Abstract
Glioblastoma multiforme (GBM), the most malignant of all gliomas is characterized by a high degree of heterogeneity and poor response to treatment. The sub-ventricular zone (SVZ) is the major site of neurogenesis in the brain and is rich in neural stem cells. Based on the proximity of the GBM tumors to the SVZ, the tumors can be further classified into SVZ+ and SVZ−. The tumors located in close contact with the SVZ are classified as SVZ+, while the tumors located distantly from the SVZ are classified as SVZ−. To gain an insight into the increased aggressiveness of SVZ+ over SVZ− tumors, we have used proteomics techniques like 2D-DIGE and LC-MS/MS to investigate any possible proteomic differences between the two subtypes. Serum proteomic analysis revealed significant alterations of various acute phase proteins and lipid carrying proteins, while tissue proteomic analysis revealed significant alterations in cytoskeletal, lipid binding, chaperone and cell cycle regulating proteins, which are already known to be associated with disease pathobiology. These findings provide cues to molecular basis behind increased aggressiveness of SVZ+ GBM tumors over SVZ− GBM tumors and plausible therapeutic targets to improve treatment modalities for these highly invasive tumors.
Introduction
Glioblastoma Multiforme (GBM) is a malignant brain tumor, characterized by heterogeneity within the tumor and poor response to treatment1. It is also the most aggressive of all known gliomas with patients having a survival period lower than one year from the time of diagnosis in most cases2. WHO categorizes GBMs as grade IV gliomas3. Progenitor astrocytes that go on to form glial cells colonize the subventricular zone (SVZ), the largest germinal zone in the brain found along the lateral walls of the lateral ventricles4. This zone of the brain has been extensively studied as a site of neurogenesis5 and is responsible for the development of neurons in an adult. The aggressive nature of GBM tumors makes their treatment almost impossible thereby making them the most dangerous of all known gliomas. Surgical resection of the tumor tissues followed by radiotherapy (RT) and biopsy followed by radiotherapy (RT) have been reported to prolong the survival period in the patients with GBMs6.
Despite all the advances in proteomics there still exists a huge void in our understanding of GBMs and the proteins with possible role in survival of the patients. A proteome based clustering of gliomas was correlated to survival in patients7. Haskin et al. compared the tissue proteome of GBM tumors (tumors in close proximity to the SVZ region) with the control tissues and identified various protein signatures responsible for migratory potential and tumor growth in GBM tumors8. Till date there has been no report of any study involving two different sub-groups of GBMs based on SVZ involvement. Tumor proximity to the subventricular zone has been recently reported to play a significant role in the survival of GBM patients, where patients with tumors in SVZ contact had an overall survival period less than those with tumors distantly located to SVZ9. However, there has been no proteomic study to support the findings at a molecular level. In the current study, we have performed a comprehensive serum and tissue proteome analysis of two sub-populations of GBMs to explore if SVZ involvement had any direct influence on tumor aggressiveness.
Results
Serum proteomic alterations in SVZ+ patients with respect to SVZ− patients
The 2D-DIGE gel for serum of SVZ+ and SVZ− GBM patients yielded ~700 protein spots. Of these ~700 spots, 10 protein spots were found to be significantly altered with a p-value ≤ 0.05. Out of the 10 protein spots, six protein spots were found to be up-regulated and four were down-regulated in the serum of SVZ+ GBM patients. Protein identity was revealed for nine out of the 10 significantly altered protein spots using MALDI-TOF/TOF. It was found that 10 protein spots corresponded to four different proteins because of the presence of isoforms and multiple sub-units in the proteins, thereby resulting in same protein hits for multiple spots. Out of the four proteins identified, two were up-regulated and two were down-regulated in the SVZ+ GBM serum samples (Table 1). Figure 1 represents the 2D-DIGE gel image and 3D-views for the significantly altered proteins in serum.
Table 1.
List of the differentially expressed and statistically significant protein spots from SVZ +/− GBM patients serum and tissue samples identified using 2D-DIGE method.
| (A) Significantly altered proteins in serum of SVZ +/− GBM patients identified using 2D-DIGE | |||||||
| Spot No. | Name of the protein | Uniprot accession number | Mol. Wt (kDa) | p value ( t- test) | Fold change (SVZ+/SVZ−) | Protein score | No. of matched peptides |
| 1 | Serum albumin | P02768 | 71.31 | 0.014 | 1.63 | 191 | 21 |
| 2 | Hemopexin* | P02790 | 52.38 | 0.0065 | 1.21 | 883 | 24 |
| 3 | Serum albumin | P02768 | 71.31 | 0.011 | 1.64 | 137 | 20 |
| 4 | Serum albumin | P02768 | 71.31 | 0.049 | 1.36 | 293 | 26 |
| 5 | α1-antichymotrypsin | P01011 | 47.79 | 0.025 | −1.52 | 220 | 19 |
| 6 | α1-antichymotrypsin | P01011 | 47.79 | 0.025 | −1.51 | 244 | 18 |
| 7 | α1-antichymotrypsin$ | P01011 | 47.79 | 0.026 | −1.58 | 284 | 20 |
| 8 | Apolipoprotein A1* | P02647 | 30.75 | 0.044 | −1.82 | 308 | 25 |
| 9 | Serum albumin | P02768 | 71.31 | 0.0039 | 1.68 | 181 | 18 |
| (B) Significantly altered proteins in tissue of SVZ +/− GBM patients identified using 2D-DIGE | |||||||
| Spot No. | Name of the protein | UniProt accession number | Mol. Wt (kDa) | p value (t-test) | Fold change (SVZ+/SVZ−) | Sequest score | Unique peptides |
| 1 | Autoantigen La (Lupus La) | P05455 | 46.8 | 0.024 | −1.87 | 146.41 | 23 |
| 2 | Proliferation associated 2G4 homologue | Q9UQ80 | 40.9 | 0.049 | −2.33 | 176.5 | 23 |
| 3 | Retinol binding protein 1 | P09455 | 15.8 | 0.011 | −2.89 | 35.14 | 7 |
| 4 | Rab GDP dissociation protein alpha | P31150 | 50.6 | 0.044 | 2.1 | 382.23 | 32 |
| Vimentin | P08670 | 53.6 | 0.044 | 2.1 | 287.8 | 41 | |
| 5 | Vimentin | P08670 | 53.6 | 0.049 | 2.4 | 224.25 | 36 |
| Rab GDP Dissociation Protein Alpha | P31150 | 50.6 | 0.049 | 2.4 | 214.65 | 27 | |
Note: The proteins with *mark are significant in only 18 out of 24 gels and the proteins with $mark are significant in 15 out of 24 gels. Remaining proteins are significant in all 24 out of 24 gels.
Figure 1.

Representative overlapped 2D-DIGE gel image of SVZ+ and SVZ- GBM patient’s serum proteome and 3D-views of significantly altered proteins. Extracted serum proteome from SVZ+ (n = 8) and SVZ- (n = 8) GBM patients were labeled with respective CyDyes and separated on IPG strips of pH 4–7; 18 cm followed by separation on 12.5% SDS polyacrylamide gels. 2D-DIGE analysis was performed using DeCyder 2D software version 7.0 and the identities of significantly altered protein spots were established using MALDI-TOF/TOF analysis (Green colour indicates Cy3 labeled protein and red colour indicates Cy5 labeled protein).
Serum proteomic analysis of SVZ+ and SVZ− GBM patients using iTRAQ method identified a total of 190 proteins, of which 148 proteins were identified with quantifiable peptides. Out of these 148 proteins, only 135 proteins showed at least ~1.1 fold change and only 75 proteins were identified with at least two peptide signatures. Of these 75 proteins, only 18 proteins showed more than 1.3 fold change (Table 2). Since the comparison involved two sub-types of GBMs, a low fold change cut-off (1.3 fold) was used to make sure that the biologically significant proteins were not missed in the discovery phase. The mass spectra of two significantly altered proteins have been provided in Fig. 2c.
Table 2.
