuvant Chemotherapy with Cisplatin and Pemetrexed versus Cisplatin and Vinorelbine (the TREAT Study) Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2016;11:85–93. doi: 10.1016/j.jtho.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Goldstraw P, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of clinical epidemiology. 2000;53:1258–1267. doi: 10.1016/S0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Trotti A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 18.Meyer RM. Generalizing the results of cancer clinical trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:187–189. doi: 10.1200/JCO.2009.25.8608. [DOI] [PubMed] [Google Scholar]
- 19.Schnell FM. Chemotherapy-induced nausea and vomiting: the importance of acute antiemetic control. The oncologist. 2003;8:187–198. doi: 10.1634/theoncologist.8-2-187. [DOI] [PubMed] [Google Scholar]
- 20.Carmichael J, et al. Use of granisetron in patients refractory to previous treatment with antiemetics. Anti-cancer drugs. 1998;9:381–385. doi: 10.1097/00001813-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Newgard CD, Hedges JR, Arthur M, Mullins RJ. Advanced statistics: the propensity score–a method for estimating treatment effect in observational research. Acad Emerg Med. 2004;11:953–961. doi: 10.1197/j.aem.2004.02.530. [DOI] [PubMed] [Google Scholar]
- 22.Kreuter M, et al. Trial on refinement of early stage non-small cell lung cancer. Adjuvant chemotherapy with pemetrexed and cisplatin versus vinorelbine and cisplatin: the TREAT protocol. BMC cancer. 2007;7:77. doi: 10.1186/1471-2407-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edelman MJ, Gandara DR. Promising new agents in the treatment of non-small cell lung cancer. Cancer chemotherapy and pharmacology. 1996;37:385–393. doi: 10.1007/s002800050402. [DOI] [PubMed] [Google Scholar]
- 24.Sandler A, Ettinger DS. Gemcitabine: single-agent and combination therapy in non-small cell lung cancer. The oncologist. 1999;4:241–251. [PubMed] [Google Scholar]
- 25.Wang Y, et al. Clinical effectiveness and clinical toxicity associated with platinum-based doublets in the first-line setting for advanced non-squamous non-small cell lung cancer in Chinese patients: a retrospective cohort study. BMC cancer. 2014;14:940. doi: 10.1186/1471-2407-14-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buque A, et al. Thymidylate synthase expression determines pemetrexed targets and resistance development in tumour cells. PloS one. 2013;8:e63338. doi: 10.1371/journal.pone.0063338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takezawa K, et al. Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. British journal of cancer. 2011;104:1594–1601. doi: 10.1038/bjc.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Z, et al. Thymidylate synthase in situ protein expression and survival in stage I nonsmall-cell lung cancer. Cancer. 2008;112:2765–2773. doi: 10.1002/cncr.23491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto H, et al. Significance of thymidylate synthase gene expression level in patients with adenocarcinoma of the lung. Cancer. 2006;106:1595–1601. doi: 10.1002/cncr.21777. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, et al. Thymidylate synthase and ERCC1 as predictive markers in patients with pulmonary adenocarcinoma treated with pemetrexed and cisplatin. Lung cancer. 2013;81:102–108. doi: 10.1016/j.lungcan.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Pepe C, et al. Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:1553–1561. doi: 10.1200/JCO.2006.09.5570. [DOI] [PubMed] [Google Scholar]
- 32.Fruh M, et al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:3573–3581. doi: 10.1200/JCO.2008.16.2727. [DOI] [PubMed] [Google Scholar]
- 33.Sequist LV, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:2442–2449. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 34.Mok TS, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England journal of medicine. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 35.Butts CA, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennouna J, Senellart H, Hiret S, Vaissiere N, Douillard JY. Impact of histology on survival of resected non-small cell lung cancer (NSCLC) receiving adjuvant chemotherapy: subgroup analysis of the adjuvant vinorelbine (NVB) cisplatin (CDDP) versus observation in the ANITA trial. Lung cancer. 2011;74:30–34. doi: 10.1016/j.lungcan.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. Bmj311, 899-909 (1995). [PMC free article] [PubMed]
- 38.Group NM-aC, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375:1267–1277. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douillard JY, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2010;5:220–228. doi: 10.1097/JTO.0b013e3181c814e7. [DOI] [PubMed] [Google Scholar]


