Abstract
Metabolites containing amino groups cover multiple pathways and play important roles in redox homeostasis and biosyntheses of proteins, nucleotides and neurotransmitters. Here, we report a new method for simultaneous quantification of 124 such metabolites. This is achieved by derivatization-assisted sensitivity enhancement with 5-aminoisoquinolyl-N-hydroxysuccinimidyl carbamate (5-AIQC) followed with comprehensive analysis using ultra-high performance liquid chromatography and electrospray ionization tandem mass spectrometry (UHPLC-MS/MS). In an one-pot manner, this quantification method enables simultaneous coverage of 20 important metabolic pathways including protein biosynthesis/degradation, biosyntheses of catecholamines, arginine and glutathione, metabolisms of homocysteine, taurine-hypotaurine etc. Compared with the reported ones, this method is capable of simultaneously quantifying thiols, disulfides and other oxidation-prone analytes in a single run and suitable for quantifying aromatic amino metabolites. This method is also much more sensitive for all tested metabolites with LODs well below 50 fmol (at sub-fmol for most tested analytes) and shows good precision for retention time and quantitation with inter-day and intra-day relative standard deviations (RSDs) below 15% and good recovery from renal cancer tissue, rat urine and plasma. The method was further applied to quantify the amino metabolites in silkworm hemolymph from multiple developmental stages showing its applicability in metabolomics and perhaps some clinical chemistry studies.
Introduction
Metabolism denotes all chemical transformations in living systems and quantifying the metabolite composition (metabonome/metabolome) of such integrated biological systems is vitally important for understanding the molecular basis of such systems. Metabonomics and metabolomics are science for accurate metabonomic (and/or metabolomic) analysis of the dynamic metabolic changes in cells, tissues and whole organisms1–5. Therefore, metabonomic/metabolomic analyses have already found widespread applications in revealing the biochemistry details for some basic living processes6–9, pathogenesis and progressions10–12, systems responses towards xenobiotics13–17 and clinical interventions18–21, symbiotic interactions in mammals22–27 and disease diagnosis and prognosis28–32. These analyses ideally require quantification of all metabolites including amino acids, nucleic acids, carboxylic acids, carbohydrates, lipids, and small peptides in complex biological matrices33 so as to define the overall metabonomic phenotypes of the studied systems. In practice, however, a single such analysis nowadays can only cover some of all these metabolites due to the diversity of molecular types, matrices, physicochemical properties, dynamic ranges of concentration for these metabolites.
Quantitative analyses of certain targeted metabolomes are often required to obtain accurate and detailed information about some specific metabolites especially in answering biological questions in the hypothesis-driven studies. For this purpose, both gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) approaches have been widely employed due to their outstanding metabolite selectivity and sensitivity34. Chromatographic separations enable reduction of the sample complexity at detectors alleviating ionization suppression in the subsequent mass spectral acquisitions. Recently developed UHPLC techniques using sub-two μm particles have offered much higher chromatographic resolution and efficiency (or shorter analytical time)35, 36 than conventional HPLC. The hyphenated UHPLC and tandem mass spectrometry (UHPLC-MS/MS) with multiple reaction monitoring (MRM) have found widespread applications in quantitative analyses of various sets of specific metabolomes37–40 with greatly enhanced throughtput, dynamic range, specificity and sensitivity35–40.
Amino group containing metabolites representing an important subset of metabonome cover many important metabolic pathways and possess a variety of vital biological functions. These metabolites include proteinogenic and non-proteinogenic amino acids carrying amino and acidic (e.g., carboxyl or sulfonic) groups, post-translationally modified (methylated, acetylated and phosphorylated) amino acids, aliphatic and aromatic amines, small peptides, catecholamines, thiol and disulfide containing amino metabolites. These metabolites cover dozens of important metabolic pathways and quantitative analysis of them is hence critically important for pathophysiology studies and biomarkers discoveries41, 42. Since most of these amino metabolites are fairly hydrophilic, they are often not suitable for straightforward reverse-phase separation and, in theory, can be analyzable with HILIC or ion-pair chromatography40. However, these techniques have limited potentials in quantitative metabonomic phenotyping due to their poor chromatographic reproducibility, sensitivity, peak shapes and long equilibration times. Reagents used in ion-pair chromatography also cause undesirable ion suppression effects in the positive ion mode so that a dedicated spectrometer is often required as reported43.
Derivatization-based reversed-phase LC-MS analysis is an excellent approach for quantification of amino metabolites especially with efficient amino-group specific tags employed44. The traditional tagging reagents include O-phthalaldehyde (OPA)45, 9-fluorenylmethylchloroformate (FMOC-Cl)46, 5-(dimethylamino)-naphthalene-1-sulfonyl chloride (Dansyl-Cl)38, phenylisothiocyanate (PITC)47 and 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (6-AQC)37, 48. Amongst them, 6-AQC-based method showed good promising by simultaneously quantifying 46 amino analytes with excellent selectively for both primary and secondary amino groups and suitability for the oxidation-prone analytes (e.g., cysteine, dopamine, N-acetyl-5-hydroxytryptamine) by employing antioxidants (ascorbic acid and TCEP)37. However, this method is neither suitable for some important aromatic amino metabolites (such as 3-aminosalicylic acid, 3-hydroxyanthranilic acid, 4-aminobenzoic acids and 4-aminohippuric acid), nor for simultaneous quantification of metabolites containing thiol groups (e.g., cysteine and glutathione) and their corresponding disulfides (cystine and GSSG)37 in an “one-pot” manner (in a single run). These thiol- and disulfide-containing metabolites often have to be quantified separately49–51 leading to substantial compromise for analytical throughputs with multiple analyses required for different subclasses of amino metabolites. It is also worth-noting that quantities of thiols and disulfides have completely different biological implications. For instance, GSH often plays vital roles in signaling and redox homeostasis and the GSH-to-GSSG ratio is an indicator for oxidative stress49–51. For the time being, however, no methods are available for simultaneous quantification of all amino metabolites carrying thiol and disulfide groups concurrently with large number of other amino metabolites in an “one-pot” fashion.
In this work, we report a new derivatization-assisted sensitivity enhancement for quantitative metabolomics method for simultaneous quantification of amino compounds tagged with 5-aminoisoquinolyl-N-hydroxysuccinimidylcarbamate (5-AIQC) using UHPLC-MS/MS techniques. This method showed excellent suitability for quantifying many aromatic amino metabolites which could not be analyzed with the 6-AQC method37 and better sensitivity for most metabolites than the 6-AQC-based method37. This method enabled simultaneous quantification of multiple subclasses of analytes in a one-pot fashion (in a single run) including both the thiol- and disulfide-containing metabolites, amino acids, biogenic amines, small peptides and monoamine neurotransmitters. This new method further showed excellent applicability in quantitative analysis of amino metabolites in different matrices including rat urine and plasma, human kidney tissue and silkworm hemolymph.
Results and Discussion
Derivatization of amino analytes by 5-aminoisoquinolyl-N-hydroxysuccinimidylcarbamate(5-AIQC)
5-AIQC was readily prepared at ambient temperature by simply adding 5-aminoisoquinoline to excess N,N′-disuccinimidylcarbonate (Fig. 1). 5-AIQC rapidly reacts with both the primary and secondary amino groups of analytes (within 10 mins) at the ambient temperature with excellent selectivity producing asymmetric ureas (Fig. 2, Supplementary Fig. S1) which are stable at room temperature. Although 5-AIQC also reacts with phenolic hydroxyl groups (e.g., in tyrosine), mild heating (55 °C) easily facilitates degradation of such adducts leaving only the amino-5-AIQC adducts intact as in the case of 6-AQC37, 48. So far, 5-AIQC has not been employed for analysis of amino compounds using mass spectrometry to the best of our knowledge though synthesis of 5-AIQC was reported in 1991 as a potential fluorescent tag for amino acids52. With higher pKa for isoquinoline than quinoline, 5-AIQC derivatized amino compounds is expected to have better sensitivities in the positive ion mass spectrometry than 6-AQC-adducts, which will be discussed later.
Figure 1.
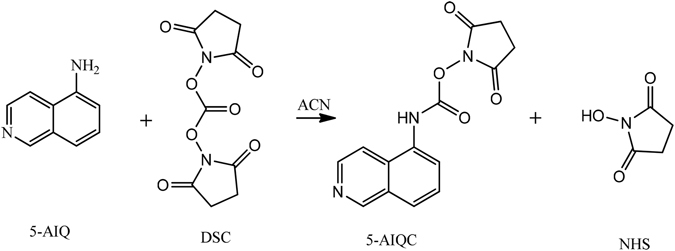
Synthesis of 5-aminoisoquinolyl-N-hydroxysuccinimidyl carbamate (5-AIQC).
Figure 2.
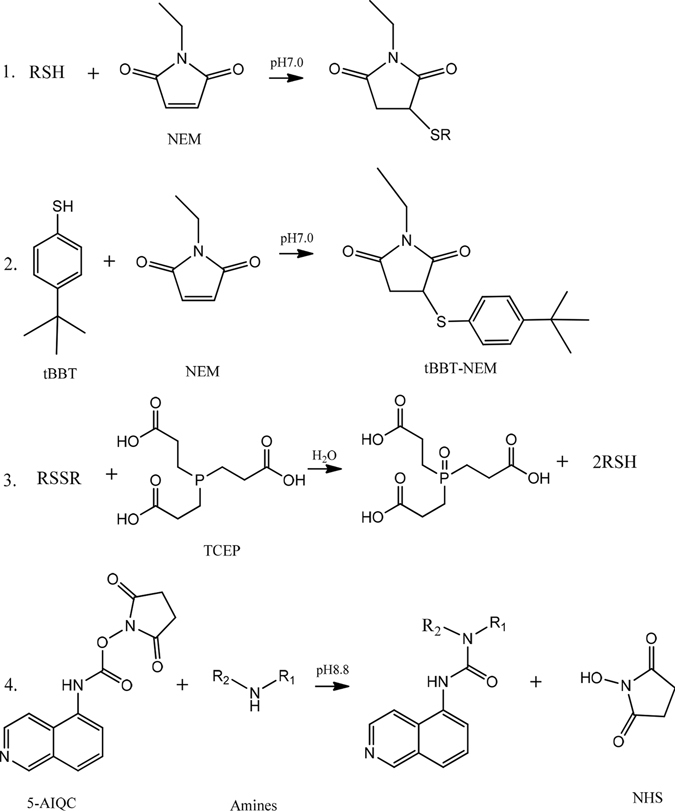
Schemes for 5-AIQC derivatization of amino analytes with thiol and disulfide groups in one pot.
To make the analytical method applicable for the oxidation-prone metabolites including thiols and catecholamines in biological samples, addition of antioxidants including TCEP and ascorbic acid is necessary37. However, TCEP will convert disulfides into thiols37. To enable simultaneous quantification of the amino-containing thiols (such as cysteine and GSH) and disulfides (such as cystine and glutathione disulfide), we employed N-ethylmaleimide (NEM) here to trap thiols through click reaction forming RSH-NEM adducts (Fig. 2, Supplementary Fig. S1) which completed within 2 min at pH7.0 avoiding very slow reactions of NEM with amino group50, 53. The remaining NEM was then quenched by click reaction with excess 4-tert-butylbenzenethiol (tBBT) to form stable NEM-tBBT adduct. After trapping of original thiols, 20 mM TCEP solution in borate buffer (200 mM, pH 8.8) containing 1 mM ascorbic acid was added to convert all disulfides into thiols to avoid multiple tagging and to prevent oxidation-prone metabolites (e.g., dopamine, tryptamine and norepinephrine) together with the TCEP-generated thiols from oxidization during analysis37. Therefore, the newly produced thiols from disulfides (RSSR) can be readily distinguished from the original thiols during quantification. After these treatments, the amino compounds were then easily quantified as 5-AIQC adducts and the thiol-containing ones were quantified as 5-AIQC-RSH-NEM adducts but disulfide-containing ones as 5-AIQC-RSH adducts in an one-pot manner. Since both tBBT and its stable NEM-tBBT adduct formed from quenching NEM are much more hydrophobic than all 5-AIQC-derivatised compounds, these two by-products can be conveniently flushed into waste with 95% CH3OH after elution of all the 5-AIQC-analyte adducts. Although NEM can be hydrolyzed slowly at pH8.8 to open its ring, such hydrolysis was minimal within the total period required for derivatization (less than 15 mins) and LC-MS analysis. For instance, we found that only less than 3% 5-AIQC-NEM-Cys was hydrolyzed in this study (Supplementary Fig. S2). With good NEM stability under acidic condition (pH < 5.0)54, sufficient formic acid was added immediately after derivatization to lower pH to about 2.5. Under such acidic condition, the thiol-NEM adducts were all very stable within 48 h without extra hydrolysis detectable (Supplementary Fig. S2) and a 20-fold excess of 5-AIQC against the total amino groups was sufficient to complete derivatization of all analytes (Supplementary Fig. S3). Interestingly, the 5-AIQC derivatives of GSH, homocysteine, γ-Glu-Cys, DL-2,6-diaminopimelic acid and DL-lanthionine (i.e., GSH-NEM-AIQC, Hcys-NEM-AIQC, γ-Glu-Cys-NEM-AIQC, DL-2,6-diaminopimelate-AIQC2 and DL-lanthionine-AIQC2) all showed two chromatographic peaks (Supplementary Fig. S4) probably due to the presence of two different ionization forms (for their carboxyl groups) at the given pH of mobile phase. This is further supported by the observable alterations of these peaks and only a single chromatographic peak for DL-2,6-diaminopimelate-AIQC2 with an increase of elution solvent acidity (to pH~2.36). Nevertheless, they all showed excellent sensitivity, linearity, retention time precision and peak shapes taking both these peaks into considerations (Table 1). However, 5-AIQC failed in tagging adenine, amide, guanido groups as in the case of 6-AQC37.
Table 1.