List of significantly altered serum and tissue proteins in SVZ +/− GBM tumor samples with respect to normal brain tissue identified using iTRAQ method (Partial list of significantly altered proteins is given in the table).
| A. Significantly altered serum proteins in SVZ+/- GBM tumors identified using iTRAQ method | |||||
| S. No. | Protein Name | Accession number | Fold Change (SVZ+/SVZ−) | No. of unique peptides | |
| 1 | Vitamin D-binding protein | P02774 | 1.59 | 2 | |
| 2 | Putative uncharacterized protein DKFZp686I04196 | Q6N093 | 1.56 | 4 | |
| 3 | cDNA, FLJ94361, highly similar to Homo sapiens serine (or cysteine) proteinase inhibitor | B2R9F2 | 1.45 | 5 | |
| 4 | Apolipoprotein E | P02649 | 1.42 | 8 | |
| 5 | cDNA FLJ60316, highly similar to Apolipoprotein-L1 | B4DNT5 | 1.37 | 8 | |
| 6 | Immunoglobulin J chain | C9JA05 | 1.33 | 2 | |
| 7 | Apolipoprotein C-III | P02656 | 1.30 | 4 | |
| 8 | cDNA FLJ56954, highly similar to Inter-alpha-trypsin inhibitor heavy chain H1 | B7Z539 | 1.30 | 10 | |
| 9 | Hemoglobin subunit delta | P02042 | 0.77 | 3 | |
| 10 | Apolipoprotein A-IV | P06727 | 0.76 | 23 | |
| 11 | Immunoblobulin light chain | Q0KKI6 | 0.74 | 5 | |
| 12 | cDNA FLJ58441, highly similar to Attractin | B4DZ36 | 0.74 | 3 | |
| 13 | cDNA FLJ54622, highly similar to Prothrombin | B4DDT3 | 0.73 | 4 | |
| 14 | cDNA FLJ53950, highly similar to Angiotensinogen | B4E1B3 | 0.70 | 9 | |
| 15 | cDNA FLJ41552 fis, clone COLON2004478, highly similar to Protein Tro alpha1 H, myeloma | Q6ZW64 | 0.69 | 4 | |
| 16 | Serum amyloid A protein | Q15423 | 0.59 | 2 | |
| 17 | Carbonic anhydrase 1 | E5RJF6 | 0.58 | 2 | |
| 18 | Hemoglobin subunit beta | P68871 | 0.39 | 2 | |
| B. Significantly altered tissue proteins in SVZ+/- GBM tumors identified using iTRAQ method | |||||
| S. No. | Protein Name | Accession number | Avg. Fold Change | ||
| SVZ−/Nor. | SVZ+/Nor. | SVZ+/SVZ− | |||
| 1 | Alpha-1-acid glycoprotein 1 | P02763 | 0.91 | 2.52 | 2.78 |
| 2 | Band 4.1-like protein 3 | Q9Y2J2 | 0.33 | 0.74 | 2.20 |
| 3 | Thymosin beta-4-like protein 3 | Q08EQ4 | 1.20 | 2.54 | 2.12 |
| 4 | Myelin basic protein | P02686 | 0.30 | 0.63 | 2.09 |
| 5 | Alpha-1-antitrypsin | P01009 | 1.41 | 2.71 | 1.93 |
| 6 | Hemoglobin subunit beta | P68871 | 1.27 | 2.41 | 1.90 |
| 7 | Haptoglobin | P00738 | 1.06 | 1.94 | 1.82 |
| 8 | 2′,3′-cyclic-nucleotide 3′-phosphodiesterase | P09543 | 0.32 | 0.57 | 1.79 |
| 9 | Ferritin light chain | P02792 | 1.76 | 3.12 | 1.77 |
| 10 | V-type proton ATPase catalytic subunit A | P38606 | 0.60 | 1.02 | 1.70 |
| 11 | Hemoglobin subunit alpha | P69905 | 1.31 | 2.22 | 1.70 |
| 12 | Vitamin D-binding protein | P02774 | 2.04 | 3.46 | 1.70 |
| 13 | Ferritin heavy chain | P02794 | 0.90 | 1.52 | 1.69 |
| 14 | Citrate synthase, mitochondrial | O75390 | 0.48 | 0.80 | 1.68 |
| 15 | Astrocytic phosphoprotein PEA-15 | Q15121 | 1.09 | 1.69 | 1.55 |
| 16 | Cytochrome c oxidase subunit 5A, mitochondrial | P20674 | 0.33 | 0.51 | 1.54 |
| 17 | Inter-alpha-trypsin inhibitor heavy chain H4 | Q14624 | 2.00 | 3.06 | 1.53 |
| 18 | Ceruloplasmin | P00450 | 1.32 | 1.96 | 1.48 |
| 19 | Alpha-2-macroglobulin | P01023 | 1.04 | 1.54 | 1.48 |
| 20 | Serum albumin | P02768 | 2.17 | 3.19 | 1.47 |
| 21 | Tenascin | P24821 | 1.67 | 2.46 | 1.47 |
| 22 | Dihydropteridine reductase | P09417 | 0.61 | 0.89 | 1.45 |
| 23 | T-complex protein 1 subunit beta | P78371 | 1.55 | 2.24 | 1.44 |
| 24 | Spectrin alpha chain, non-erythrocytic 1 | Q13813 | 0.47 | 0.67 | 1.44 |
| 25 | Ig alpha-1 chain C region | P01876 | 1.42 | 2.05 | 1.44 |
| 26 | V-type proton ATPase subunit B, brain isoform | P21281 | 0.50 | 0.72 | 1.43 |
| 27 | Microtubule-associated protein tau | P10636 | 0.39 | 0.55 | 1.42 |
| 28 | Septin-2 | Q15019 | 1.19 | 1.70 | 1.42 |
| 29 | Ig gamma-1 chain C region | P01857 | 1.46 | 2.06 | 1.41 |
| 30 | Aspartate aminotransferase, cytoplasmic | P17174 | 0.51 | 0.71 | 1.41 |
| 31 | Guanine nucleotide-binding protein G(o) subunit alpha | P09471 | 0.37 | 0.52 | 1.40 |
| 32 | Serotransferrin | P02787 | 1.43 | 2.00 | 1.39 |
| 33 | S-formylglutathione hydrolase | P10768 | 0.91 | 1.26 | 1.38 |
| 34 | Tryptophan–tRNA ligase, cytoplasmic | P23381 | 1.30 | 1.79 | 1.37 |
| 35 | Microtubule-associated protein 2 | P11137 | 0.52 | 0.71 | 1.35 |
| 36 | Coactosin-like protein | Q14019 | 1.18 | 1.59 | 1.35 |
| 37 | Synapsin-1 | P17600 | 0.35 | 0.47 | 1.34 |
| 38 | Band 4.1-like protein 2 | O43491 | 0.74 | 0.99 | 1.34 |
| 39 | Sodium/potassium-transporting ATPase subunit alpha-1 | P05023 | 1.15 | 1.51 | 1.32 |
| 40 | Ig mu chain C region | P01871 | 0.86 | 1.13 | 1.31 |
| 41 | Glutamate dehydrogenase 1, mitochondrial | P00367 | 0.75 | 0.98 | 1.31 |
| 42 | T-complex protein 1 subunit zeta | P40227 | 1.03 | 1.35 | 1.31 |
| 43 | Myosin-9 | P35579 | 1.48 | 1.93 | 1.30 |
| 44 | Pyruvate kinase isozymes M1/M2 | P14618 | 1.65 | 1.28 | 0.77 |
| 45 | Alpha-crystallin B chain | P02511 | 1.64 | 1.27 | 0.77 |
| 46 | Glial fibrillary acidic protein | P14136 | 1.91 | 1.47 | 0.77 |
| 47 | Proteasome subunit beta type-1 | P20618 | 1.36 | 1.05 | 0.77 |