Data for 126 amino analytes in the form of their 5-AIQC-adducts including the neutral formula, theoretical m/z, precursor (Q1) and fragment (Q3) ions, retention time (RT), collision energy (CE), fragmentor voltage (FV), linear range and coefficients (R2) and the on-column limits of detection for the 5-AIQC-adducts (LODa) and 6-AQC-adducts (LODb) from the same method.
| NO. | Analytes | Neutral formula | 5-AIQC-adduct m/z | Q1 (m/z) | Q3 (m/z) | FV | CE | RT | LODa (fmol) | LODb (fmol) | Linear range (μM) | R2 | Categories |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | D-Mannosamine | C16H19N3O6 | 350.1347 | 350 | 171 | 140 | 40 | 1.328 | 17.3 | 80.6 | 5.8–500 | 0.9978 | AS |
| 2 | D-(+)-Glucosamine | C16H19N3O6 | 350.1347 | 350 | 171 | 140 | 40 | 1.498 | 9.4 | 61.2 | 3.1–500 | 0.9941 | AS |
| 3 | D-(+)-Galactosamine | C16H19N3O6 | 350.1347 | 350 | 171 | 140 | 40 | 1.892 | 15.4 | 113.6 | 5.1–500 | 0.9958 | AS |
| 4 | 1-Deoxynojirimycin | C16H19N3O5 | 334.1397 | 334 | 171 | 120 | 20 | 3.347 | 0.1 | 0.2 | 0.02–200 | 0.9977 | AS |
| 5 | L-Asparagine | C14H14N4O4 | 303.1088 | 303 | 171 | 120 | 10 | 3.847 | 1.3 | 2.2 | 0.4–200 | 0.9986 | PAA |
| 6 | L-Histidine | C16H15N5O3 | 326.1248 | 326 | 171 | 100 | 20 | 3.957 | 1.8 | 5.5 | 0.6–200 | 0.9997 | PAA |
| 7 | L-Serine | C13H13N3O4 | 276.0979 | 276 | 171 | 100 | 10 | 4.024 | 0.5 | 0.9 | 0.2–200 | 0.9986 | PAA |
| 8 | Glycine | C12H11N3O3 | 246.0873 | 246 | 171 | 100 | 10 | 4.278 | 0.6 | 0.9 | 0.2–200 | 0.9998 | PAA, NT |
| 9 | L-Glutamine | C15H16N4O4 | 317.1244 | 317 | 171 | 120 | 10 | 4.74 | 2.9 | 6.7 | 1–200 | 0.9998 | PAA |
| 10 | L-Arginine | C16H20N6O3 | 345.167 | 345 | 171 | 120 | 40 | 5.016 | 15.0 | 30.0 | 5–200 | 0.9992 | PAA |
| 11 | L-Aspartic acid | C14H13N3O5 | 304.0928 | 304 | 171 | 120 | 20 | 5.021 | 1.4 | 5.5 | 0.5–200 | 0.9998 | PAA, NT |
| 12 | L-Glutamic acid | C15H15N3O5 | 318.1084 | 318 | 171 | 120 | 10 | 5.703 | 1.2 | 3.2 | 0.4–200 | 0.9999 | PAA, NT |
| 13 | L-Threonine | C14H15N3O4 | 290.1135 | 290 | 171 | 100 | 20 | 5.979 | 0.7 | 2.3 | 0.2–200 | 0.9987 | PAA |
| 14 | L-Alanine | C13H13N3O3 | 260.103 | 260 | 171 | 100 | 20 | 6.27 | 0.3 | 0.3 | 0.1–200 | 0.999 | PAA |
| 15 | L-Proline | C15H15N3O3 | 286.1186 | 286 | 171 | 100 | 20 | 6.538 | 0.5 | 7.0 | 0.2–200 | 0.996 | PAA |
| 16 | L-Tyrosine | C19H17N3O4 | 352.1292 | 352 | 171 | 120 | 10 | 8.709 | 0.4 | 0.5 | 0.1–100 | 0.999 | PAA |
| 17 | L-Methionine | C15H17N3O3S | 320.1063 | 320 | 171 | 120 | 20 | 8.911 | 1.1 | 1.9 | 0.4–200 | 0.9971 | PAA, S-AA |
| 18 | L-Lysine | C26H26N6O4 | 244.1081 | 244 | 171 | 100 | 20 | 9.202 | 0.6 | 1.3 | 0.2–200 | 0.9997 | PAA |
| 19 | L-Valine | C15H17N3O3 | 288.1343 | 288 | 171 | 120 | 20 | 9.515 | 0.8 | 1.5 | 0.3–200 | 0.9975 | PAA |
| 20 | L-Isoleucine | C16H19N3O3 | 302.1499 | 302 | 171 | 120 | 20 | 12.671 | 1.8 | 2.4 | 0.6–200 | 0.9988 | PAA |
| 21 | L-Leucine | C16H19N3O3 | 302.1499 | 302 | 171 | 120 | 20 | 12.738 | 1.6 | 2.7 | 0.5–200 | 0.9955 | PAA |
| 22 | DL-Phenylalanine | C19H17N3O3 | 336.1343 | 336 | 171 | 120 | 20 | 12.783 | 0.5 | 0.5 | 0.2–200 | 0.9998 | PAA |
| 23 | L-Tryptophan | C21H18N4O3 | 375.1452 | 375 | 171 | 140 | 20 | 12.992 | 1.0 | 1.0 | 0.3–200 | 0.9985 | PAA |
| 24 | D-Homoserine | C14H15N3O4 | 290.1135 | 290 | 171 | 100 | 20 | 4.942 | 0.9 | 2.5 | 0.3–200 | 0.9997 | N-PAA |
| 25 | Saccharopine | C21H26N4O7 | 447.1874 | 447 | 171 | 120 | 20 | 5.518 | 4.2 | 15.8 | 1.4–280 | 0.9986 | N-PAA |
| 26 | Argininosuccinic acid | C20H24N6O7 | 461.1779 | 461 | 171 | 160 | 40 | 5.561 | 7.7 | 79.4 | 2.6–500 | 0.9726 | N-PAA, MAA |
| 27 | β-alanine | C13H13N3O3 | 260.103 | 260 | 171 | 100 | 10 | 5.844 | 0.3 | 0.4 | 0.1–200 | 0.9996 | N-PAA |
| 28 | L-Citrulline | C16H19N5O4 | 346.151 | 346 | 171 | 120 | 20 | 5.867 | 2.1 | 2.9 | 0.7–200 | 0.9996 | N-PAA |
| 29 | L-Homoarginine | C17H22N6O3 | 359.1826 | 359 | 171 | 120 | 20 | 5.934 | 8.9 | 26.7 | 3–100 | 0.9997 | N-PAA |
| 30 | γ-Aminobutyric acid | C14H15N3O3 | 274.1186 | 274 | 171 | 120 | 20 | 6.665 | 1.2 | 2.7 | 0.4–200 | 0.9983 | N-PAA, NT |
| 31 | L-Homocitrulline | C17H21N5O4 | 360.1666 | 360 | 171 | 100 | 20 | 6.946 | 1.5 | 5.8 | 0.5–200 | 0.9985 | N-PAA |
| 32 | L-2-aminoadipic acid | C16H17N3O5 | 332.1241 | 332 | 171 | 120 | 20 | 7.075 | 2.5 | 4.2 | 0.8–100 | 0.9998 | N-PAA |
| 33 | DL-3-Aminoisobutyric acid | C14H15N3O3 | 274.1186 | 274 | 171 | 120 | 20 | 7.172 | 1.1 | 2.1 | 0.4–200 | 0.9997 | N-PAA |
| 34 | 2-Aminoisobutyric acid | C14H15N3O3 | 274.1186 | 274 | 171 | 100 | 20 | 7.613 | 2.6 | 8.6 | 0.9–200 | 0.991 | N-PAA |
| 35 | 5-Aminovaleric acid | C15H17N3O3 | 288.1343 | 288 | 171 | 120 | 20 | 7.754 | 2.2 | 5.9 | 0.7–200 | 0.9992 | N-PAA |
| 36 | L-2-Aminobutyric acid | C14H15N3O2 | 274.1186 | 274 | 171 | 100 | 20 | 7.792 | 0.8 | 1.5 | 0.3–200 | 0.9995 | N-PAA |
| 37 | 2,4-diaminobutanoic acid | C24H22N6O4 | 230.0924 | 230 | 171 | 80 | 20 | 7.859 | 3.2 | 8.3 | 1.1–200 | 0.9988 | N-PAA |
| 38 | DL-2,6-Diaminopimelic acid | C27H26N6O6 | 266.103 | 266 | 171 | 100 | 20 | 8.084, 8.208 | 2.5 | 7.0 | 0.8–200 | 0.9996 | N-PAA |
| 39 | L-Ornithine | C25H24N6O4 | 237.1002 | 237 | 171 | 100 | 10 | 8.321 | 0.9 | 1.8 | 0.3–200 | 0.9966 | N-PAA |
| 40 | 6-Aminocaproic acid | C16H19N3O3 | 302.1499 | 302 | 171 | 120 | 20 | 9.053 | 2.1 | 6.3 | 0.7–200 | 0.9993 | N-PAA |
| 41 | 3-hydroxykynurenine | C20H18N4O5 | 395.135 | 395 | 171 | 110 | 20 | 9.479 | 3.1 | 13.2 | 1.0–200 | 0.9914 | N-PAA |
| 42 | L-Norvaline | C15H17N3O3 | 288.1343 | 288 | 171 | 120 | 20 | 9.709 | 0.8 | 1.7 | 0.3–200 | 0.9994 | N-PAA |
| 43 | D–(−)-α-Phenylglycine | C18H15N3O3 | 322.1186 | 322 | 171 | 100 | 20 | 10.224 | 2.4 | 3.2 | 0.8–200 | 0.9983 | N-PAA |
| 44 | L-Pipecolic acid | C16H17N3O3 | 300.1343 | 300 | 171 | 100 | 20 | 10.336 | 22.0 | 249.6 | 7.3–500 | 0.9962 | N-PAA |
| 45 | L-Kynurenine | C20H18N4O4 | 379.1401 | 379 | 171 | 100 | 20 | 11.753 | 1.9 | 3.2 | 0.6–100 | 0.9997 | N-PAA |
| 46 | L-Norleucine | C16H19N3O3 | 302.1499 | 302 | 171 | 120 | 20 | 13.156 | 2.0 | 2.2 | 0.7–100 | 0.9998 | N-PAA |
| 47 | Histamine | C15H15N5O | 282.1349 | 282 | 171 | 100 | 20 | 4.237 | 2.9 | 4.4 | 1.0–500 | 0.9928 | NT, ALA |
| 48 | (−)-Norepinephrine | C18H17N3O4 | 340.1292 | 340 | 171 | 120 | 20 | 6.882 | 2.7 | 4.4 | 0.9–200 | 0.9991 | NT, ALA |
| 49 | (±)-Octopamine | C18H17N3O3 | 324.1343 | 324 | 171 | 100 | 20 | 7.59 | 1.3 | 1.5 | 0.4–200 | 0.9994 | NT, ALA |
| 50 | Dopamine | C18H17N3O3 | 324.1343 | 324 | 171 | 100 | 20 | 8.336 | 1.4 | 2.0 | 0.5–200 | 0.9988 | NT, ALA |
| 51 | Serotonin | C20H18N4O2 | 347.1503 | 347 | 171 | 100 | 20 | 8.606 | 1.6 | 14.8 | 0.5–500 | 0.9926 | NT, ALA |
| 52 | Tyramine | C18H17N3O2 | 308.1394 | 308 | 171 | 120 | 20 | 9.358 | 2.3 | 4.7 | 0.8–200 | 0.9993 | NT, ALA |
| 53 | 3-Methoxytyramine | C19H19N3O3 | 338.1499 | 338 | 171 | 100 | 20 | 10.008 | 0.8 | 1.3 | 0.3–50 | 0.9998 | NT, ALA |
| 54 | Tryptamine | C20H18N4O | 331.1553 | 331 | 171 | 120 | 20 | 13.305 | 3.2 | 4.6 | 1.1–200 | 0.9984 | NT, ALA |
| 55 | 4-Aminophenol | C16H13N3O2 | 280.1081 | 280 | 171 | 100 | 20 | 8.165 | 2.9 | 50.6 | 1–200 | 0.9978 | ARA |
| 56 | 3-hydroxyanthranilic acid | C17H13N3O4 | 324.0979 | 324 | 171 | 100 | 20 | 8.866 | 19.0 | — | 6.3–100 | 0.9962 | ARA, N-PAA |
| 57 | 4-aminohippuric acid | C19H16N4O4 | 365.1244 | 365 | 171 | 120 | 20 | 9.202 | 30.0 | — | 10–200 | 0.9995 | ARA, N-PAA |
| 58 | Procaine | C23H26N4O3 | 407.2078 | 407 | 171 | 140 | 20 | 9.612 | 174.4 | — | 58.1–200 | 0.9899 | ARA |
| 59 | 5-Hydroxyindoleacetic acid | C20H15N3O4 | 362.1135 | 362 | 171 | 100 | 20 | 11.373 | 16.8 | 105.0 | 5.6–500 | 0.973 | ARA |
| 60 | 3-Aminobenzoic acid | C17H13N3O3 | 308.103 | 308 | 171 | 120 | 10 | 11.477 | 4.1 | — | 1.4–200 | 0.9939 | ARA, N-PAA |
| 61 | 3-Aminosalicylic acid | C17H13N3O4 | 324.0979 | 324 | 171 | 120 | 20 | 11.574 | 8.7 | — | 2.9–100 | 0.9984 | ARA, N-PAA |
| 62 | 4-Aminobenzoic acid | C17H13N3O3 | 308.103 | 308 | 171 | 120 | 10 | 11.649 | 1.7 | — | 0.6–200 | 0.9989 | ARA, N-PAA |
| 63 | N-acetyl-5-hydroxytryptamine | C22H20N4O3 | 389.1608 | 389 | 171 | 120 | 20 | 11.761 | 10.1 | 62.0 | 3.4–200 | 0.9997 | ARA |
| 64 | L-Cysteic acid | C13H13N3O6S | 340.0598 | 340 | 171 | 120 | 20 | 3.473 | 5.8 | 8.7 | 1.9–200 | 0.999 | S-AA, N-PAA |
| 65 | Taurine | C12H13N3O4S | 296.07 | 296 | 171 | 100 | 10 | 4.733 | 2.5 | 7.4 | 0.8–200 | 0.9998 | S-AA, N-PAA |
| 66 | Hypotaurine | C12H13N3O3S | 280.075 | 280 | 171 | 120 | 10 | 4.875 | 0.8 | 1.1 | 0.3–100 | 0.9995 | S-AA, N-PAA |
| 67 | S-(5’-Adenosyl)-L-methionine | C25H28N8O6S | 569.1925 | 569 | 171 | 140 | 20 | 5.146 | 19.5 | 36.3 | 6.5–200 | 0.9973 | S-AA, N-PAA |
| 68 | DL-Methionine sulfone | C15H17N3O6S | 352.0962 | 352 | 171 | 100 | 10 | 5.546 | 0.9 | 3.1 | 0.3–200 | 0.9997 | S-AA, N-PAA |
| 69 | DL-methionine sulfoxide | C15H17N3O4S | 336.1013 | 336 | 171 | 100 | 20 | 5.606 | 2.0 | 7.1 | 0.7–200 | 0.9998 | S-AA, N-PAA |
| 70 | Glutathione disulfide | C20H23N5O7S | 478.1391 | 478 | 171 | 160 | 20 | 6.844 | 3.6 | 4.3 | 1.2–40 | 0.9924 | S-AA, SP |
| 71 | L-Cystine | C13H14N3O3S | 292.075 | 292 | 171 | 120 | 20 | 6.882 | 1.2 | 1.6 | 0.4–50 | 0.9917 | S-AA, N-PAA |
| 72 | Cystamine | C12H13N3OS | 248.0852 | 248 | 171 | 100 | 10 | 7.232 | 2.9 | 3.5 | 1–100 | 0.998 | S-AA, ALA |
| 73 | L-Homocystine | C14H15N3O3S | 306.0907 | 306 | 171 | 100 | 20 | 7.896 | 2.7 | 2.8 | 0.9–50 | 0.9989 | S-AA, N-PAA |
| 74 | S-(5’-adenosyl)-L-homocysteine | C24H26N8O6S | 555.1769 | 555 | 171 | 160 | 20 | 8.344 | 19.0 | 52.3 | 6.3–120 | 0.9862 | S-AA, N-PAA |
| 75 | Cystathionine | C27H26N6O6S | 282.089 | 282 | 171 | 100 | 10 | 8.956 | 2.8 | 8.2 | 0.9–100 | 0.9992 | S-AA, N-PAA |
| 76 | S-(2-Aminoethyl)-L-cysteine | C25H24N6O4S | 253.0863 | 253 | 171 | 80 | 10 | 8.985 | 1.3 | 3.0 | 0.4–200 | 0.9996 | S-AA |
| 77 | L-Cysteine | C19H20N4O5S | 417.1227 | 417 | 171 | 140 | 20 | 9.261 | 3.6 | 4.0 | 1.2–100 | 0.9934 | PAA, S-AA |
| 78 | Djenkolic Acid | C27H26N6O6S2 | 298.075 | 298 | 171 | 100 | 20 | 10.112 | 2.2 | 2.1 | 0.7–200 | 0.9991 | S-AA |
| 79 | Cysteamine | C18H20N4O3S | 373.1329 | 373 | 171 | 120 | 20 | 10.246 | 4.1 | 4.0 | 1.4–100 | 0.9943 | S-AA, ALA |
| 80 | DL-Ethionine | C16H19N3O3S | 334.122 | 334 | 171 | 100 | 20 | 11.201 | 1.4 | 4.0 | 0.5–100 | 0.9996 | S-AA, N-PAA |
| 81 | DL-Homocysteine | C20H22N4O5S | 431.1384 | 431 | 171 | 130 | 20 | 10.291, 10.611 | 6.9 | 5.9 | 2.3–100 | 0.9912 | S-AA, N-PAA |
| 82 | DL-Lanthionine | C26H24N6O6S | 275.0812 | 275 | 171 | 100 | 10 | 7.986, 8.217 | 3.1 | 8.3 | 1.0–400 | 0.9986 | S-AA, N-PAA |
| 83 | Glutathione | C26H30N6O9S | 603.1868 | 603 | 171 | 120 | 20 | 8.784, 9.053 | 4.1 | 6.8 | 1.4–100 | 0.9995 | SP, S-AA |
| 84 | γ-Glu-Cys | C24H27N5O8S | 546.1653 | 546 | 171 | 140 | 20 | 8.791, 8.933 | 0.9 | 2.3 | 0.3–250 | 0.9916 | SP, S-AA |
| 85 | L-Carnosine (β-ala-L-his) | C19H20N6O4 | 397.1619 | 397 | 171 | 120 | 20 | 4.964 | 18.3 | 28.4 | 6.1–200 | 0.9989 | SP |
| 86 | L-Anserine (β-ala-N-methyl-his) | C20H22N6O4 | 411.1775 | 411 | 171 | 140 | 40 | 5.203 | 2.8 | 5.7 | 0.9–200 | 0.9935 | SP |
| 87 | Ala-leu | C19H24N4O4 | 373.187 | 373 | 171 | 130 | 20 | 11.574 | 0.7 | 1.7 | 0.2–200 | 0.9998 | SP |
| 88 | Ala-Trp | C24H23N5O4 | 446.1823 | 446 | 171 | 140 | 20 | 11.753 | 1.6 | 5.3 | 0.5–50 | 0.9998 | SP |
| 89 | Leu-Pro | C21H26N4O4 | 399.2027 | 399 | 171 | 130 | 20 | 13.678 | 0.3 | 0.8 | 0.1–500 | 0.9967 | SP |
| 90 | trans-4-Hydroxy-L-proline | C15H15N3O4 | 302.1135 | 302 | 171 | 120 | 20 | 3.091 | 2.2 | 2.1 | 0.7–200 | 0.999 | N-PAA, MAA |
| 91 | O-Phospho-L-serine | C13H14N3O7P | 356.0642 | 356 | 171 | 100 | 10 | 3.633 | 23.4 | 65.2 | 7.8–1000 | 0.999 | N-PAA, MAA |
| 92 | O-Phosphorylethanolamine | C12H14N3O5P | 312.0744 | 312 | 171 | 100 | 10 | 3.875 | 1.2 | 4.3 | 0.4–200 | 0.9993 | N-PAA, MAA |
| 93 | Sarcosine | C13H13N3O3 | 260.103 | 260 | 171 | 100 | 10 | 4.412 | 4.1 | 6.2 | 1.4–200 | 0.9947 | N-PAA, MAA |
| 94 | 3-Methyl-L-histidine | C17H17N5O3 | 340.1404 | 340 | 171 | 120 | 20 | 4.457 | 7.5 | 11.4 | 2.5–100 | 0.9998 | N-PAA, MAA |
| 95 | Nε,Nε,Nε-Trimethyllysine | C19H26N4O3 | 180.1075 | 180 | 171 | 120 | 20 | 4.524 | 1.0 | 3.6 | 0.3–500 | 0.9919 | N-PAA, MAA |
| 96 | O-Phospho-L-threonine | C14H16N3O7P | 370.0799 | 370 | 171 | 120 | 20 | 4.817 | 3.8 | 20.7 | 1.3–1000 | 0.9904 | N-PAA, MAA |
| 97 | 1-Methyl-L-histidine | C17H17N5O3 | 340.1404 | 340 | 171 | 100 | 20 | 4.897 | 9.4 | 19.4 | 3.1–100 | 0.9995 | N-PAA, MAA |
| 98 | Asymmetric dimethylarginine | C18H24N6O3 | 373.1983 | 373 | 171 | 120 | 20 | 6.24 | 31.3 | 37.5 | 10.4–50 | 0.9996 | N-PAA, MAA |
| 99 | O-acetyl-L-serine | C15H15N3O5 | 318.1084 | 318 | 171 | 120 | 10 | 6.941 | 1.9 | 8.6 | 0.6–200 | 0.9995 | N-PAA, MAA |
| 100 | O-phospho-L-tyrosine | C19H18N3O7P | 432.0955 | 432 | 171 | 120 | 20 | 7.179 | 17.8 | 85.2 | 5.9–1000 | 0.9932 | N-PAA, MAA |
| 101 | Nα-Acetyl-L-lysine | C18H22N4O4 | 359.1714 | 359 | 171 | 120 | 20 | 7.59 | 2.0 | 3.7 | 0.7–200 | 0.9996 | N-PAA, MAA |
| 102 | DL-5-Hydroxylysine | C26H26N6O5 | 252.1055 | 252 | 171 | 100 | 10 | 7.986 | 1.7 | 3.2 | 0.6–100 | 0.9992 | N-PAA, MAA |
| 103 | 5-Hydroxy-L-tryptophan | C21H18N4O4 | 391.1401 | 391 | 171 | 100 | 20 | 8.001 | 2.6 | 2.5 | 0.9–200 | 0.9958 | N-PAA, MAA |
| 104 | 4-Hydroxy-L-isoleucine | C16H19N3O4 | 318.1448 | 318 | 171 | 80 | 20 | 8.404 | 5.2 | 5.2 | 1.7–50 | 0.9998 | N-PAA, MAA |
| 105 | Ethanolamine | C12H13N3O2 | 232.1081 | 232 | 171 | 100 | 20 | 5.024 | 1.0 | 5.6 | 0.3–200 | 0.9998 | ALA |
| 106 | Methylamine | C11H11N3O | 202.0975 | 202 | 171 | 80 | 10 | 5.076 | 0.6 | 1.9 | 0.2–200 | 0.999 | ALA |
| 107 | Agmatine | C15H20N6O | 301.1771 | 301 | 171 | 120 | 20 | 5.68 | 1.8 | 6.3 | 0.6–200 | 0.9992 | NT, ALA |
| 108 | Ethylamine | C12H13N3O | 216.1131 | 216 | 171 | 100 | 20 | 6.613 | 1.7 | 20.1 | 0.6–200 | 0.9997 | ALA |
| 109 | Putrescine | C24H24N6O2 | 215.1053 | 215 | 171 | 120 | 20 | 9.008 | 2.5 | 11.3 | 0.8–200 | 0.9998 | ALA |
| 110 | Cadaverine | C25H26N6O2 | 222.1131 | 222 | 171 | 100 | 20 | 10.284 | 2.5 | 6.5 | 0.8–200 | 0.9996 | ALA |
| 111 | Spermidine | C37H37N9O3 | 219.4413 | 219 | 171 | 80 | 20 | 12.641 | 3.5 | 19.9 | 1.2–500 | 0.9945 | ALA |
| 112 | Spermine | C50H50N12O4 | 442.2112 | 442 | 171 | 140 | 20 | 13.292 | 8.1 | 40.0 | 2.7–200 | 0.9959 | ALA |
| 113 | NH4Cl | C10H9N3O | 188.0818 | 188 | 171 | 80 | 10 | 3.076 | 0.4 | 0.7 | 0.1–200 | 0.9979 | Ammonium |
| 114 | Prolinamide | C15H16N4O2 | 285.1346 | 285 | 171 | 80 | 20 | 5.501 | 1.2 | 3.3 | 0.4–200 | 0.9993 | ALA |
| 115 | Allantoin | C14H12N6O4 | 329.0993 | 329 | 171 | 120 | 20 | 6.521 | 15.8 | 25.9 | 5.3–100 | 0.9989 | ALA |
| 116 | 5-Hydroxydopamine | C18H17N3O4 | 340.1292 | 340 | 171 | 120 | 20 | 7.061 | 3.6 | 6.1 | 1.2–50 | 0.9998 | ALA |
| 117 | 3,4-dihydroxy-DL-phenylalanine | C19H17N3O5 | 368.1241 | 368 | 171 | 120 | 20 | 7.725 | 2.7 | 3.1 | 0.9–200 | 0.9996 | ALA |
| 118 | DL-Normetanephrine | C19H19N3O4 | 354.1448 | 354 | 171 | 120 | 20 | 8.045 | 2.5 | 3.2 | 0.8–100 | 0.9997 | ALA |
| 119 | 2-Amino-2-methyl-1-propanol | C14H17N3O2 | 260.1394 | 260 | 171 | 100 | 10 | 8.091 | 0.2 | 0.3 | 0.1–400 | 0.9992 | ALA |
| 120 | 1,3-Diaminopropane | C23H22N6O2 | 208.0975 | 208 | 171 | 80 | 10 | 8.418 | 1.8 | 7.4 | 0.6–200 | 0.9993 | ALA |
| 121 | 1,2-Diaminopropane | C23H22N6O2 | 208.0975 | 208 | 171 | 80 | 10 | 8.821 | 0.9 | 4.4 | 0.3–200 | 0.9992 | ALA |
| 122 | L-Tryptophanamide | C21H19N5O2 | 374.1612 | 374 | 171 | 100 | 20 | 10.649 | 1.1 | 3.2 | 0.4–100 | 0.9991 | ALA |
| 123 | Isopentylamine | C15H19N3O | 258.1601 | 258 | 171 | 120 | 20 | 13.382 | 0.9 | 2.7 | 0.3–500 | 0.9957 | ALA |
| 124 | Desipramine | C28H28N4O | 437.2336 | 437 | 171 | 130 | 20 | 14.072 | 0.3 | 0.4 | 0.1–100 | 0.9995 | ALA |
| 125 | Methylguanidine | C22H19N7O2 | 207.5873 | — | — | — | — | — | — | — | — | — | guanidines |
| 126 | Adenosine | C20H19N7O5 | 438.152 | — | — | — | — | — | — | — | — | — | NT |
AS: amino-saccharides; PAA: proteinogenic amino acids; NT: neurotransmitters; N-PAA: Non-proteinogenic amino acids; ALA: aliphatic amines; ARA: aromatic amines; S-AA: sulfur-containing analytes; SP: small peptides; MAA: modified amino acids.