| 48 | Angiotensinogen | P01019 | 1.49 | 1.14 | 0.77 |
| 49 | Annexin A2 | P07355 | 2.40 | 1.85 | 0.77 |
| 50 | Fructose-bisphosphate aldolase A | P04075 | 1.03 | 0.80 | 0.77 |
| 51 | Chitinase-3-like protein 1 | P36222 | 2.38 | 1.83 | 0.77 |
| 52 | Stress-induced-phosphoprotein 1 | P31948 | 1.12 | 0.86 | 0.77 |
| 53 | Sorcin | P30626 | 1.68 | 1.29 | 0.77 |
| 54 | Elongation factor 1-delta | P29692 | 1.75 | 1.34 | 0.76 |
| 55 | Heterogeneous nuclear ribonucleoproteins A2/B1 | P22626 | 1.47 | 1.13 | 0.76 |
| 56 | Phosphoglucomutase-1 | P36871 | 1.62 | 1.23 | 0.76 |
| 57 | RNA-binding motif protein, X chromosome | P38159 | 2.08 | 1.57 | 0.75 |
| 58 | Far upstream element-binding protein 2 | Q92945 | 2.98 | 2.23 | 0.75 |
| 59 | Neural cell adhesion molecule 1 | P13591 | 0.52 | 0.38 | 0.74 |
| 60 | Profilin-1 | P07737 | 2.19 | 1.62 | 0.74 |
| 61 | Ubiquitin-conjugating enzyme E2 N | P61088 | 0.84 | 0.62 | 0.74 |
| 62 | Transthyretin | P02766 | 1.99 | 1.44 | 0.72 |
| 63 | ES1 protein homolog, mitochondrial | P30042 | 1.15 | 0.83 | 0.72 |
| 64 | Elongation factor 1-gamma | P26641 | 2.25 | 1.62 | 0.72 |
| 65 | Malate dehydrogenase, mitochondrial | P40926 | 0.87 | 0.62 | 0.72 |
| 66 | Gamma-synuclein | O76070 | 0.39 | 0.28 | 0.71 |
| 67 | Actin-related protein 2 | P61160 | 2.01 | 1.42 | 0.71 |
| 68 | 6-phosphogluconolactonase | O95336 | 2.21 | 1.56 | 0.70 |
| 69 | Keratin, type I cytoskeletal 10 | P13645 | 1.20 | 0.83 | 0.70 |
| 70 | Heat shock protein beta-1 | P04792 | 2.02 | 1.40 | 0.69 |
| 71 | Peroxiredoxin-6 | P30041 | 1.49 | 1.02 | 0.69 |
| 72 | Trifunctional enzyme subunit beta, mitochondrial | P55084 | 1.43 | 0.97 | 0.68 |
| 73 | Voltage-dependent anion-selective channel protein 2 | P45880 | 0.39 | 0.27 | 0.68 |
| 74 | Adenosylhomocysteinase | P23526 | 2.04 | 1.39 | 0.68 |
| 75 | Proteasome activator complex subunit 2 | Q9UL46 | 4.95 | 3.36 | 0.68 |
| 76 | T-complex protein 1 subunit gamma | P49368 | 1.32 | 0.89 | 0.68 |
| 77 | ADP/ATP translocase 3 | P12236 | 0.78 | 0.52 | 0.67 |
| 78 | Alpha-actinin-1 | P12814 | 2.65 | 1.77 | 0.67 |
| 79 | Retinal dehydrogenase 1 | P00352 | 1.30 | 0.84 | 0.65 |
| 80 | Cytosol aminopeptidase | P28838 | 2.26 | 1.45 | 0.64 |
| 81 | Peptidyl-prolyl cis-trans isomerase B | P23284 | 2.48 | 1.57 | 0.63 |
| 82 | Nucleoside diphosphate kinase B | P22392 | 1.68 | 1.06 | 0.63 |
| 83 | Peptidyl-prolyl cis-trans isomerase A | P62937 | 1.04 | 0.64 | 0.62 |
| 84 | Transgelin | Q01995 | 2.31 | 1.38 | 0.60 |
| 85 | Phosphoglycerate kinase 1 | P00558 | 1.63 | 0.98 | 0.60 |
| 86 | Myristoylated alanine-rich C-kinase substrate | P29966 | 1.27 | 0.76 | 0.59 |
| 87 | Annexin A1 | P04083 | 2.92 | 1.73 | 0.59 |
| 88 | Tenascin-R | Q92752 | 0.98 | 0.58 | 0.59 |
| 89 | X-ray repair cross-complementing protein 6 | P12956 | 2.28 | 1.29 | 0.56 |
| 90 | Fibrinogen beta chain | P02675 | 2.79 | 1.33 | 0.48 |
| 91 | Polypyrimidine tract-binding protein 1 | P26599 | 1.91 | 0.90 | 0.47 |
| 92 | Histone H3.1t | Q16695 | 3.65 | 1.62 | 0.44 |
| 93 | Histone H2B type 1-M | Q99879 | 2.36 | 1.04 | 0.44 |
| 94 | Protein dpy-30 homolog | Q9C005 | 2.31 | 1.01 | 0.44 |
| 95 | Protein S100-A9 | P06702 | 3.92 | 1.65 | 0.42 |
| 96 | X-ray repair cross-complementing protein 5 | P13010 | 2.01 | 0.83 | 0.41 |
Figure 2.
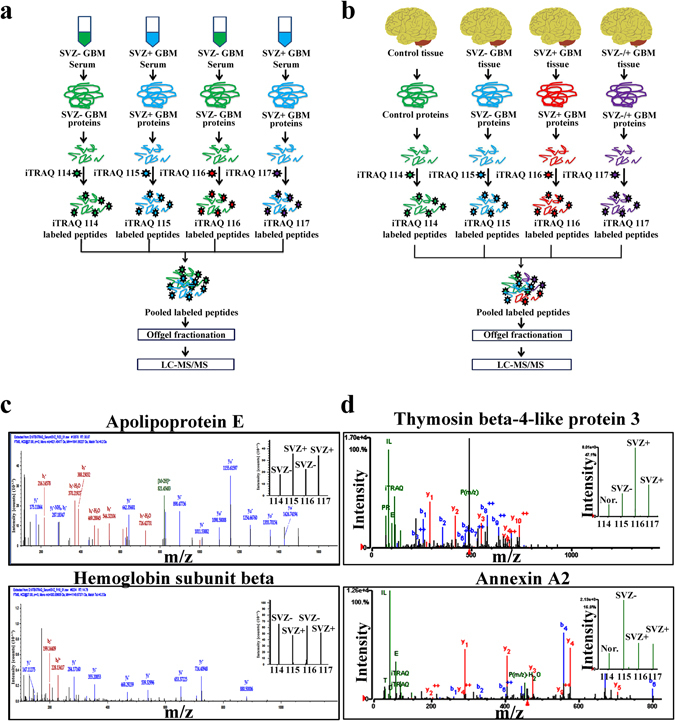
Quantitative serum and tissue proteomic analysis of SVZ+ and SVZ- GBMs. Schematic of iTRAQ based proteomic analysis of (a) serum and (b) tissue specimens for the identification of quantitative proteomic alterations. (c) Serum proteomic analysis of SVZ+ (n = 12) and SVZ- (n = 12) GBMs was performed using iTRAQ method. Representative mass spectra for one of the highly up-regulated (apolipoprotein E) and down-regulated (hemoglobin subunit beta) proteins in SVZ+ GBMs (w.r.t SVZ− GBMs). (d) Tissue proteomic analysis of SVZ+ (n = 6) and SVZ- (n = 6) GBMs was performed using iTRAQ approach. Representative mass spectra of two differentially expressed proteins, thymosin beta-4-like protein 3 and annexin A2 in SVZ+ GBM tumors (w.r.t SVZ− GBMs). Relative abundance of thymosin beta-4-like protein 3 and annexin A2 protein are shown in the inset as the intensities of iTRAQ reporter ions.
Tissue proteomic alterations in the sub-groups of GBM patients
Tissue proteome of the SVZ+ and SVZ− GBM patients was studied using 2D-DIGE and iTRAQ methods. 2D-DIGE analysis was performed for the identification of tissue proteomic alterations in SVZ+ and SVZ− GBM tumor tissue samples (n = 6 each). Analysis of the 2D-DIGE gels of the GBM tumor tissue proteome was performed using DeCyder software. Approximately, 1650 protein spots were identified on each 2D-DIGE gel. After matching the gels, only five protein spots were found to be statistically significant having a p-value ≤ 0.05. Of the five significantly altered protein spots, two were up-regulated and three were down-regulated in SVZ+ GBM patients. These five protein spots were subjected to mass spectrometric analysis to establish their identity (Table 1; Fig. 3).