UHPLC-ESI-MS/MS Analysis of Amino Compounds
Asymmetric ureas formed from 5-AIQC and all amino metabolites were readily detectable in the positive ion mass spectrometry with MRM mode by showing a common fragment ion at m/z 171 derived from the amino isoquinoline moiety (Table 1). Such derivatized amino analytes have higher hydrophobicity than analytes themselves making reverse-phase UHPLC-MS/MS suitable technique for more sensitive quantitative analysis. We developed an UHPLC-MS/MS method for simultaneous quantification of 124 amino compounds with all parameters systematically optimized for UHPLC (including columns and temperature, mobile phases and gradients, buffers, flow rate and injection volume) and mass spectrometry. With these optimized parameters, both excess by-products, 5-AIQ and NEM-tBBT, were eluted either at the beginning (in the case of 5-AIQ) or end of chromatography and discarded to avoid contaminating source. This new method demonstrated easy coverage of 124 analytes in this work representing 4 amino-saccharides, 20 proteinogenic amino acids, 57 non-proteinogenic amino acids, 17 modified amino acids, 26 aliphatic and 8 aromatic amines, 22 sulfur-containing compounds, 14 monoamine neurotransmitters and 8 small peptides (Table 1, Supplementary Fig. S5).
This optimized method enabled many sets of isomers to be chromatographically separated (Fig. 3) making them readily quantifiable. For intance, 5-AIQC derivatives of 5 leucine isomers (isoluecine, leucine, norleucine, hydroxyproline and 6-aminocaproic acid) were separated on column though they all had the same ion m/z 302 (Fig. 3A). 5-AIQC adducts of five three-metabolite sets were readily separated on column and quantified (Fig. 3B), respectively, such as 3 valine isomers with ion m/z 288 (5-aminovaleric acid, L-valine, L-norvaline) and 3 aromatic metabolites with ion m/z 308 (tyramine, 3-aminobenzoic acid, 4-aminobenzoic acid), etc. In the same manner, eight pairs of the 5-AIQC derivatives of analytes were separated and simultaneously quantified (Fig. 3C), respectively, including hypotaurine and 4-aminophenol (m/z 280), histamine and cystathionine (m/z 282), etc. These indicate that the 5-AIQC-tagging method has wide suitability for isomers and analytes having the same ions at unit mass when they have distinctive chromatographic behavior. However, higher resolution mass spectrometers will be required for analytes having the same unit mass and similar chromatographic behavior; some extra chromatographic resolution measures may also be needed for some isomers having similar fragmentation patterns in mass spectrometry.
Figure 3.
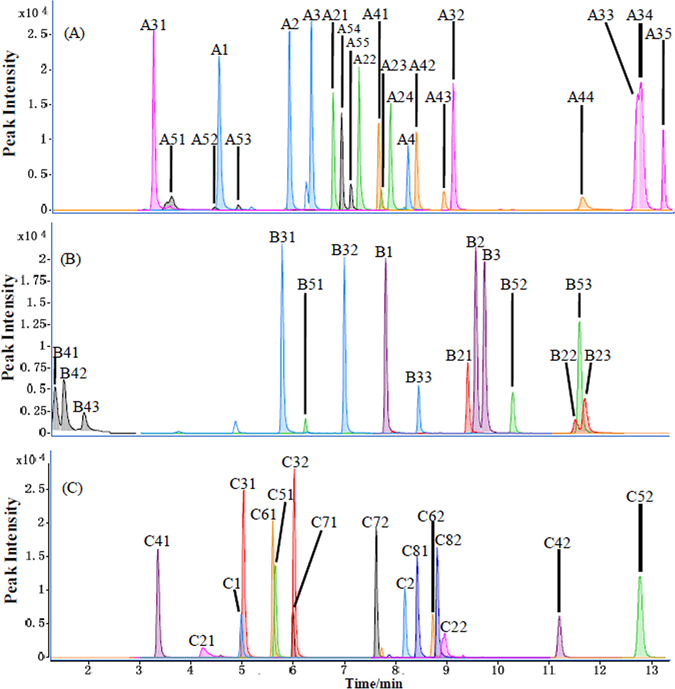
UHPLC-MS/MS chromatograms for some sets of the 5-AIQC-tagged amino analytes having the same pseudomolecular ions (m/z at unit resolution). (A) ion m/z 260 (A1: sarcosine; A2: β-alanine; A3: L-alanine; A4: 2-amino-2-methyl-1-propanol); ion m/z 274 (A21: γ-aminobutyric acid; A22: DL-3-aminoisobutyric acid; A23: 2-aminoisobutyric acid; A24: L-2-aminobutyric acid); ion m/z 302 (A31: trans-4-hydroxy-L-proline; A32: 6-aminocaproic acid; A33: L-isoleucine; A34: L-leucine; A35: L-norleucine); ion m/z 324 (A41: (±)-octopamine; A42: dopamine; A43: 3-hydroxyanthranilic acid; A44: 3-aminosalicylic acid); ion m/z 340 (A51: L-cysteic acid; A52: 3-methyl-L-histidine; A53: 1-methyl-L-histidine; A54: (−)-norepinephrine; A55: 5-hydroxydopamine). (B) ion m/z 288 (B1: 5-aminovaleric acid; B2: L-valine; B3: L-norvaline); ion m/z 308 (B21: tyramine; B22: 3-aminobenzoic acid; B23: 4-aminobenzoic acid); ion m/z 318 (B31: L-glutamic acid; B32: o-acetyl-L-serine; B33: 4-hydroxy-L-isoleucine); ion m/z 350 (B41: D-mannosamine; B42: D-(+)-glucosamine; B43: D-(+)-galactosamine); ion m/z 373 (B51: asymmetric dimethylarginine; B52: cysteamine; B53: Ala-Leu). (C) ion m/z 280 (C1: hypotaurine; C2: 4-aminophenol); ion m/z 282 (C21: histamine; C22: cystathionine); ion m/z 290 (C31: D-homoserine; C32: L-threonine); ion m/z 334 (C41: 1-deoxynojirimycin; C42: DL-ethionine); ion m/z 336 (C51: DL-methionine sulfoxide; C52: DL-phenylalanine); ion m/z 352 (C61: DL-methionine sulfone; C62: L-tyrosine); ion m/z 359 (C71: L-homoarginine; C72: Nα-acetyl-L-lysine); ion m/z 208 (C81: 1,3-diaminopropane; C82: 1,2-diaminopropane).
This method further facilitated simultaneous quantification of 22 sulfur-containing analytes together with some other oxidation-prone aromatic analytes. In particular, the method enabled simultaneous quantification of a number of thiols and disulfides in the same sample in an “one-pot” manner (Table 1, Fig. 4a). To the best of our knowledge, such approach has not been reported previously. It is important to note that aromatic amines such as 3-aminosalicylic acid, 4-aminohippuric acid, 3-aminobenzoic acid and 4-aminobenzoic acid were also readily derivatized and hence quantified by our 5-AIQC approach but not by 6-AQC method37 (Table 1). Furthermore, our method enables simultaneous quantification of many oxidation-prone metabolites including dopamine and tyramine metabolites together with these containing thiol and disulfide groups including cysteine-containing metabolites (Table 1, Fig. 4b). The results have also indicated that this 5-AIQC-based method is also applicable for quantification of small peptides including dipeptides (L-carnosine, L-anserine, Ala-Trp, Ala-Leu, Leu-Pro, γ-Glu-Cys) and tripeptides (GSH, GSSG) (Table 1, Supplementary Fig. S6).
Figure 4.
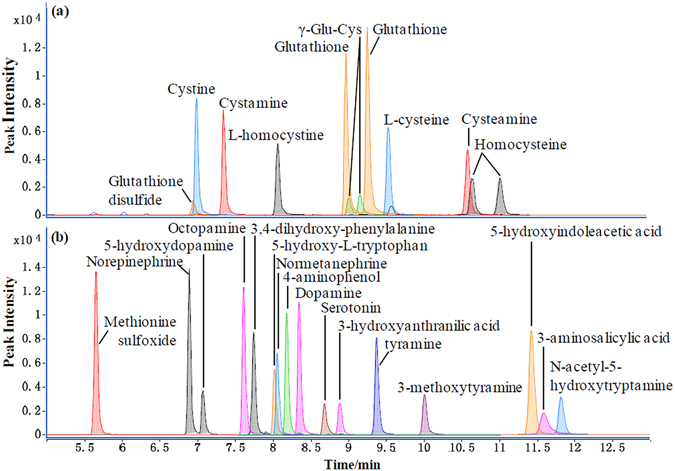
UHPLC-MS/MS chromatograms for the 5-AIQC-tagged oxidation-prone amino analytes including (a) these containing thiol and disulfide groups and (b) the aromatic metabolites from three aromatic amino acids (phenylalanine, tyrosine and tryptophan).
In a single run, moreover, this method enabled simultaneous quantification of multiple metabolites having important functions with the coverage of more than twenty metabolic pathways (Fig. 5, Supplementary Table S2). Quantification of proteinogenic amino acids will be vital for understanding protein biosynthesis/degradation (Fig. 5a) whilst quantification of the arginine-metabolism-related metabolites is important for quantitative understanding the urea cycle (or ornithine cycle) (Fig. 5b). Quantification of cysteine metabolism and the folate-related homocysteine metabolism was also highlighted by multiple intermediates in such metabolic pathway including cysteine, L-methionine, SAM, SAH, homocysteine and cystathionine (Fig. 5c). Furthermore, monoamine neurotransmitters including amino acids themselves, metabolites derived from both aliphatic amino acids (e.g., agmatine) and aromatic ones (e.g., catecholamines) including phenylalanine-tyrosine metabolism and tryptophan-metabolism mediated 5-hydroxytryptamine pathway (Fig. 5d–e). The coverage of the tryptophan-metabolism mediated kynurenine pathway was reflected by eight major metabolites in the pathway (Fig. 5e) whilst such coverage of polyamine pathway was well highlighted by spermine, putrescine, cadaverine and spermidine (Supplementary Table S2).
Figure 5.
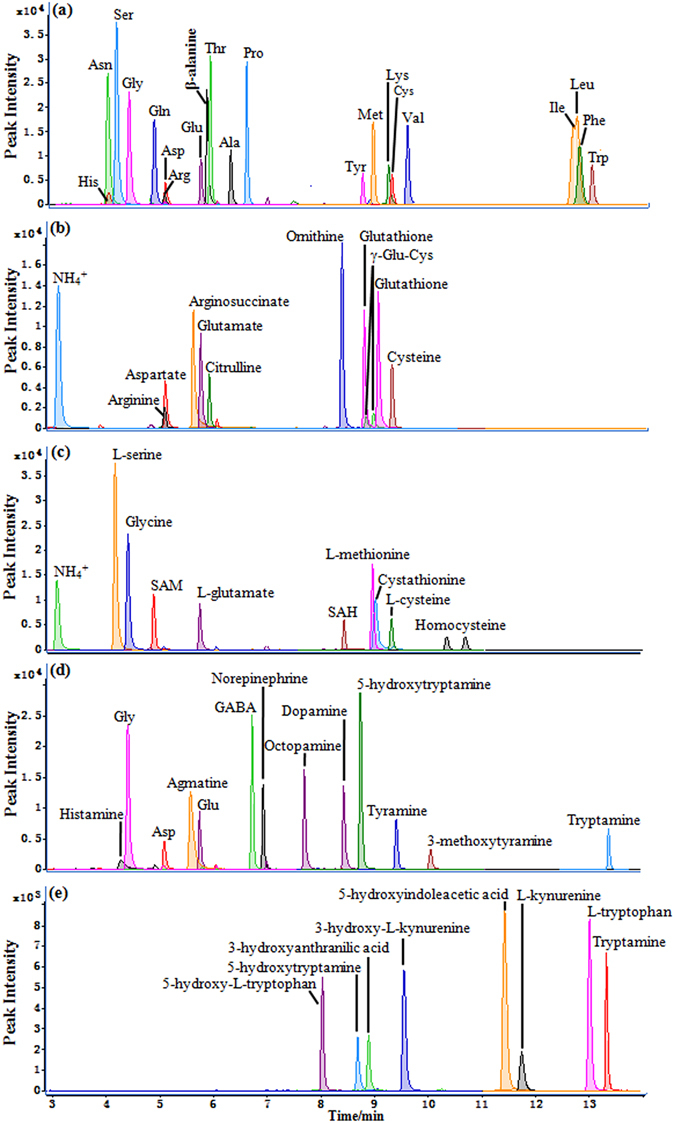
UHPLC-MS/MS chromatograms for the 5-AIQC-tagged amino metabolites in multiple metabolic pathways including (a) protein biosynthesis/degradation, (b) urea cycle, (c) folate-associated homocysteine metabolism, (d) biosynthesis of monoamine neurotransmitters and (e) tryptophan-mediated kynurenine pathway.
However, we found that 5-AIQC was not suitable for derivatization of adenine, amide, guanido and urea groups as in the case of 6-AQC37.
Sensitivity, precision, accuracy and recovery for this quantification method
To validate this method, 95 amino compounds with their concentration in the range of 0.02–200 μM were employed to respectively represent proteinogenic and non-proteinogenic amino acids, modified amino acids, small peptides, aliphatic and aromatic amines, oxidation-prone analytes (such as thiols, disulfides and catecholamines). Their mixtures (Mix1-Mix9) were prepared in volumetric flasks from solution of each standard with gradual dilution of the stock solution using phosphate buffer (0.1 M, pH7.0) (Supplementary Table S1) and used for method validation.
The chromatographic reproducibility was evaluated by computing the retention time of each analyte obtained over 3 days using the mixed analytes Mix2, Mix5, Mix6 and Mix7 representing high, intermediate and low concentration situations, respectively (Supplementary Table S1). The intra-day RSDs of the retention times for 95 amino compounds were all below 5% (Supplementary Table S3) and the inter-day RSDs were about 1–6.8%.
Sensitivity was assessed for all 124 metabolites by determination of the limit of detection (LOD) and quantification (LOQ) for amino analytes on column. Linearity of detection response was excellent for all analytes in the concentration range of 0.0002–2 μM (on column) with R2 well above 0.99 (Table 1). Amongst 124 analytes tested here, only procaine had LOD above 50 fmol. The LOD was below 32 fmol (on column) for the rest 123 analytes, below 9.5 fmol for 108 analytes, below 5 fmol for 98 analytes and sub-fmol for 26 analytes (Table 1). When the Jet Stream ion source and iFunnel technology was jointly employed (with an Agilent 6495 Mass Spectrometer), sensitivity was further improved (up to 8 folds) with the LOD reached sub-fmol level for most 95 analytes tested (Supplementary Table S4).
Our method had superior LODs for all analytes when compared with the results from the 6-AQC approach37 under the same analytical conditions (Table 1). Noticeably, our method was 5 times more sensitive for His, Thr, Asp, taurine and ethanolamine whilst 10 times more sensitive for L-proline, ethylamine, and 4-aminophenol than the 6-AQC method (Table 1). The only exception was homocysteine that showed only slightly lower sensitivity. Such sensitivity enhancement is probably due to the fact that isoquinoline is more basic (pKa ~ 5.40) than quinoline (pKa ~ 4.95)55. Consistently, our data measured from an NMR method56 showed the pKa values of 5.31 ± 0.07 and 4.95 ± 0.03, respectively, for the 5-aminoisoquinoline and 6-aminoquinoline ring nitrogen (Supplementary Fig. S7).
It is particularly important to note that our 5-AIQC approach can be used to derivatize numerous aromatic amines successfully including 3-aminosalicylic acid, 4-aminohippuric acid, 3-aminobenzoic acid and 4-aminobenzoic acid. In contrast, 6-AQC approach cannot be used to analyze them37. Although 4-aminophenol can be analyzed by both 5-AIQC and 6-AQC methods, LOD was more than an order of magnitude (17 times) lower for 5-AIQC method than 6-AQC approach (Table 1). Nonetheless, both 5-AIQC and 6-AQC failed to tag the amino group of adenosine.
The intra-and inter-day variations for quantification of analytes were assessed by using four mixed standard solutions (i.e., Mix2, Mix5, Mix6, and Mix7) representing high, intermediate and low concentration cases respectively. In any case, both the intra- and inter-day RSDs were below 15% (Supplementary Table S3) for most analytes except cystine, sarcosine and 2-aminoisobutyrate. Cystine had inter-day RSDs just over 16% at intermediate to high concentration. However, the intra- and inter-day variations for both sarcosine and 2-aminoisobutyrate were surprisingly poor ranging from 27% to 90% (Supplementary Table S3) though ionization efficiency of them was not problematic and such remained to be understood.
Accuracy for the simultaneous quantification of these amino analytes were evaluated by calculated recoveries from three mixed standard solutions (Mix1, Mix4 and Mix6), respectively, in which Mix4 was spiked. The results showed that such recoveries for most analytes were about 88–116% (Table 2) with most of the oxidation-prone compounds around 88.5–110.9%. Tyr had such recovery over 120% at mediate to high concentrations. We have also found that such recoveries were about 80–120% for 43 and 29 representative analytes in rat urine and serum samples, respectively. The obvious exceptions were again observed for sarcosine and 2-aminoisobutyric acid in rat urine with virtual recoveries of 193.8% and 189.2%, respectively (Table 2) for unknown reasons though this might be related to their poor inter- and intra-day quantification precision.
Table 2.
Recoveries for 95 representative amino analytes with low (L), intermediate (M) and high concentration (H) from standard mixtures (Stds, n = 5), human renal tumor and adjacent non-involved tissues (ANIT) (n = 6), rat urine and serum (n = 6) samples.
| Stds (L) | Stds (M) | Stds (H) | Renal tumor | Renal ANIT | Rat urine | Rat serum | |
|---|---|---|---|---|---|---|---|
| L-Asparagine | 111.8(1.0) | 113.2(6.3) | 112.9(4.4) | 116.2(3.0) | 112.3(0.5) | 119.7(6.8) | 118.7(6.5) |
| L-Histidine | 115.6(2.8) | 107.8(10.6) | 99.3(5.5) | 106.6(4.4) | 111.7(2.1) | 109.7(7.6) | 124.9(7.7) |
| L-Serine | 101.0(5.4) | 105.5(12.5) | 107.5(3.5) | 86.4(1.5) | 80.8(0.9) | 117(11.9) | 120.2(6.1) |
| Glycine | 109.7(5.0) | 99.6(4.6) | 105.4(3.2) | 86.4(1.5) | 80.8(0.9) | 105.2(6.6) | 96.4(8.4) |
| L-Glutamine | 104.1(2.2) | 111.5(8.5) | 107.6(6.1) | 114.3(1.1) | 109.4(2.5) | 106.1(9.1) | 98.9(10.1) |
| L-Arginine | 101.3(2.0) | 89.2(6.4) | 91.6(4.2) | 115.0(3.1) | 117.6(2.6) | 105.6(16.1) | 109.4(10.7) |
| L-Aspartic acid | 98.9(4.6) | 107.9(6.1) | 107.9(0.6) | 116.0(1.5) | 114.1(2.0) | 117.1(8.8) | 119.8(1.7) |
| L-Glutamic acid | 116.1(2.1) | 113.8(5.0) | 108.1(0.9) | 114.4(3.2) | 119.9(2.2) | 113.2(10.7) | 108.5(6.5) |
| L-Threonine | 110.3(4.5) | 109.3(2.9) | 109.3(0.2) | 117.6(2.4) | 116.6(1.7) | 115.5(11.7) | 114.5(8.4) |
| L-Alanine | 102.9(2.2) | 107.0(6.3) | 111.3(3.1) | 120.8(2.4) | 120.4(1.3) | 112.1(7.2) | 118.6(8.1) |
| L-Proline | 111.1(0.8) | 108.2(5.3) | 107.4(3.7) | 112.5(3.0) | 115.1(2.0) | 106.5(5.9) | 101.9(7.1) |
| L-Tyrosine | 113.7(4.7) | 126.6(6.3) | 135.7(2.1) | 131.7(2.2) | 116.1(4.2) | 99.7(4.6) | 119.4(6.3) |
| L-Methionine | 107.9(2.2) | 106.5(6.4) | 108.7(4.7) | 108.6(1.8) | 105.6(2.2) | 100.8(8.4) | 108.1(6.9) |
| L-Lysine | 106.4(2.8) | 106.3(5.1) | 106.7(3.5) | 114.3(1.9) | 110.5(6.3) | 109.3(9.3) | 110.5(10.0) |
| L-Valine | 109.2(1.2) | 107.4(4.2) | 108.8(4.7) | 112.1(1.8) | 110.0(1.9) | 119.3(1.9) | 105.4(7.5) |
| L-Isoleucine | 110.0(2.4) | 113.2(7.3) | 108.8(3.5) | 114.9(2.7) | 108.1(1.3) | 84.9(14.2) | 119.3(4.3) |
| L-Leucine | 99.6(4.5) | 104.2(6.6) | 112.5(3.4) | 113.9(3.7) | 107.1(3.3) | 99.3(16.4) | 96.2(13) |
| DL-Phenylalanine | 103.7(2.5) | 107.3(7.8) | 107.9(3.3) | 108.2(2.3) | 115.7(0.7) | 115.9(1.9) | 105.4(6.1) |
| L-Tryptophan | 103.3(4.5) | 101.6(7.5) | 103.4(4.8) | 105.8(2.8) | 103.7(1.9) | 117.3(1.3) | 115.6(4.3) |
| D-Homoserine | 110.0(3.9) | 112.9(4.9) | 111.6(1.3) | 116.2(1.7) | 109.3(3.4) | 105.6(7.4) | 103.4(5.3) |
| β-alanine | 108.1(2.0) | 105.3(4.4) | 106.6(0.7) | 114.8(4.3) | 114.7(2.7) | 101.1(8.3) | 110.0(6.7) |
| L-Citrulline | 113.4(3.8) | 109.9(5.6) | 108.4(2.3) | 114.0(5.4) | 108.7(3.5) | 117.1(3.9) | 103.9(6.7) |
| L-Homoarginine | 102.4(6.2) | 97.0(7.4) | 79.9(8.6) | 119.5(0.5) | 108.6(8.8) | 103.5(5.0) | 82.7(6.9) |
| γ-aminobutyric acid | 112.6(2.4) | 105.0(3.9) | 108.2(2.5) | 111.4(5.0) | 107.8(0.5) | 112.6(3.1) | 106.6(5.5) |
| L-Homocitrulline | 110.8(1.4) | 112.7(3.4) | 108.9(3.3) | 115.4(5.4) | 115.7(1.9) | 116.5(6.0) | 108.0(5.3) |
| L-2-aminoadipic acid | 112.7(2.4) | 111.8(10.8) | 111.5(4.1) | 110.8(4.6) | 114.5(0.9) | 108.7(10.0) | 104.2(4.4) |
| DL-3-aminoisobutyrate | 110.9(3.3) | 107.5(4.5) | 106.6(1.5) | 110.5(3.1) | 108.2(2.8) | 108.3(4.8) | 96.7(5.9) |
| 2-Aminoisobutyric acid | 47.6(8.8) | 163.7(11.9) | 157.1(8.4) | 144.3(1.6) | 113.5(2.4) | 189.2(1.2) | 158.0(0.4) |
| 5-Aminovaleric acid | 117.4(1.8) | 111.3(5.9) | 110.6(2.2) | 115.3(2.0) | 110.1(3.4) | 114.1(3.3) | 105.5(3.7) |
| L-2-Aminobutyric acid | 108.8(3.2) | 107.5(6.3) | 109.6(2.0) | 111.7(4.6) | 108.5(2.8) | 86.8(6.7) | 106.8(4.9) |
| 2,4-diaminobutyric acid | 113.1(1.8) | 114.8(5.9) | 113.8(0.7) | 115.8(4.4) | 110.6(2.0) | 100.5(5.0) | 101.9(4.6) |
| DL-2,6-diaminopimelate | 104.2(3.1) | 108.2(5.7) | 114.3(4.9) | 111.7(0.4) | 101.0(2.4) | 106.4(6.4) | 104.7(4.9) |
| L-Ornithine | 102.2(2.4) | 108.2(5.6) | 111.4(1.3) | 111.9(1.6) | 108.1(4.8) | 120.2(1.1) | 113.5(4.2) |
| 6-Aminocaproic acid | 104.0(2.6) | 106.3(5.6) | 106.1(2.4) | 107.1(1.2) | 103.3(2.9) | 99.6(3.2) | 118.6(3.7) |
| L-Norvaline | 107.5(1.3) | 106.3(7.0) | 108.8(3.7) | 117.9(2.7) | 112.5(4.1) | 107.2(7.1) | 108.7(4.9) |
| D–(−)-α-Phenylglycine | 99.2(3.5) | 107.9(3.7) | 107.9(4.5) | 109.5(3.1) | 104.1(2.9) | 103.2(4.3) | 117.0(5.9) |
| L-Kynurenine | 106.2(4.7) | 105.8(1.7) | 110.5(2.8) | 111.6(1.9) | 110.3(3.4) | 106.5(13.5) | 115.0(8.2) |
| L-Norleucine | 105.4(4.8) | 102.5(2.8) | 104.8(3.6) | 111.6(1.9) | 109.1(1.9) | 109.2(4.0) | 97.3(4.2) |
| (−)-Norepinephrine | 107.2(2.1) | 108.0(7.2) | 108.7(2.4) | 111.1(3.1) | 104.2(2.8) | 103.0(8.1) | 102.1(3.4) |