Figure 3.

Tissue proteomic analysis of SVZ+ (n = 6) and SVZ- (n = 6) GBMs using 2D-DIGE method. Cy3 and Cy5 overlapped 2D-DIGE gel image of SVZ+ and SVZ- GBM brain tumor tissue proteome and the 3D-views for the significantly altered proteins. The tissue proteins were separated based on their pI using IPG strips of pH 4–7; 18 cm length, followed by separation based on molecular weights using 12.5% SDS-polyacrylamide gels and image analysis was performed using DeCyder 2D software. Significantly altered protein spots were subjected to MALDI-TOF/TOF analysis to establish their identity. Dot plots and 3D-views represent the relative abundance of significantly altered proteins in the SVZ+ and SVZ- GBMs (Green colour indicates Cy3 labeled protein and red colour indicates Cy5 labeled protein).
Tissue proteome of normal brain tissue was compared with the tissue proteome of SVZ+ and SVZ− GBMs using iTRAQ method. In the six comparisons possible, proteins with two peptide signatures following similar trends in more than three comparisons and with an average fold change of 1.3 were considered significant. In the first comparison involving SVZ− GBM tissue proteome and normal brain tissue proteome, 165 proteins were found to be differentially regulated with 112 proteins being up-regulated and 53 proteins down-regulated in the SVZ− GBM tumors. The comparison of SVZ+ GBM tissue proteome with the normal brain tissue proteome, yielded 164 proteins with altered levels of expression (107 proteins being up-regulated while 57 proteins being down-regulated in SVZ+ tumors). The comparison between proteomes of SVZ+ GBM tumors and SVZ− GBM tumors indicated 96 significantly differentially regulated proteins. Of these, 43 proteins were found to be up-regulated while 53 proteins were found to be down-regulated in SVZ+ tumors in comparison with SVZ− tumors. Representative mass spectra for thymosin beta-4-like protein 3 and annexin A2 have been provided in Fig. 2d. Comparison of the results arising from serum and tissue proteomic analyses using 2D-DIGE and iTRAQ methods revealed a few common proteins as mentioned in Supplementary Table s4.
Tissue proteomic alterations in SVZ+ and SVZ- GBM tumors were further compared with the mRNA expression data of long-term survivors and short-term survivors of GBM patients from TCGA. We identified 42 common proteins/genes from our proteomics data and TCGA mRNA data. Of the 42 common proteins/genes only 18 proteins/genes showed similar expression trends some of which included adenosylhomocysteinase, protein disulfide-isomerase, endoplasmin and alpha-2-macroglobulin (Supplementary Table s5). Though the TCGA survival data does not mention about SVZ involvement with tumors, we believe that our analysis would be useful in understanding the factors affecting survival in GBM patients.
Bioinformatics analysis was performed for significantly altered proteins identified from the 3 comparisons (SVZ− vs Nor., SVZ+ vs Nor., SVZ+ vs SVZ- tissue proteome analysis) using DAVID version 6.7. Most of the pathways affected in both SVZ+ and SVZ- GBMs were found to be common and associated with sugar metabolism and blood coagulation cascades.
The data was further subjected to partial least square discriminant analysis and resulted in separation of SVZ+ GBMs from SVZ- GBMs on the 3D-score plot (Supplementary Fig. s2).
Validation of few significantly altered serum and tissue proteins
Hemopexin, one of the significantly up-regulated proteins in the serum samples of SVZ+ patients was validated using ELISA and western blotting methods. Serum levels of hemopexin from SVZ+ (n = 10) and SVZ− (n = 10) GBM patients were determined using competitive sandwich ELISA method. The serum levels of hemopexin in SVZ+ GBM patients (1.72 mg/ml) were found to be marginally higher than the SVZ− GBM patients (n = 1.25 mg/ml) and have been represented in graphical form in Fig. 4a.
Figure 4.
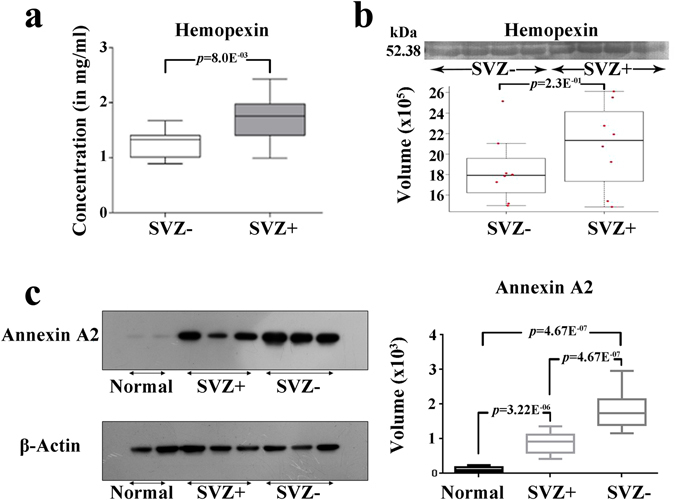
Validation of significantly altered serum (hemopexin-HPX) and tissue (annexin A2-ANXA2) proteins in SVZ+ and SVZ- GBMs. (a) Box plots representing the serum levels of HPX in SVZ− (n = 10) and SVZ+ (n = 10) GBMs estimated using ELISA and (b) western blotting results representing the serum levels of HPX in SVZ− (n = 8) and SVZ+ (n = 8) GBMs. (c) Validation of ANXA2 levels in normal brain tissue (n = 2), SVZ+ (n = 3) and SVZ- (n = 3) GBM tumors using western blotting method.
Serum western blotting analysis for hemopexin indicated a marginal increase in the levels of the protein in SVZ+ individuals in comparison to SVZ− individuals (Fig. 4b).
Tissue western blotting analysis for annexin A2 revealed two fold up-regulation of the protein in SVZ− GBM patients as compared to SVZ+ GBM patients (Fig. 4c).
Discussion
The current study aimed at offering an insight into the proteomic differences in serum and tissues from the two subgroups of GBMs and was based on available literature suggesting a possible role of SVZ in survival of GBM patients9. The proteomics study performed by Haskins et al. involved comparison of SVZ+ GBM tumor proteome with control samples8. However, protein expression analysis of both SVZ+ and SVZ− GBM tumors is likely to reveal protein signatures specific to SVZ+ GBMs. Hence, we have performed proteomic analysis of serum and tissues from SVZ+ and SVZ- GBMs in an attempt to identify protein markers responsible for aggressive nature of these tumors.
Proteomics analysis using 2D-DIGE on serum from the GBM patients revealed differential expression of four proteins-hemopexin (HPX), apolipoprotein A1 (APOA1), alpha-1-antichymotrypsin (SERPINA3)and serum albumin (ALB). A significant change in levels of acute phase proteins, HPX and SERPINA3 was observed. Besides maintaining homeostasis, hemopexin protects cells from heme mediated oxidation10 and plays an important role in differentiation of oligodendrocytes and myelin sheath formation11. SERPINA3, a serine protease inhibitor, is known to protect tissues from action of proteases12. Two major events in the process of metastasis include release of the tumor cells from the site of tumor by the action of proteases on the extracellular matrix and adhesion of the migrated tumor cells to the tissues in a new location with the help of cell surface molecules. Mass spectrometric analysis revealed a decrease in serum SERPINA3 levels in SVZ+ patients with respect to SVZ− patients, though the difference was negligible. The reduced levels of SERPINA3 in serum of SVZ+ GBM patients could be one of the plausible explanations for the increased aggressiveness of SVZ+ tumors over SVZ− tumors.