| (±)-Octopamine | 113.2(6.9) | 110.8(5.1) | 110.7(1.3) | 112.9(1.0) | 111.6(2.5) | 114.4(9.9) | 110.0(14.1) |
| Dopamine | 112.3(2.4) | 105.9(6.7) | 109.7(1.4) | 108.0(3.6) | 109.1(4.6) | 116.6(1.2) | 112.3(4.2) |
| Tyramine | 106.4(2.7) | 101.7(5.1) | 106.0(3.0) | 104.2(3.7) | 107.5(5.2) | 122.1(2.5) | 93.4(3.1) |
| 3-Methoxytyramine | 105.1(2.1) | 103.4(5.8) | 107.4(0.5) | 108.6(4.8) | 107.7(5.2) | 111.4(11.4) | 106.0(5.7) |
| Tryptamine | 111.1(3.9) | 109.4(5.0) | 108.7(5.2) | 110.0(2.8) | 118.7(1.6) | 119.7(1.8) | 79.1(0.9) |
| 4-Aminophenol | 107.2(1.3) | 107.8(6.3) | 109.4(3.2) | 106.8(3.0) | 107.0(1.9) | 86.2(3.5) | 116.9(3.0) |
| 4-Aminohippuric acid | 117.7(6.2) | 111.4(19.6) | 100.4(5.3) | 107.3(0.5) | 109.3(6.3) | 103.2(6.4) | 100.8(8.5) |
| 3-Aminobenzoic acid | 114.4(1.9) | 99.8(6.3) | 101.9(8.2) | 104.1(2.3) | 99.9(6.9) | 95.1(0.8) | 97.0(0.9) |
| 3-Aminosalicylic acid | 113.3(2.1) | 106.9(4.6) | 106.4(3.8) | 103.3(3.0) | 115.0(2.6) | 120.8(3.0) | 104.1(10.2) |
| 4-Aminobenzoic acid | 104.8(5.6) | 105.8(5.3) | 108.2(1.5) | 105.0(2.6) | 110.0(2.3) | 82.4(0.9) | 84.3(1.0) |
| L-Cysteic acid | 100.5(1.0) | 105.9(7.4) | 113.6(3.2) | 120.1(1.6) | 110.5(3.5) | 101.0(6.1) | 106.8(7.2) |
| Taurine | 116.4(3.0) | 105.5(9.0) | 107.5(3.9) | 114.3(1.9) | 127.6(3.2) | 108.6(3.1) | 93.5(15.0) |
| Hypotaurine | 113.5(2.4) | 106.0(6.7) | 103.0(1.6) | 109.3(2.5) | 106.8(2.7) | 120.6(2.2) | 105.5(2.6) |
| DL-Methionine sulfone | 109.4(0.5) | 112.7(4.5) | 111.2(2.2) | 114.0(3.7) | 109.9(2.1) | 103.2(6.4) | 103.8(3.9) |
| DL-methionine sulfoxide | 118.9(1.7) | 112.4(3.7) | 112.5(5.4) | 116.7(4.6) | 111.3(0.7) | 108.4(5.9) | 107.5(5.0) |
| Glutathione disulfide | 88.5(4.3) | 95.9(15.5) | 97.7(2.9) | 94.3(6.6) | 99.0(8.2) | 104.0(5.8) | 95.4(3.6) |
| L-Cystine | 103.2(3.4) | 101.3(5.4) | 107.1(2.8) | 108.5(2.9) | 117.3(1.7) | 84.6(2.2) | 90.7(1.4) |
| Cystamine | 106.2(0.7) | 102.5(9.5) | 104.0(4.0) | 105.7(1.8) | 105.3(3.3) | 89.1(0.7) | 84.1(2.6) |
| L-Homocystine | 101.0(6.7) | 102.5(6.8) | 105.5(4.0) | 108.3(4.7) | 104.3(3.2) | 100.2(2.3) | 83.5(1.6) |
| Cystathionine | 104.7(2.0) | 112.0(8.0) | 109.3(4.8) | 118.3(5.4) | 83.2(10.7) | 108.3(3.8) | 87.7(3.4) |
| S-(2-Aminoethyl)-L-Cys | 104.3(1.9) | 110.0(7.4) | 109.0(2.5) | 110.4(2.5) | 104.6(3.0) | 112.4(1.0) | 105.9(4.7) |
| L-Cysteine | 102.9(1.7) | 110.9(7.5) | 107.8(2.4) | 108.1(1.2) | 102.9(2.1) | 109.9(3.8) | 99.9(3.5) |
| Djenkolic Acid | 94.9(6.3) | 103.7(5.7) | 105.3(7.8) | 102.7(7.8) | 97.0(5.4) | 111.8(2.2) | 109.6(3.5) |
| Cysteamine | 108.7(1.9) | 104.4(4.7) | 105.8(1.6) | 111.3(2.2) | 108.7(4.1) | 92.1(0.7) | 90.4(3.6) |
| DL-Ethionine | 104.9(0.8) | 109.2(6.5) | 108.1(3.5) | 108.5(1.2) | 108.6(2.6) | 106.1(6.6) | 108.2(3.3) |
| DL-Homocysteine | 109.0(2.6) | 110.3(6.3) | 108.3(3.8) | 108.3(4.1) | 107.6(6.9) | 100.8(5.9) | 92.8(7.0) |
| Glutathione | 93.2(12.1) | 104.9(8.8) | 105.1(10.9) | 105.2(13.6) | 112.8(10.6) | 95.0(9.9) | 82.8(7.2) |
| L-Carnosine | 104.9(6.7) | 101.4(10.3) | 97.7(7.6) | 134.3(5.8) | 138.0(6.3) | 103.0(8.0) | 95.6(6.3) |
| Ala-leu | 113.5(9.3) | 108.0(4.2) | 109.0(2.9) | 107.2(2.6) | 105.1(2.0) | 113.7(5.2) | 104.3(4.6) |
| Ala-Trp | 105.3(4.3) | 109.6(6.5) | 108.6(4.7) | 116.7(0.9) | 112.4(1.9) | 113.6(3.1) | 116.6(1.9) |
| trans-4-Hydroxy-L-Pro | 106.2(7.3) | 107.9(8.1) | 116.6(3.9) | 98.8(2.5) | 100.2(3.1) | 84.3(6.6) | 95.6(13.1) |
| O-PE | 114.4(9.0) | 107.3(9.4) | 106.9(5.6) | 110.3(1.7) | 114.9(1.1) | 108.0(2.6) | 99.5(1.8) |
| Sarcosine | 18.4(7.3) | 76.8(16.1) | 75.2(11.5) | 110.1(7.4) | 86.4(5.1) | 193.8(2.7) | 154.4(4.4) |
| 3-Methyl-L-histidine | 107.2(4.9) | 96.4(2.9) | 91.1(6.3) | 112.8(1.3) | 104.5(11.7) | 106.3(9.9) | 107(9.3) |
| 1-Methyl-L-histidine | 90.9(12.1) | 90.9(14.5) | 88.5(2.1) | 106.9(9.7) | 106.9(3.6) | 109.6(12.2) | 120.3(2.5) |
| ADMA | 114.2(16.8) | 112.8(10.2) | 108.2(4.7) | 102.1(9.6) | 104.8(16.6) | 108.1(4.1) | 87.7(4.3) |
| O-acetyl-L-serine | 106.3(3.7) | 107.8(5.0) | 107.7(2.9) | 110.2(2.7) | 105.5(2.6) | 103.4(3.8) | 120.3(4.3) |
| Nα-Acetyl-L-lysine | 109.5(0.9) | 109.9(7.1) | 112.6(2.8) | 111.9(3.3) | 106.5(2.4) | 114.4(3.8) | 100.3(5.3) |
| DL-5-Hydroxylysine | 103.1(3.0) | 105.0(8.5) | 108.0(3.0) | 111.2(2.8) | 103.0(2.7) | 105.9(5.7) | 103.9(3.6) |
| 5-Hydroxy-L-tryptophan | 106.3(4.9) | 103.5(1.6) | 103.4(3.0) | 104.0(3.8) | 104.9(2.1) | 109.2(2.3) | 103.6(3.0) |
| 4-Hydroxy-L-isoleucine | 88.6(2.1) | 117.2(2.7) | 124.0(1.4) | 117.9(8.0) | 97.4(1.6) | 110.6(4.6) | 120.6(3.5) |
| Ethanolamine | 110.2(2.4) | 104.0(8.7) | 103.5(3.3) | 113.0(2.4) | 116.4(2.9) | 114.6(2.2) | 102.6(5.8) |
| Methylamine | 115.3(2.2) | 107.4(6.8) | 110.9(5.8) | 114.2(1.2) | 113.1(3.2) | 110.2(15.2) | 110.3(6.6) |
| Agmatine | 108.4(7.2) | 105.6(4.5) | 113.5(1.6) | 118.4(0.3) | 113.8(3.0) | 110.8(6.2) | 100.2(2.6) |
| Ethylamine | 116.0(0.8) | 107.5(5.8) | 108.2(3.7) | 108.5(1.4) | 111.6(1.3) | 107.5(1.4) | 105.7(6.4) |
| Putrescine | 105.2(3.3) | 104.5(6.2) | 105.1(7.2) | 109.4(1.5) | 110.9(6.4) | 110.1(2.2) | 104.2(3.0) |
| Cadaverine | 103.3(3.8) | 105.9(3.0) | 106.5(1.4) | 106.8(1.6) | 110.1(2.2) | 105.7(2.0) | 106.7(5.1) |
| Spermine | 99.2(3.2) | 105.9(9.9) | 101.1(9.8) | 100.0(8.9) | 103.1(3.6) | 82.3(0.5) | 102.7(7.0) |
| Prolinamide | 110.3(7.2) | 104.8(5.6) | 109.4(4.6) | 109.2(2.5) | 104.7(4.6) | 110.2(5.1) | 98.6(5.5) |
| Allantoin | 98.1(0.8) | 98.9(6.5) | 113.7(9.4) | 113.6(10.5) | 108.7(12.3) | 120.1(1.3) | 100.0(11.9) |
| 5-Hydroxydopamine | 116.1(6.2) | 108.9(4.4) | 102.9(7.5) | 105.5(3.7) | 106.1(3.9) | 94.4(4.5) | 103.1(6.0) |
| 3,4-dihydroxy-DL-Phe | 116.2(0.4) | 106.7(4.9) | 106.8(3.8) | 108.7(5.4) | 108.4(2.4) | 105.3(10.7) | 110.4(2.7) |
| DL-Normetanephrine | 112.3(6.0) | 108.2(6.5) | 107.9(3.3) | 107.1(1.8) | 103.5(3.6) | 110.5(1.3) | 107.0(4.3) |
| 1,3-Diaminopropane | 109.8(2.1) | 107.8(5.8) | 111.6(6.6) | 111.6(1.0) | 109.5(2.8) | 106.1(0.9) | 103.0(3.6) |
| 1,2-Diaminopropane | 104.8(1.5) | 105.2(7.0) | 106.2(1.2) | 106.2(4.2) | 102.3(3.4) | 100.6(1.3) | 90.3(3.6) |
| L-Tryptophanamide | 104.0(3.1) | 105.4(9.0) | 107.8(3.8) | 111.2(3.1) | 111.1(1.6) | 100.3(2.6) | 112.0(3.9) |
Data in parathesis are RSD (%); data in bold letters were from metabolites detected in real sample whereas these in italics were not. ADMA: Asymmetric dimethylarginine; Cys: cysteine, Pro: proline; O-PE: O-Phosphorylethanolamine; Phe: phenylalanine.
Quantification of amino-group containing metabolites in haemolymph of silkworm (Bombyx mori L.)
We further applied this newly developed method to analyze the amino metabolites in the silkworm haemolymph at three developmental stages (Table 3). 45 amino metabolites were quantified including 20 proteinogenic and 11 non-proteinogenic amino acids (4-hydroxy-proline, 1-methyl-histidine, 3-methyl-histidine, ornithine, citrulline, β-alanine, γ-aminobutyric acid, 2-aminobutyric acid, 3-aminoisobutyric acid, 2-aminoadipic acid, Nε,Nε,Nε-trimethyllysine), 6 sulfur-containing metabolites (methionine sulfoxide, methionine sulfone, cysteine, cystine, GSSG, cystathionine), 2 polyamines (putrescine, 1,3-diaminopropane), 2 catecholamines (3,4-dihydroxy-phenylalanine, dopamine) and 2 ethanolamines (ethanolamine and o-phosphorylethanolamine) (Table 3). Amongst them, many amino metabolites were not reported in the classical studies of silkworm haemolymph with ion-exchange and paper chromatographic57 and/or more recent NMR studies7, 58 including 3-methyl-histidine, 2-aminobutyric acid, 3-aminoisobutyric acid, 2-aminoadipic acid, methionine sulfone, GSSG, 1,3-diaminopropane and 3,4-dihydroxy-phenylalanine (Table 3). This is probably due to much higher sensitivity of our present method than these used previously. The rich amino metabolites in silkworm haemolymph clearly showed concentration variations with the developmental processes (Table 3) as reported previously7 reflecting the functions of the metabolites for silkworm’s growth, activities and ecdysis in energy metabolism, biosynthesis of proteins57, 59 and pigments. These will be discussed in details elsewhere.
Table 3.
Concentration of amino metabolites (mM) in haemolymph of silkworm (Bombyx mori L strain P50) (the first number denotes day and second one instars, e.g, 5d5I: day 5 in the fifth instar; pP: pre-pupa).
| Amino metabolites | 3d3I | 5d5I | pP |
|---|---|---|---|
| 1-Deoxynojirimycin | 6.136 ± 0.748 | 5.027 ± 0.608 | 0.467 ± 0.115 |
| L-Glutamine | 10.248 ± 3.674 | 10.073 ± 1.397 | 15.326 ± 0.678 |
| L-Asparagine | 2.157 ± 0.545 | 3.179 ± 0.388 | 2.873 ± 1.058 |
| L-Glutamic acid | 0.487 ± 0.179 | 0.107 ± 0.046 | 5.166 ± 3.227 |
| L-Aspartic acid | 0.071 ± 0.038 | 0.049 ± 0.013 | 0.643 ± 0.421 |
| Glycine | 6.078 ± 1.382 | 5.620 ± 0.546 | 12.242 ± 1.484 |
| L-Alanine | 3.065 ± 0.643 | 3.197 ± 1.194 | 5.441 ± 1.688 |
| L-Serine | 7.318 ± 1.556 | 10.807 ± 0.892 | 7.629 ± 0.284 |
| L-Threonine | 4.748 ± 1.570 | 2.998 ± 0.583 | 7.158 ± 0.777 |
| L-Valine | 5.508 ± 1.674 | 1.570 ± 0.582 | 7.570 ± 0.401 |
| L-Isoleucine | 4.548 ± 0.743 | 0.880 ± 0.319 | 8.857 ± 1.393 |
| L-Leucine | 3.794 ± 0.602 | 0.664 ± 0.258 | 7.287 ± 0.815 |
| L-Proline | 2.293 ± 0.866 | 1.085 ± 0.298 | 8.104 ± 1.160 |
| L-Methionine | 1.067 ± 0.285 | 0.520 ± 0.140 | 1.766 ± 0.390 |
| L-Histidine | 5.010 ± 1.642 | 18.247 ± 1.514 | 38.693 ± 2.239 |
| L-Lysine | 11.175 ± 4.222 | 3.955 ± 0.713 | 8.848 ± 1.069 |
| L-Arginine | 3.514 ± 1.394 | 0.723 ± 0.145 | 2.696 ± 0.215 |
| L-Phenylalanine | 0.450 ± 0.101 | 0.465 ± 0.073 | 1.588 ± 0.240 |
| L-Tyrosine | 3.696 ± 0.588 | 0.110 ± 0.058 | 4.343 ± 0.944 |
| L-Tryptophan | 0.315 ± 0.076 | 0.098 ± 0.025 | 1.412 ± 0.323 |
| β-alanine | 0.459 ± 0.200 | 0.256 ± 0.042 | 0.284 ± 0.046 |
| L-Ornithine | 3.660 ± 1.900 | 9.224 ± 2.090 | 0.613 ± 0.296 |
| L-Citrulline | 0.235 ± 0.038 | 0.083 ± 0.030 | 0.013 ± 0.001 |
| 4-Hydroxy-L-proline | 0.057 ± 0.014 | 0.146 ± 0.017 | 0.033 ± 0.004 |
| L-2-aminoadipic acid | 0.004 ± 0.001 | 0.051 ± 0.022 | 0.041 ± 0.012 |
| γ-Aminobutyric acid | 0.003 ± 0.001 | 0.019 ± 0.006 | 0.012 ± 0.003 |
| DL-3-aminoisobutyric acid | 0.023 ± 0.017 | 0.057 ± 0.007 | 0.018 ± 0.003 |
| L-2-Aminobutyric acid | 0.047 ± 0.009 | 0.064 ± 0.014 | 0.131 ± 0.021 |
| 3-hydroxykynurenine | 1.662 ± 0.103 | 0.074 ± 0.005 | 0.402 ± 0.090 |
| 1-Methyl-L-histidine | 0.050 ± 0.012 | 0.014 ± 0.002 | 0.340 ± 0.054 |
| 3-Methyl-L-histidine | 0.064 ± 0.022 | 0.017 ± 0.003 | 0.022 ± 0.003 |
| Nε,Nε,Nε-trimethyllysine | 0.880 ± 0.166 | 1.270 ± 0.158 | 1.980 ± 0.197 |
| Dopamine | 0.032 ± 0.011 | 0.006 ± 0.001 | 0.059 ± 0.018 |
| 3,4-dihydroxy-DL-phenylalanine | 0.611 ± 0.182 | 0.847 ± 0.206 | 1.422 ± 0.328 |
| Cysteine | 0.010 ± 0.001 | 0.020 ± 0.004 | 0.049 ± 0.013 |
| Cystine | 0.098 ± 0.028 | 0.091 ± 0.027 | 0.113 ± 0.031 |
| DL-Methionine sulfoxide | 0.053 ± 0.022 | 0.017 ± 0.004 | 10.881 ± 1.776 |
| DL-Methionine sulfone | 0.003 ± 0.000 | 0.008 ± 0.001 | 0.019 ± 0.001 |
| Cystathionine | 2.632 ± 0.373 | 2.380 ± 0.472 | 4.502 ± 0.767 |
| Glutathione disulfide | 0.134 ± 0.018 | 0.028 ± 0.008 | 0.155 ± 0.032 |
| Putrescine | 2.262 ± 0.374 | 4.366 ± 0.691 | 15.754 ± 1.940 |
| 1,3-Diaminopropane | 0.011 ± 0.003 | 0.021 ± 0.003 | 0.050 ± 0.005 |
| O-Phosphorylethanolamine | 0.209 ± 0.081 | 0.165 ± 0.031 | 4.778 ± 0.845 |
| Ethanolamine | 0.036 ± 0.006 | 0.011 ± 0.001 | 0.043 ± 0.004 |
| NH4 + | 0.188 ± 0.118 | 0.031 ± 0.013 | 1.151 ± 0.059 |
Conclusions
We developed a new and parameter-optimized UHPLC-MS/MS method for simultaneous quantification of the amino-group containing metabolites based on derivatization-assisted sensitivity enhancement by 5-aminoisoquinolyl-N-hydroxysuccinimidyl carbamate (5-AIQC). By using an N-ethylmaleimide-based click reaction followed with addition of antioxidants (TCEP and ascorbic acid), our method enabled simultaneous quantification of thiols, disulfides and oxidation-prone metabolites concurrently with other amino analytes in an one-pot manner (and in a single run). This method is also applicable to quantify aromatic amines which cannot be done with the 6-AQC-based method37. This 5-AIQC-based method had higher sensitivity than the 6-AQC-based one37 for an extensive coverage of analytes including 4 amino saccharides, 20 proteinogenic amino acids, 57 non-proteinogenic amino acids, 17 modified amino acids, 26 aliphatic and 8 aromatic amines, 22 sulfur-containing analytes, 14 monoamine neurotransmitters and 8 small peptides. Amongst them, many sets of isomeric analytes (or having the same ion) were also separable on a common reversed-phase column and quantifiable with tandem-mass spectrometry. This method enables simultaneous quantification of 124 important functional metabolites in more than twenty metabolic pathways such as protein biosynthesis/degradation, gut microbiota metabolism, biosynthesis of arginine, glutathione and catecholamine neurotransmitters, urea cycle, uridine catabolism, polyamine pathway together with the metabolisms of phenylalanine, histidine, tryptophan, cysteine-methionine, taurine-hypotaurine and homocysteine. Our method had excellent precision, accuracy, linearity and recovery for most of analytes including thiols, disulfides and other oxidation-prone metabolites in mixed standards, renal tumor extracts, rat urine and plasma samples. We further applied this method to measure the amino metabolites in hemolymph of silkworms at multiple developmental stages and discovered dozens of metabolites which were not reported previously confirming applicability of this method in cohort studies of biological samples. However, sarcosine and 2-aminoisobutyric acid had unexpected poor behavior in terms of their quantification precision and accuracy with unknown reasons at this stage. As the 6-AQC-based method37, this method is not suitable for adenine, guanido and amide groups. Nevertheless, with a unique single-charged fragment ion at m/z 171.0550, it is expected that all the 5-AIQC-derivatized amino compounds can be comprehensively analyzed in a semi-targeted and discovery fashion using UHPLC-QTOF-MS approaches. This will be particularly useful for screening large cohort samples. With many classes of metabolites simultaneously quantified in an one-pot manner, this method may also have useful potentials in clinical chemistry settings as well.
Methods
Reagents
HPLC grade methanol and acetonitrile were purchased from TEDIA (Shanghai, China) and Sigma-Aldrich (Shanghai, China), respectively. Na2HPO4·12H2O and NaH2PO4·2H2O, boric acid, sodium hydroxide, ethylenediaminetetraacetic acid (EDTA), dimethylsulfoxide (DMSO) were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China) all as analytical grade reagents. Formic acid, ascorbic acid, tris(2-carboxyethyl)phosphinehydrochloride (TCEP), N,N’-disuccinimidyl carbonate (DSC), N-ethylmaleimide (NEM), 4-tert-butylbenzenethiol (tBBT), 5-aminoisoquinoline (5-AIQ) and 6-aminoquinoline (6-AQ) were purchased from Sigma-Aldrich (Shanghai, China) together with 126 analyte standards used here. (see details in Table 1).
Buffer solutions
Phosphate buffer and borate buffer were prepared in a normal manner with their pH adjusted to 7.0 and 8.8, respectively, using sodium hydroxide solution. Phosphate buffer (0.1 M) contained 10 mM ascorbic acid and 10 mM EDTA whereas borate buffer (0.2 M) contained 20 mM TCEP and 1 mM ascorbic acid.
Standard Solutions
Each amino-containing analyte standard was weighed accurately and dissolved in aqueous solution of formic acid (0.1%) or phosphate buffer as appropriate. The combined solution from known quantity of these standards gave a stock solution of mixed standards. A series of solutions for mixed standards (Supplementary Table S1) was prepared in volumetric flasks by gradual dilution of the stock solution using phosphate buffer (0.1 M, pH7.0) to make such solutions contain 0.1 M phosphate, 10 mM ascorbic acid and 10 mM EDTA. Solutions of analytes containing thiol and disulfide groups (about 50–500 μM) were prepared in air-tight containers in phosphate buffer (0.1 M, pH7.0) containing 10 mM ascorbic acid and 10 mM EDTA followed with storage at −20 °C until further use.
Collection and treatments of rat urine and serum samples
The animal experiment was approved by the local committee in the Chinese Academy of Sciences and conducted in accordance with the national guidelines for animal research (Ministry of Science and Technology of China, 2006). Urine and serum samples were from 8-weeks old Wistar rats allowing free access to normal chow and water in a standard manner followed with storage at −80 °C. In 100 μL biological fluids (urine and serum), 300 μL methanol was respectively added directly followed with vortex mixing and 10 min centrifugation (11060 × g, 4 °C). The supernatant of each sample was then snap-frozen and stored at −80 °C until further analysis.
Extracts of human renal cancer tissue samples
The human renal cancer and adjacent non involved tissues from tissue bank at Fudan University Shanghai Cancer Center were used with approval by the local ethic committee (050432-4-1212B). Each tissue sample (about 50 mg) was extracted with 600 μL pre-cooled methanol-water mixture (2:1, v/v) using a tissuelyzer (QIAGEN TissueLyser II, Germany) at 20 Hz for 90 s as previously described60. Such extracts were respectively redissolved in 600 μL phosphate buffer (0.1 M, pH7.4) as stock solution for UHPLC-MS/MS analysis.
Hemolymph Sample Collection and Preparation
Hemolymph samples were obtained from silkworms (Bombyx mori L. strain p50) at three developmental stages in a previously reported study7. These samples were collected on the day 3 in the third instar (3d3I), day 1 in the fourth instar (1d4I), day 4 in the fourth instar (4d4I), and day 1, 3, 5, 7, 8 in the fifth instar (1d5I, 3d5I, 5d5I, 7d5I, 8d5I) as well as at the pre-pupa stage (pP). All these samples were collected in tubes containing thiourea (as an antioxidant) and stored at −80 °C until analysis. Ten biological replicates were employed in this study. For amino metabolites analysis, each hemolymph sample was individually centrifuged (4000 × g, 4 °C) for 10 minutes to obtain supernatants; 10 μL supernatant from each sample was mixed with 40 μL phosphate buffer (0.1 M, pH7.0), snap-frozen and stored at −80 °C till analysis.
Synthesis of 5-aminoisoquinolyl-N-hydroxysuccinimidylcarbamate (5-AIQC)
5-Aminoisoquinolyl-N-hydroxysuccinimidylcarbamate (5-AIQC) for tagging amino groups was synthesized (Fig. 1) by drop-wise addition of 5-aminoisoquinoline (5-AIQ) solution (2 mmol in 50 mL ACN) to N,N′-disuccinimidylcarbonate (DSC) solution (3 mmol in 40 mL ACN). This was done over about 2 hours at ambient temperature with magnetic stirring. After further stirring for 24 h and removal of acetonitrile by rotary evaporation, 5-AIQC was obtained as crystals from the concentrated solution through filtration (650 mg, 82% yield). Its 1H-NMR and ESI-QTOFMS spectral data are shown in Supplementary Fig. S8.
Derivatization of amino metabolites by tagging amino group with 5-AIQC
The amino analytes were derivatized individually and in the forms of their mixtures with 5-AIQC in dry acetonitrile (Fig. 2, Supplementary Fig. S1). First, each aliquot of standards (10 μL) or biological samples was vortex-mixed with 80 µL of NEM solution (2.5 mM) in phosphate buffer (0.1 M, pH7.0) containing 10 mM ascorbic acid, 10 mM EDTA and 7% DMSO for 1 min. 10 μL tBBT solution (1 M in DMSO) was added followed with addition of 700 μL borate buffer (0.2 M, pH 8.8) containing 20 mM TCEP and 1 mM ascorbic acid. After vortex-mixing and standing for 2 min, 200 µL 5-AIQC solution was then added and incubated at 55 °C for 10 min. The mixture was cooled down to the ambient temperature and added with 10 µL formic acid followed with storage in air-tight tubes at −20 °C until UHPLC-MS/MS analysis.
UHPLC-ESI-MS/MS Analysis
UHPLC-MS/MS analyses were conducted on an Agilent UHPLC-MS/MS system consisting of an 1290 UHPLC-system coupled with an Agilent 6460 and 6495 triple-quadrupole mass spectrometer (Agilent Technologies, USA) with Jet Stream ion source in both. The latter also employed iFunnel technology to improve detection sensitivity. MassHunter Workstation software was used for data analysis.
The 5-AIQC-tagged samples (1 μL) were individually injected on an UHPLC column (Agilent Zorbax Eclipse XDB-C18 Rapid Resolution HD, 2.1 × 100 mm, 1.8 μm) with its temperature set to 50 °C. Ultrapure water (MilliQ) and methanol containing 0.1% (v/v) formic acid were used as two mobile phases A and B, respectively, with flow rate of 0.6 mL/min. An optimized gradient elution scheme was employed as 1% B (0–2 min), 1–3.8% B (2–4 min), 3.8–22% B (4–8 min), 22–25% B (8–12 min), 25–60% B (12–13 min), 60–80% B (13–13.51 min) and 80–95% B (13.51–16 min).
Mass spectrometers were operated in the positive ion mode. The MS parameters including source, collision energies and fragmentor voltages were optimized for each analyte by directly infusing the derivatized standard. Gas flow was 10 L/min with gas temperature of 315 °C; nebulizer pressure was 50 psi with temperature of 350 °C and sheath gas flow was 10 L/min. Nozzle voltage was 500 V and capillary voltage was 4000 V. Spectra were acquired in the MRM mode with a common fragment ion at m/z 171 for all analytes. All parameters especially collision energies and fragmentor voltages were optimized for each individual analyte by directly infusing the derivatized individual standard.
Validation of the UHPLC-ESI-MS/MS Analytical Method for Amino Metabolites
Nine mixed solutions of 95 known standards (amino compounds) were employed for method validation and denoted as Mix1-Mix9 (Supplementary Table S1). Precision for retention times was evaluated by using the retention time of each amino compound in the mixed standards recorded on three different days whilst MassHunter Workstation software (Agilent, USA) was used to calculate the linearity (correlation coefficients), limit of detection (with S/N = 3) and limit of quantification (with S/N = 10). For intra-day and inter-day precision of quantification, four different mixed solutions of known standards (Mix2, Mix5, Mix6 and Mix7) were employed to represent high, intermediate and low concentration situations, respectively (Supplementary Table S1) with each mixed-solution repeatedly analyzed five times per day for three days. Quantification accuracy of the method was measured from the Mix1, Mix4 and Mix6 (representing high, intermediate and low concentration situations, respectively) spiked with an equal volume of Mix4. Recovery of each amino metabolite was measured by using the extracts of renal tumor and adjacent non-involved tissues, and deproteinated rat urine and serum samples spiked with Mix3.