Levels of APOA1, a major component of HDL involved in cholesterol transport13, have been reported to increase in serum of patients with pancreatic cancer, gastric cancer, ovarian cancer and glioblastomas14–16. Survival studies in mouse models with ovarian cancer revealed higher levels of APOA1 to play a role in increased survival13. Serum APOA1 levels in our study were found to be down-regulated in SVZ+ GBMs. Apolipoprotein E (APOE) forms a major component of LDL carrying cholesterol from liver to extra hepatic tissues and activates cell proliferation and anti-apoptotic cascades upon binding to LDL receptors thereby helping the cells in proliferation and evading apoptosis. It has been reported to be up-regulated in breast, colon, stomach and prostate cancers17. Increased levels of APOE in serum of SVZ+ GBMs possibly indicates its role in membrane synthesis and evading apoptosis.
Vitamin-D has been reported to inhibit tumor angiogenesis, increase cell adhesion and induce cellular apoptosis18, 19. Free vitamin-D is known to cross the blood brain barrier freely20. Vitamin-D binding protein binds to free vitamin-D in the serum and transports it to the target sites. We observed decreased levels of vitamin-D binding protein in SVZ− GBM serum which supports our hypothesis that the decreased protein levels possibly inhibits tumor growth by increasing availability of free vitamin-D.
iTRAQ based quantitative mass spectrometric analysis of GBM tissue (SVZ+/-) proteome revealed significant changes in expression of proteins in many important metabolic pathways. Pathways involved in carbohydrate metabolism (glycolysis/gluconeogenesis and pentose phosphate pathway), amino acid metabolism (cys and met metabolism), complement and coagulation cascades, ECM-receptor interaction and neurodegenerative diseases (Parkinson’s, Alzheimer’s and Huntington’s disease) were found to be affected in SVZ− and SVZ+ GBMs (Refer Supplementary Tables s6, s7 and s8). Mitochondrial proteins involved in oxidative phosphorylation were down-regulated in both SVZ+ and SVZ- GBMs.
Cancer cells require a higher flux of glucose into glycolysis to meet the energy needs of rapidly proliferating tumor cells and to provide pentose phosphate pathway (PPP) intermediates for the synthesis of nucleic acid precursors21. Also, many key enzymes involved in glycolysis and PPP show increased activity in cancerous cells22. However, our study indicated down-regulation of a few glycolytic enzymes. It is possible that post translational modifications like phosphorylation/dephosphorylation of these enzymes control the flux of glucose into the glycolytic pathway. Except aldolase-C, most pentose phosphate pathway proteins identified in our study were up-regulated in both SVZ+ and SVZ- GBM tumors.
Proteins involved in complement pathway and blood coagulation cascades were found to be altered in both SVZ+ and SVZ- GBMs. Coagulation cascade related genes were found to be over expressed in epithelial cancer, ovarian cancer23 and astrocytomas24. Activation of the blood coagulation system was associated with poor prognosis25, while increased tissue factor (activator of blood coagulation system) correlated with the decreased survival of breast cancer patients26. Many proteins associated with blood coagulation system were found to be up-regulated in SVZ+ GBMs with respect to SVZ− GBMs in our study. Protease inhibitors like alpha-2-macroglobulin (A2M), alpha-1-antitrypsin (SERPINA1) and antithrombin-III (SERPINC1) associated with blood coagulation system, were found to be up-regulated in SVZ+ GBM tumors as compared to SVZ− GBM tumors. A2M binds to a number of proteases at their active sites using its bait region. On proteolysis, A2M changes its conformation rendering the protease inactive27. A2M was found to be expressed in many different cancers like human gliomas, melanomas, colon cancer etc.28–30 indicating its role in tumorigenicity30. SERPINA1, another important protease inhibitor with affinity for inhibition of neutrophil elastase31, is known to play an important role in modulating immunity, inflammation, apoptosis etc.32 and has been reported to protect brain tumor cells from the action of other proteases33. Fibrin/fibrinogen, a blood coagulation factor was found to be deposited in various cancers like brain tumors, prostate cancers, mesothelioma, colon cancer and lymphoma34–38. Fibrinogen plays critical role in angiogenesis, tumor cell growth, proliferation and development by sequestering the growth factors like fibroblast growth factor-2 and vascular endothelial growth factor39, 40. Tumor cells utilize sequestered growth factors by degrading the fibrinogen41.
Fibrin increases the metastatic capacity of tumor by virtue of its ability to bind to various cell surface molecules like integrins and non integrin receptors, thereby helping the circulating tumor cells to adhere to the cells at new location. Increased levels of fibrinogen induce plasmin, a protease and increase the invasive and metastatic nature of tumors by degrading the extracellular matrix42. Fibrin also protects the tumor cells from host mediated inflammatory response against the tumor cells43, 44.
Extracellular matrix (ECM)-receptor interaction pathways which play a role in tumor cell migration and adhesion were found to be altered in GBMs in the current study. Extracellular matrix proteins like collagen and fibronectin (FN1) were found to be up-regulated in SVZ+ GBM tumors. Collagen VI is an important extracellular matrix protein and plays a role in providing structural support to the cells, cell signaling and in increasing the availability of various growth factors45. Cattaruzza et al. studied the role of collagen VI and NG2/chondroitin sulphate proteoglycan 4 (CSPG4) molecular interactions in progression of soft tissue sarcomas. Patients suffering from soft tissue sarcomas with over-expressed Collagen VI and CSPG4 together were found to have the worst disease free survival rates46. Interaction of Collagen VI with NG2/CSPG4 resulted in increased cell motility in human glioma cell lines47. Col VI was found to protect the cells from apoptosis by activating Akt/PI3K pathway48 and has been reported to be over expressed in breast cancer, ovarian cancer and gliomas45, 49, 50. Fibronectin, a glycoprotein with cell adhesive regions on it, plays a major role in the tumor cell migration and invasion through its interaction with partner proteins called integrins51. Increased levels of fibronectin have been reported to cause an increase in migration rate of glial tumors52, in addition to increasing the invasive nature of the tumors by up-regulating a protein tyrosine kinase tie2, which activates PI3-kinase and PAK53. We found increased levels of Collagen alpha-3 (VI) chain and fibronectin in SVZ+ GBM tumors (with respect to SVZ− GBM tumors), providing a plausible explanation for their increased invasiveness over SVZ− GBM tumors. Tenascin-C, an extracellular protein majorly expressed during embryonic development is known to disappear in adults but reappears at the site of wound healing in cases of brain cancer and colorectal carcinoma54–56. It interferes with syndecan-4 binding to fibronectin and enhances proliferation of glioma and breast cancer cells57. Increase in levels of tenascin were correlated with increase in grade of tumors and invading tumor cells besides tumor cell proliferation and angiogenesis in gliomas58. In the current study, levels of tenascin-C were found to be increased in both SVZ+ and SVZ− tumors as compared to peritumoral tissue, with the levels of tenascin-C in SVZ+ tumors being higher than SVZ− tumors.
Some cytoskeleton associated proteins like thymosin beta 4 (TMSB4X) and brain acid soluble protein 1 (BASP1) were found to be significantly altered in SVZ+ GBMs, and showed good correlation with the aggressive nature of SVZ+ tumors. Thymosin beta-4, an actin binding protein plays a major role in actin polymerization and maintains dynamic equilibrium between G-actin and F-actin. It is also involved in the proliferation, migration, survival and aggressiveness of colorectal cancer and pancreatic cancer via activation of Akt and JNK signaling pathways, respectively59, 60. Expression of thymosin beta-4 was found to have a negative correlation with survival period of patients with non-small cell lung cancer (NSCLC), where the protein expression was associated with increased metastasis and poor prognosis61. We observed an up-regulation of thymosin beta-4 like protein 3 in SVZ+ GBM patients as compared to SVZ− GBM in the current study. BASP1 is involved in organization of cytoskeletal elements and determines the morphology of plasma membrane62. Hartl et al. showed a decrease in expression levels of BASP1 in cells transformed with the Myc, whereas the ectopic expression of BASP1 in the cells transfected with v-myc oncogene showed resistance to v-myc induced transformation63. BASP1 over expression was shown to result in death of cells by apoptosis, whereas decreased expression of BASP1 was found to increase cell survival64. In the current study, both SVZ+ and SVZ− GBM tumors showed reduced levels of BASP1, which possibly helps the tumor cells in evading apoptosis.