Electronic supplementary material
Acknowledgements
We acknowledge the National Natural Science Foundation of China (91439102, 81590953, 21375144, 21405020) for financial supports.
Author Contributions
H.R.T. and J.W. designed the experiments. H.R.T., L.X. and Y.L.W. obtained the funding. L.H.Z. performed silkworm animal experiments and J.W. performed LC-MS experiments. H.R.T., J.W. and Y.L.W. analyzed, interpreted data and wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01435-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 2.Tang H, Wang Y. Metabonomics: a revolution in progress. Progress in Biochemistry and Biophysics. 2005;33:401–417. [Google Scholar]
- 3.Fan TW-M. Metabolite profiling by one-and two-dimensional NMR analysis of complex mixtures. Progress in nuclear magnetic resonance spectroscopy. 1996;28:161–219. doi: 10.1016/0079-6565(96)90002-3. [DOI] [Google Scholar]
- 4.Wang Y, et al. Magic angle spinning NMR and 1H-31P heteronuclear statistical total correlation spectroscopy of intact human gut biopsies. Analytical chemistry. 2008;80:1058–1066. doi: 10.1021/ac701988a. [DOI] [PubMed] [Google Scholar]
- 5.Holmes E, et al. Detection of urinary drug metabolite (xenometabolome) signatures in molecular epidemiology studies via statistical total correlation (NMR) spectroscopy. Analytical chemistry. 2007;79:2629–2640. doi: 10.1021/ac062305n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson JK, Lindon JC. Systems biology: metabonomics. Nature. 2008;455:1054–1056. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L, et al. Developmental Changes for the Hemolymph Metabolome of Silkworm (Bombyx mori L.) Journal of proteome research. 2015;14:2331–2347. doi: 10.1021/acs.jproteome.5b00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholson G, et al. Human metabolic profiles are stably controlled by genetic and environmental variation. Molecular systems biology. 2011;7:525. doi: 10.1038/msb.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, et al. Antagonist of Prostaglandin E2 Receptor 4 Induces Metabolic Alterations in Liver of Mice. Journal of proteome research. 2015;14:1566–1573. doi: 10.1021/pr501236y. [DOI] [PubMed] [Google Scholar]
- 10.Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134:714–717. doi: 10.1016/j.cell.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Li D, et al. Metabonomic changes associated with atherosclerosis progression for LDLR−/− mice. Journal of proteome research. 2015;14:2237–2254. doi: 10.1021/acs.jproteome.5b00032. [DOI] [PubMed] [Google Scholar]
- 12.An Y, et al. High-fat diet induces dynamic metabolic alterations in multiple biological matrices of rats. Journal of proteome research. 2013;12:3755–3768. doi: 10.1021/pr400398b. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nature Reviews Drug Discovery. 2002;1:153–161. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, et al. Selective metabolic effects of gold nanorods on normal and cancer cells and their application in anticancer drug screening. Biomaterials. 2013;34:7117–7126. doi: 10.1016/j.biomaterials.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 15.Zheng, X. et al. Melamine-induced renal toxicity is mediated by the gut microbiota. Science translational medicine 5, 172ra122–172ra122 (2013). [DOI] [PubMed]
- 16.Shi X, Xiao C, Wang Y, Tang H. Gallic acid intake induces alterations to systems metabolism in rats. Journal of proteome research. 2012;12:991–1006. doi: 10.1021/pr301041k. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, et al. Metabonomic profiling revealed an alteration in purine nucleotide metabolism associated with cardiac hypertrophy in rats treated with thiazolidinediones. Journal of proteome research. 2013;12:5634–5641. doi: 10.1021/pr400587y. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson JK, et al. Metabolic phenotyping in clinical and surgical environments. Nature. 2012;491:384–392. doi: 10.1038/nature11708. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, et al. Metabonomic analysis reveals efficient ameliorating effects of acupoint stimulations on the menopause-caused alterations in mammalian metabolism. Scientific reports. 2014;4:3641. doi: 10.1038/srep03641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinross JM, Holmes E, Darzi AW, Nicholson JK. Metabolic phenotyping for monitoring surgical patients. The Lancet. 2011;377:1817–1819. doi: 10.1016/S0140-6736(11)60171-2. [DOI] [PubMed] [Google Scholar]
- 21.Holmes E, Wijeyesekera A, Taylor-Robinson SD, Nicholson JK. The promise of metabolic phenotyping in gastroenterology and hepatology. Nature Reviews Gastroenterology & Hepatology. 2015;12:458–471. doi: 10.1038/nrgastro.2015.114. [DOI] [PubMed] [Google Scholar]
- 22.Li M, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proceedings of the National Academy of Sciences. 2008;105:2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin FPJ, et al. A top‐down systems biology view of microbiome‐mammalian metabolic interactions in a mouse model. Molecular systems biology. 2007;3:112. doi: 10.1038/msb4100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clayton TA, Baker D, Lindon JC, Everett JR, Nicholson JK. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proceedings of the National Academy of Sciences. 2009;106:14728–14733. doi: 10.1073/pnas.0904489106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, et al. Gut microbiota composition modifies fecal metabolic profiles in mice. Journal of proteome research. 2013;12:2987–2999. doi: 10.1021/pr400263n. [DOI] [PubMed] [Google Scholar]
- 26.Claus SP, et al. Colonization-induced host-gut microbial metabolic interaction. MBio. 2011;2:e00271–00210. doi: 10.1128/mBio.00271-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, H., An, Y., Hao, F., Wang, Y. & Tang, H. Correlations of fecal metabonomic and microbiomic changes induced by high-fat diet in the pre-obesity state. Scientific reports6, 21618 (2016). [DOI] [PMC free article] [PubMed]
- 28.Brindle JT, et al. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nature medicine. 2002;8:1439–1445. doi: 10.1038/nm1202-802. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, et al. Rapid diagnosis and prognosis of de novo acute myeloid leukemia by serum metabonomic analysis. Journal of proteome research. 2013;12:4393–4401. doi: 10.1021/pr400403p. [DOI] [PubMed] [Google Scholar]
- 30.Ni Y, Xie G, Jia W. Metabonomics of human colorectal cancer: new approaches for early diagnosis and biomarker discovery. Journal of proteome research. 2014;13:3857–3870. doi: 10.1021/pr500443c. [DOI] [PubMed] [Google Scholar]
- 31.Tian, Y. et al. Integrative metabonomics as potential method for diagnosis of thyroid malignancy. Scientific reports5, 14869 (2015). [DOI] [PMC free article] [PubMed]
- 32.Tian, Y. et al. Tissue metabonomic phenotyping for diagnosis and prognosis of human colorectal cancer. Scientific reports6, 20790 (2016). [DOI] [PMC free article] [PubMed]
- 33.Wishart DS, et al. HMDB: the human metabolome database. Nucleic acids research. 2007;35:D521–D526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dettmer K, Aronov PA, Hammock BD. Mass spectrometry‐based metabolomics. Mass spectrometry reviews. 2007;26:51–78. doi: 10.1002/mas.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fekete S, Schappler J, Veuthey J-L, Guillarme D. Current and future trends in UHPLC. TrAC Trends in Analytical Chemistry. 2014;63:2–13. doi: 10.1016/j.trac.2014.08.007. [DOI] [Google Scholar]
- 36.Walter TH, Andrews RW. Recent innovations in UHPLC columns and instrumentation. TrAC Trends in Analytical Chemistry. 2014;63:14–20. doi: 10.1016/j.trac.2014.07.016. [DOI] [Google Scholar]
- 37.Boughton BA, et al. Comprehensive profiling and quantitation of amine group containing metabolites. Analytical chemistry. 2011;83:7523–7530. doi: 10.1021/ac201610x. [DOI] [PubMed] [Google Scholar]
- 38.Guo K, Li L. Differential 12C-/13C-isotope dansylation labeling and fast liquid chromatography/mass spectrometry for absolute and relative quantification of the metabolome. Analytical chemistry. 2009;81:3919–3932. doi: 10.1021/ac900166a. [DOI] [PubMed] [Google Scholar]
- 39.Guo K, Li L. High-performance isotope labeling for profiling carboxylic acid-containing metabolites in biofluids by mass spectrometry. Analytical chemistry. 2010;82:8789–8793. doi: 10.1021/ac102146g. [DOI] [PubMed] [Google Scholar]
- 40.Buescher JM, Moco S, Sauer U, Zamboni N. Ultrahigh performance liquid chromatography– tandem mass spectrometry method for fast and robust quantification of anionic and aromatic metabolites. Analytical chemistry. 2010;82:4403–4412. doi: 10.1021/ac100101d. [DOI] [PubMed] [Google Scholar]
- 41.Batch BC, Hyland K, Svetkey LP. Branch chain amino acids: biomarkers of health and disease. Current Opinion in Clinical Nutrition & Metabolic Care. 2014;17:86–89. doi: 10.1097/MCO.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 42.Van Doorn M, et al. Evaluation of metabolite profiles as biomarkers for the pharmacological effects of thiazolidinediones in Type 2 diabetes mellitus patients and healthy volunteers. British journal of clinical pharmacology. 2007;63:562–574. doi: 10.1111/j.1365-2125.2006.02816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rütters H, Möhring T, Rullkötter J, Griep‐Raming J, Metzger JO. The persistent memory effect of triethylamine in the analysis of phospholipids by liquid chromatography/mass spectrometry. Rapid Communications in Mass Spectrometry. 2000;14:122–123. doi: 10.1002/(SICI)1097-0231(20000130)14:2<122::AID-RCM844>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 44.Quirke JME, Adams CL, Van Berkel GJ. Chemical derivatization for electrospray ionization mass spectrometry. 1. Alkyl halides, alcohols, phenols, thiols, and amines. Analytical chemistry. 1994;66:1302–1315. doi: 10.1021/ac00080a016. [DOI] [Google Scholar]
- 45.Mengerink Y, Kutlán D, Tóth F, Csámpai A, Molnár-Perl I. Advances in the evaluation of the stability and characteristics of the amino acid and amine derivatives obtained with the o-phthaldialdehyde/3-mercaptopropionic acid and o-phthaldialdehyde/N-acetyl-L-cysteine reagents: High-performance liquid chromatography–mass spectrometry study. Journal of Chromatography A. 2002;949:99–124. doi: 10.1016/S0021-9673(01)01282-1. [DOI] [PubMed] [Google Scholar]
- 46.Haynes PA, Sheumack D, Greig LG, Kibby J, Redmond JW. Applications of automated amino acid analysis using 9-fluorenylmethyl chloroformate. Journal of Chromatography A. 1991;588:107–114. doi: 10.1016/0021-9673(91)85012-5. [DOI] [PubMed] [Google Scholar]
- 47.Heinrikson RL, Meredith SC. Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Analytical biochemistry. 1984;136:65–74. doi: 10.1016/0003-2697(84)90307-5. [DOI] [PubMed] [Google Scholar]
- 48.Cohen SA, Michaud DP. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Analytical biochemistry. 1993;211:279–287. doi: 10.1006/abio.1993.1270. [DOI] [PubMed] [Google Scholar]
- 49.Navarro J, et al. Blood glutathione as an index of radiation-induced oxidative stress in mice and humans. Free Radical Biology and Medicine. 1997;22:1203–1209. doi: 10.1016/S0891-5849(96)00554-0. [DOI] [PubMed] [Google Scholar]
- 50.Giustarini D, Dalle-Donne I, Colombo R, Milzani A, Rossi R. An improved HPLC measurement for GSH and GSSG in human blood. Free Radical Biology and Medicine. 2003;35:1365–1372. doi: 10.1016/j.freeradbiomed.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 51.Seiwert B, Karst U. Simultaneous LC/MS/MS determination of thiols and disulfides in urine samples based on differential labeling with ferrocene-based maleimides. Analytical chemistry. 2007;79:7131–7138. doi: 10.1021/ac071016b. [DOI] [PubMed] [Google Scholar]
- 52.Cohen, S. M. D. (EP 0533200 B1, 1992).
- 53.Hong V, Kislukhin AA, Finn M. Thiol-selective fluorogenic probes for labeling and release. Journal of the American Chemical Society. 2009;131:9986–9994. doi: 10.1021/ja809345d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gregory JD. The stability of N-ethylmaleimide and its reaction with sulfhydryl groups. Journal of the American Chemical Society. 1955;77:3922–3923. doi: 10.1021/ja01619a073. [DOI] [Google Scholar]
- 55.Joule, J. A. & Mills, K. Heterocyclic chemistry. 177–178 (John Wiley & Sons, 2008).
- 56.Xiao C, Hao F, Qin X, Wang Y, Tang H. An optimized buffer system for NMR-based urinary metabonomics with effective pH control, chemical shift consistency and dilution minimization. Analyst. 2009;134:916–925. doi: 10.1039/b818802e. [DOI] [PubMed] [Google Scholar]
- 57.Wyatt GR. The biochemistry of insect hemolymph. Annual review of entomology. 1961;6:75–102. doi: 10.1146/annurev.en.06.010161.000451. [DOI] [Google Scholar]
- 58.Chikayama E, et al. Systematic NMR analysis of stable isotope labeled metabolite mixtures in plant and animal systems: coarse grained views of metabolic pathways. PloS one. 2008;3:e3805. doi: 10.1371/journal.pone.0003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andersen SO, Hojrup P, Roepstorff P. Insect cuticular proteins. Insect biochemistry and molecular biology. 1995;25:153–176. doi: 10.1016/0965-1748(94)00052-J. [DOI] [PubMed] [Google Scholar]
- 60.Jiang L, Huang J, Wang Y, Tang H. Metabonomic analysis reveals the CCl4-induced systems alterations for multiple rat organs. Journal of proteome research. 2012;11:3848–3859. doi: 10.1021/pr3003529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