The proteins identified in the current study provide a molecular evidence for the aggressive nature of SVZ+ GBM tumors over SVZ− GBM tumors. However, these proteins need to be further explored in biological models i.e., glioma cell lines and animal models to authenticate their role in increased aggressiveness of SVZ+ tumors.
Materials and Methods
Patient selection and samples collection
GBM serum and tissue samples were collected from Tata Memorial Centre’s, Advanced Centre for Treatment, Research and Education (TMC-ACTREC), Mumbai, India. The samples were categorized into two groups based on sub-ventricular zone involvement and median age of the patients. A total of 21 tissue samples (nine SVZ−, eight SVZ+ and four peritumoral samples) and 33 serum samples (16 SVZ− and 17 SVZ+) were used in the current proteomics study. The study was carried out after approval of the institutional ethics committee of ACTREC (IEC-80) and IIT Bombay. Pre-informed consent of all the subjects enrolled for this study were taken before sample collection. All experiments were performed in accordance with guidelines and regulations laid down by institutional ethics committees of TMH and IIT Bombay. Wherever possible, peritumoral tissue samples from presumed uninvolved brain included in the resection specimen (with confirmed histology) were used as normal control samples for the proteomics study.
Serum and brain tissue samples processing
Protein extraction from serum was performed using TCA–Acetone precipitation method65, whereas the brain tissue proteins were extracted using trizol method66. The pre-processing steps for the serum and tissue protein extraction are described in detail in the supplementary materials and methods section. The extracted protein samples were quantified using 2D-Quant kit (GE Healthcare) and the protein samples were stored at −20 °C until further use (For more details refer supplementary materials and methods).
Two dimensional difference gel electrophoresis (2D-DIGE)
The protein samples in rehydration buffer were minimal labeled with CyDyes as per the manufacturer’s instructions (GE Healthcare), pooled and loaded onto a 18 cm IPG strip of pH 4–7 (linear) followed by isoelectric focusing (IEF) for 64 kVh (for tissue proteins) and 78 kVh (for serum proteins). After IEF, the proteins were further separated on a 12.5% SDS-Polyacrylamide gels followed by scanning the gels using Typhoon FLA 9500 scanner (GE Healthcare) at different excitation and emission wavelengths. All the images were scanned at 100 µm resolution and the images obtained were further processed using DeCyder 2D software version 7.0 (GE Healthcare) (For more details refer supplementary materials and methods).
MALDI-TOF/TOF analysis
The significantly altered protein spots were subjected to in-gel digestion67 followed by mass spectrometric analysis using 4800 MALDI TOF/TOF MS (AB Sciex) in reflectron mode. The mass spectrometry data was analysed using MASCOT version 2.1 search engine for identification of the protein against Swiss-Prot database (For more details refer supplementary materials and methods).
In-solution digestion and iTRAQ labeling
The brain tissue and serum proteins were made LC-MS compatible by exchanging the buffer with 0.5 M TEAB using 3 kDa molecular weight cutoff filters. The protein samples were subjected to denaturation, reduction and alkylation followed by overnight trypsin digestion for 16 hr. The in-solution digested normal brain tissue proteins, SVZ− and SVZ+ GBM tumor tissue protein samples were then labeled with iTRAQ reagents 114, 115 and 116, respectively. In case of first two iTRAQ sets, iTRAQ reagent 117 was used for labeling of in-solution digested SVZ− GBM tissue proteins, whereas in the iTRAQ sets three and four, iTRAQ reagent 117 was used for labeling of in-solution digested SVZ+ GBM tissue proteins. For serum proteomic analysis, trypsin digested SVZ− serum proteins were labeled with iTRAQ reagent 114 and 116, where as the SVZ+ GBM patient’s serum proteins were labeled with iTRAQ reagent 115 and 117. The labeled samples were finally pooled and subjected to off-gel fractionation (For more details refer supplementary materials and methods).
Offgel fractionation and LC-MS/MS
200 µg of the labeled and pooled peptide sample was subjected to Offgel fractionation on 3–10 non-linear, 24 cm high resolution IPG strips using Agilent 3100 Offgel fractionator. Minor modifications were made to the manufacturer’s protocol (Agilent Technologies). Isoelectrically focused and concentrated peptide fractions were later reconstituted in 0.1% formic acid and subjected to LC-MS/MS (For more details refer supplementary materials and methods).
Bioinformatics analysis
The mRNA expression data for 558 GBM samples was downloaded from TCGA data portal (https://gdc-portal.nci.nih.gov/) and the patients were classified into short-term survivors (STS), median-term survivors (MTS) and long-term survivors (LTS) based on their overall survival. GBM patients with an overall survival of <1 year, 1–3 years and >3 years were classified into STS, MTS and LTS, respectively. In the current study, we compared the mRNA expression data of 217 STS with 38 LTS GBMs using BRB-Array tools68. Using class comparison module of BRB-Array tools, the expression levels of different genes in short-term survivors of GBMs was compared with long-term survivors of GBM patients. Further, the mRNA expression data (p-value ≤ 0.05) was correlated with protein expression data obtained from our quantitative mass spectrometric studies.
The data obtained from the tissue proteomic analysis of the SVZ+, SVZ− GBMs and normal brain tissue proteome was further subjected to bioinformatics analysis using the Database for Annotation, Visualization and Integrated Discovery (DAVID) version 6.7. Significantly altered proteins obtained from the iTRAQ analysis of SVZ+ GBM vs Normal, SVZ− GBM vs Normal and SVZ+ vs SVZ− GBM were subjected to DAVID analysis69, 70 to identify the pathways affected in the sub groups of GBM patients.
iTRAQ data for the tissue proteomic analysis of SVZ−, SVZ+ GBM tumors and normal/control tissues was subjected to PLSDA using METAGENassist, an online tool71. Both SVZ− and SVZ+ GBMs were segregated from each other on a 3D-score plot obtained from this multivariate analysis.
ELISA and Western blotting
Hemopexin levels in GBM patient (both SVZ+ and SVZ−) serum samples were determined using competitive sandwich ELISA method (AssayPro ELISA kit, USA) following manufacturer’s instructions. The absorbance of the chromogenic substrate was read at two different wavelengths, i.e., 450 nm and 570 nm using SpectraMax microplate reader (Molecular devices). The absorbance values at 570 nm were substracted from the values at 450 nm and the resulting absorbance values were used for determining the serum concentrations of hemopexin from the standard curve.
Western blotting was performed on the serum samples of SVZ+ (n = 8) and SVZ− (n = 8) GBM patients to validate the serum hemopexin levels. The serum proteins were separated using SDS-PAGE followed by their transfer onto PVDF membrane. Blocking was done in 5% skimmed milk followed by incubation with primary antibody (Rabbit polyclonal IgG, 1:500 dilution) against hemopexin (Santacruz biotechnology, lot no.J2909, sc-13443) followed by incubation with HRP conjugated secondary antibody (1:2500 dilution, Goat anti-Rabbit IgG-HRP, GeNei, lot no. 032071) against the primary antibody, followed by washes with TBST buffer. A chromogenic substrate (TMB/H2O2, GeNei, lot no. 033091) was added, followed by scanning the membrane using LabScan (GEHealthcare). Densitometric analysis of the hemopexin bands was performed using ImageQuant TL software (GE Healthcare).
Western blotting was carried out on SVZ+ and SVZ− GBM tissue lysates (n = 3 for each subtype) and compared with normal brain tissue lysates (n = 2) in order to validate the levels of Annexin A2. 10 µg of the protein lysates were resolved on 8% SDS-PAGE gels following which the proteins were transferred onto a PVDF membrane. Post transfer, the membrane was blocked using 5% non-fat dried milk in 0.1% TBST (pH- 7.4) for one hour. The blots were then probed with primary antibodies for Actin, which was used as loading control (Mouse polyclonal 1:2000) and Annexin A2 (Rabbit polyclonal 1:5000) overnight at 4 °C. Following overnight incubation, the blots were washed three times in 0.1% TBST and incubated for 1 hour with secondary antibodies anti- mouse (1:8000) and anti-rabbit (1:5000) for Actin and Annexin A2, respectively. The blots were finally washed three times in 0.1% TBST and developed using chemiluminescent reagent (GE Amersham) followed by autoradiography. Densitometric analysis of the blots was performed using ImageQuant TL software (GE Healthcare).
Electronic supplementary material
Acknowledgements
The authors would like to thank Thermo Fisher Scientific India Pvt Ltd. for their support in performing iTRAQ based mass spectrometric study of serum protein samples, Department of Biotechnology, Govt. of India (BT/PR14359/MED/30/916/2010 and BT/PR4599/BRB/10/1042/2012) for funding the project to perform proteomic experiments, Intramural grant by DAE-CTC for the IHC work done at ACTREC and CSIR for providing fellowship to Kishore Gollapalli. Authors declare that there is no conflict of interest.
Author Contributions
K.G., A.M. and S.S. have designed and conceived the study. K.G. performed tissue 2D-DIGE experiments, iTRAQ LC-MS/MS experiments for tissue and serum samples. K.G. and S.G. analysed data emerging from iTRAQ experiments and performed pathway enrichment analysis. S.K. and R.S. performed 2D-DIGE experiments and validation on serum samples using ELISA and western blotting. G.S. extracted proteins from tissue samples and carried out validation of iTRAQ tissue results using Western blotting. A.M. and S.E. collected the serum and tissue samples along with the clinical details of the patients. S.R. has facilitated MALDI-TOF/TOF analysis of significantly altered serum protein spots obtained from the 2D-DIGE experiments. S.G. and K.G. have written the manuscript. A.M., S.E. and S.S. have critically reviewed and provided expert advices for the improvement of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01202-8
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maher EA, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 2.Ichimura K, Ohgaki H, Kleihues P, Collins VP. Molecular pathogenesis of astrocytic tumours. J. Neurooncol. 2004;70:137–160. doi: 10.1007/s11060-004-2747-2. [DOI] [PubMed] [Google Scholar]
- 3.Kleihues P, et al. The WHO classification of tumors of the nervous system. J. Neuropathol. Exp. Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 4.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc. Natl. Acad. Sci. USA. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J. Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreth FW, et al. The role of tumor resection in the treatment of glioblastoma multiforme in adults. Cancer. 1999;86:2117–2123. doi: 10.1002/(SICI)1097-0142(19991115)86:10<2117::AID-CNCR33>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Iwadate Y, et al. Molecular classification and survival prediction in human gliomas based on proteome analysis. Cancer Res. 2004;64:2496–2501. doi: 10.1158/0008-5472.CAN-03-1254. [DOI] [PubMed] [Google Scholar]
- 8.Haskins WE, et al. Molecular characteristics in MRI-classified group 1 glioblastoma multiforme. Frontiers in Oncology. 2013;3:182. doi: 10.3389/fonc.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adeberg S, et al. A comparison of long-term survivors and short-term survivors with glioblastoma, subventricular zone involvement: a predictive factor for survival? Radiat Oncol. 2014;9:95. doi: 10.1186/1748-717X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolosano E, Altruda F. Hemopexin: structure, function, and regulation. DNA Cell Biol. 2002;21:297–306. doi: 10.1089/104454902753759717. [DOI] [PubMed] [Google Scholar]
- 11.Morello N, Bianchi FT, Marmiroli P, Tonoli E, et al. A role for hemopexin in oligodendrocyte differentiation and myelin formation. PLoS. One. 2011;6:e20173. doi: 10.1371/journal.pone.0020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Janciauskiene S. Multi-functional capability of proteins: alpha1-antichymotrypsin and the correlation with Alzheimer’s disease. J. Alzheimers. Dis. 2002;4:115–122. doi: 10.3233/JAD-2002-4206. [DOI] [PubMed] [Google Scholar]
- 13.Su F, et al. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc. Natl. Acad. Sci. USA. 2010;107:19997–20002. doi: 10.1073/pnas.1009010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozak KR, et al. Characterization of serum biomarkers for detection of early stage ovarian cancer. Proteomics. 2005;5:4589–4596. doi: 10.1002/pmic.200500093. [DOI] [PubMed] [Google Scholar]
- 15.Ehmann M, et al. Identification of potential markers for the detection of pancreatic cancer through comparative serum protein expression profiling. Pancreas. 2007;34:205–214. doi: 10.1097/01.mpa.0000250128.57026.b2. [DOI] [PubMed] [Google Scholar]
- 16.Takaishi S, Wang TC. Gene expression profiling in a mouse model of Helicobacter-induced gastric cancer. Cancer Sci. 2007;98:284–293. doi: 10.1111/j.1349-7006.2007.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YC, et al. Apolipoprotein E is required for cell proliferation and survival in ovarian cancer. Cancer Res. 2005;65:331–337. [PubMed] [Google Scholar]
- 18.Garland CF, et al. The role of vitamin D in cancer prevention. Am. J. Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004;79:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 20.Harms LR, Burne TH, Eyles DW, McGrath JJ. Vitamin D and the brain. Best. Pract. Res. Clin. Endocrinol. Metab. 2011;25:657–669. doi: 10.1016/j.beem.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Puzio-Kuter AM. The Role of p53 in Metabolic Regulation. Genes Cancer. 2011;2:385–391. doi: 10.1177/1947601911409738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Board M, Humm S, Newsholme EA. Maximum activities of key enzymes of glycolysis, glutaminolysis, pentose phosphate pathway and tricarboxylic acid cycle in normal, neoplastic and suppressed cells. Biochem. J. 1990;265:503–509. doi: 10.1042/bj2650503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang E, et al. Peritoneal and subperitoneal stroma may facilitate regional spread of ovarian cancer. Clin. Cancer Res. 2005;11:113–122. [PubMed] [Google Scholar]
- 24.Rong Y, et al. PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res. 2005;65:1406–1413. doi: 10.1158/0008-5472.CAN-04-3376. [DOI] [PubMed] [Google Scholar]
- 25.Lima LG, Monteiro RQ. Activation of blood coagulation in cancer: implications for tumour progression. Biosci. Rep. 2013;33:e00064. doi: 10.1042/BSR20130057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueno T, Toi M, Koike M, Nakamura S, Tominaga T. Tissue factor expression in breast cancer tissues: its correlation with prognosis and plasma concentration. Br. J. Cancer. 2000;83:164–170. doi: 10.1054/bjoc.2000.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borth W. Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992;6:3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- 28.Businaro R, Fabrizi C, Fumagalli L, Lauro GM. Synthesis and secretion of alpha 2-macroglobulin by human glioma established cell lines. Exp. Brain Res. 1992;88:213–218. doi: 10.1007/BF02259144. [DOI] [PubMed] [Google Scholar]
- 29.Matoska J, Wahlstrom T, Vaheri A, Bizik J, Grofova M. Tumor-associated alpha-2-macroglobulin in human melanomas. Int. J. Cancer. 1988;41:359–363. doi: 10.1002/ijc.2910410307. [DOI] [PubMed] [Google Scholar]
- 30.Smorenburg SM, et al. alpha2-Macroglobulin is mainly produced by cancer cells and not by hepatocytes in rats with colon carcinoma metastases in liver. Hepatology. 1996;23:560–570. doi: 10.1002/hep.510230323. [DOI] [PubMed] [Google Scholar]
- 31.Huber R, Carrell RW. Implications of the three-dimensional structure of alpha 1-antitrypsin for structure and function of serpins. Biochemistry. 1989;28:8951–8966. doi: 10.1021/bi00449a001. [DOI] [PubMed] [Google Scholar]
- 32.Hunt JM, Tuder R. Alpha 1 anti-trypsin: one protein, many functions. Curr. Mol. Med. 2012;12:827–835. doi: 10.2174/156652412801318755. [DOI] [PubMed] [Google Scholar]
- 33.Sawaya R, Zuccarello M, Highsmith R. Alpha-1-antitrypsin in human brain tumors. J. Neurosurg. 1987;67:258–262. doi: 10.3171/jns.1987.67.2.0258. [DOI] [PubMed] [Google Scholar]
- 34.Bardos H, Molnar P, Csecsei G, Adany R. Fibrin deposition in primary and metastatic human brain tumours. Blood Coagul. Fibrinolysis. 1996;7:536–548. doi: 10.1097/00001721-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Wojtukiewicz MZ, et al. Fibrin formation on vessel walls in hyperplastic and malignant prostate tissue. Cancer. 1991;67:1377–1383. doi: 10.1002/1097-0142(19910301)67:5<1377::AID-CNCR2820670517>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Wojtukiewicz MZ, et al. Absence of components of coagulation and fibrinolysis pathways in situ in mesothelioma. Thromb. Res. 1989;55:279–284. doi: 10.1016/0049-3848(89)90445-3. [DOI] [PubMed] [Google Scholar]
- 37.Wojtukiewicz MZ, et al. Indirect activation of blood coagulation in colon cancer. Thromb. Haemost. 1989;62:1062–1066. [PubMed] [Google Scholar]
- 38.Costantini V, et al. Fibrinogen deposition and macrophage-associated fibrin formation in malignant and nonmalignant lymphoid tissue. J. Lab Clin. Med. 1992;119:124–131. [PubMed] [Google Scholar]
- 39.Sahni A, Odrljin T, Francis CW. Binding of basic fibroblast growth factor to fibrinogen and fibrin. J. Biol. Chem. 1998;273:7554–7559. doi: 10.1074/jbc.273.13.7554. [DOI] [PubMed] [Google Scholar]
- 40.Sahni A, Francis CW. Vascular endothelial growth factor binds to fibrinogen and fibrin and stimulates endothelial cell proliferation. Blood. 2000;96:3772–3778. [PubMed] [Google Scholar]
- 41.Simpson-Haidaris PJ, Rybarczyk B. Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein. Ann. N. Y. Acad. Sci. 2001;936:406–425. doi: 10.1111/j.1749-6632.2001.tb03525.x. [DOI] [PubMed] [Google Scholar]
- 42.Palumbo JS, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–3309. [PubMed] [Google Scholar]
- 43.Dvorak HF, Dickersin GR, Dvorak AM, Manseau EJ, Pyne K. Human breast carcinoma: fibrin deposits and desmoplasia. Inflammatory cell type and distribution. Microvasculature and infarction. J. Natl. Cancer Inst. 1981;67:335–345. [PubMed] [Google Scholar]
- 44.Dvorak HF, Senger DR, Dvorak AM. Fibrin as a component of the tumor stroma: origins and biological significance. Cancer Metastasis Rev. 1983;2:41–73. doi: 10.1007/BF00046905. [DOI] [PubMed] [Google Scholar]
- 45.Iyengar P, et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J. Clin. Invest. 2005;115:1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cattaruzza S, et al. NG2/CSPG4-collagen type VI interplays putatively involved in the microenvironmental control of tumour engraftment and local expansion. J. Mol. Cell Biol. 2013;5:176–193. doi: 10.1093/jmcb/mjt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fang X, et al. Cytoskeletal reorganization induced by engagement of the NG2 proteoglycan leads to cell spreading and migration. Mol. Biol. Cell. 1999;10:3373–3387. doi: 10.1091/mbc.10.10.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng IH, et al. Collagen VI protects against neuronal apoptosis elicited by ultraviolet irradiation via an Akt/phosphatidylinositol 3-kinase signaling pathway. Neuroscience. 2011;183:178–188. doi: 10.1016/j.neuroscience.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 49.Sherman-Baust CA, et al. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell. 2003;3:377–386. doi: 10.1016/S1535-6108(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 50.Han J, Daniel JC, Pappas GD. Expression of type VI collagen during glioblastoma cell invasion in brain tissue cultures. Cancer Lett. 1995;88:127–132. doi: 10.1016/0304-3835(94)03627-U. [DOI] [PubMed] [Google Scholar]
- 51.Akiyama SK, Olden K, Yamada KM. Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev. 1995;14:173–189. doi: 10.1007/BF00690290. [DOI] [PubMed] [Google Scholar]
- 52.Ohnishi T, et al. Role of fibronectin-stimulated tumor cell migration in glioma invasion in vivo: clinical significance of fibronectin and fibronectin receptor expressed in human glioma tissues. Clin. Exp. Metastasis. 1998;16:729–741. doi: 10.1023/A:1006532812408. [DOI] [PubMed] [Google Scholar]
- 53.Knowles LM, Malik G, Pilch J. Plasma Fibronectin Promotes Tumor Cell Survival and Invasion through Regulation of Tie2. J. Cancer. 2013;4:383–390. doi: 10.7150/jca.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Derr LB, Chiquet-Ehrismann R, Gandour-Edwards R, Spence J, Tucker RP. The expression of tenascin-C with the AD1 variable repeat in embryonic tissues, cell lines and tumors in various vertebrate species. Differentiation. 1997;62:71–82. doi: 10.1046/j.1432-0436.1997.6220071.x. [DOI] [PubMed] [Google Scholar]
- 55.Leins A, et al. Expression of tenascin-C in various human brain tumors and its relevance for survival in patients with astrocytoma. Cancer. 2003;98:2430–2439. doi: 10.1002/cncr.11796. [DOI] [PubMed] [Google Scholar]
- 56.Emoto K, et al. Annexin II overexpression correlates with stromal tenascin-C overexpression: a prognostic marker in colorectal carcinoma. Cancer. 2001;92:1419–1426. doi: 10.1002/1097-0142(20010915)92:6<1419::AID-CNCR1465>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 57.Huang W, Chiquet-Ehrismann R, Moyano JV, Garcia-Pardo A, Orend G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res. 2001;61:8586–8594. [PubMed] [Google Scholar]
- 58.Zagzag D, et al. Tenascin expression in astrocytomas correlates with angiogenesis. Cancer Res. 1995;55:907–914. [PubMed] [Google Scholar]
- 59.Ricci-Vitiani L, et al. Thymosin beta4 targeting impairs tumorigenic activity of colon cancer stem cells. FASEB J. 2010;24:4291–4301. doi: 10.1096/fj.10-159970. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Y, et al. Thymosin Beta 4 is overexpressed in human pancreatic cancer cells and stimulates proinflammatory cytokine secretion and JNK activation. Cancer Biol. Ther. 2008;7:419–423. doi: 10.4161/cbt.7.3.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji P, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 62.Korshunova I, et al. Characterization of BASP1-mediated neurite outgrowth. J. Neurosci. Res. 2008;86:2201–2213. doi: 10.1002/jnr.21678. [DOI] [PubMed] [Google Scholar]
- 63.Hartl M, Nist A, Khan MI, Valovka T, Bister K. Inhibition of Myc-induced cell transformation by brain acid-soluble protein 1 (BASP1) Proc. Natl. Acad. Sci. USA. 2009;106:5604–5609. doi: 10.1073/pnas.0812101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanchez-Nino MD, et al. BASP1 promotes apoptosis in diabetic nephropathy. J. Am. Soc. Nephrol. 2010;21:610–621. doi: 10.1681/ASN.2009020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen YY, et al. A modified protein precipitation procedure for efficient removal of albumin from serum. Electrophoresis. 2005;26:2117–2127. doi: 10.1002/elps.200410381. [DOI] [PubMed] [Google Scholar]
- 66.Reddy PJ, et al. A simple protein extraction method for proteomic analysis of diverse biological specimens. Current Proteomics. 2013;10:298–311. doi: 10.2174/15701646113106660004. [DOI] [Google Scholar]
- 67.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 68.Simon R, et al. Analysis of gene expression data using BRB-ArrayTools. Cancer informatics. 2007;3:11–17. [PMC free article] [PubMed] [Google Scholar]
- 69.Huang dW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang dW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 71.Arndt D, et al. METAGENassist: a comprehensive web server for comparative metagenomics. Nucleic acids research. 2012;40:W88–95. doi: 10.1093/nar/gks497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


