Abstract
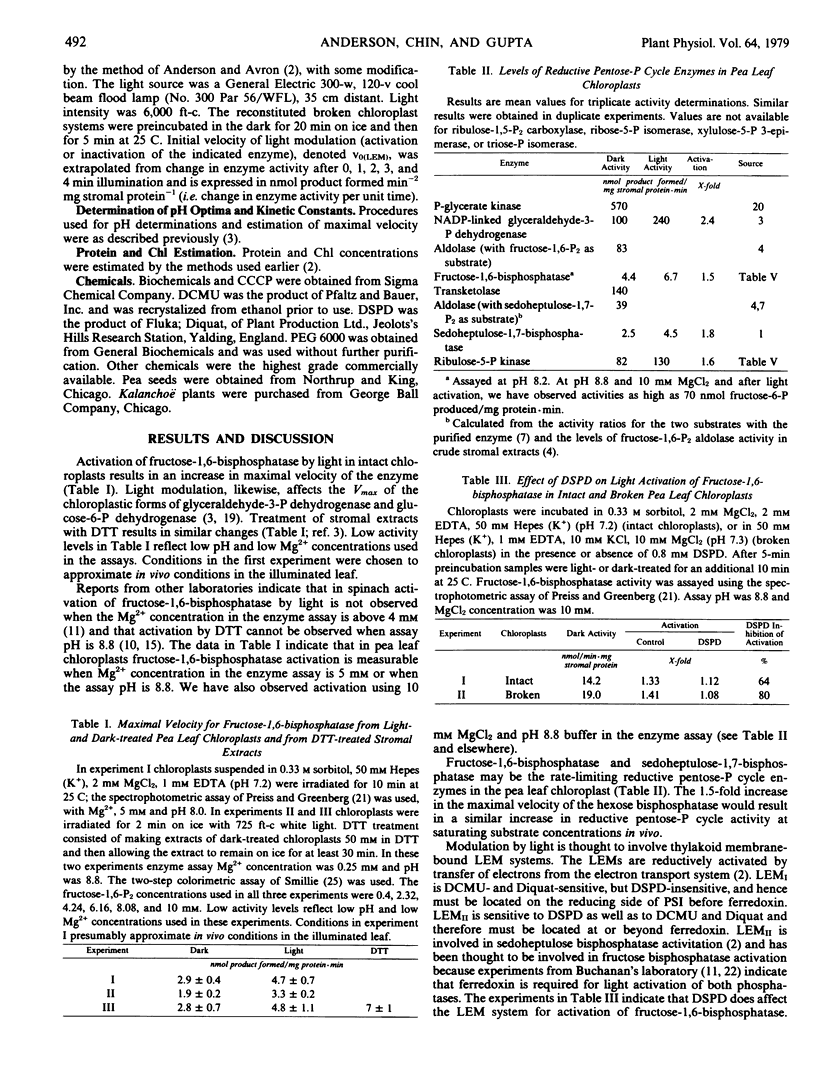
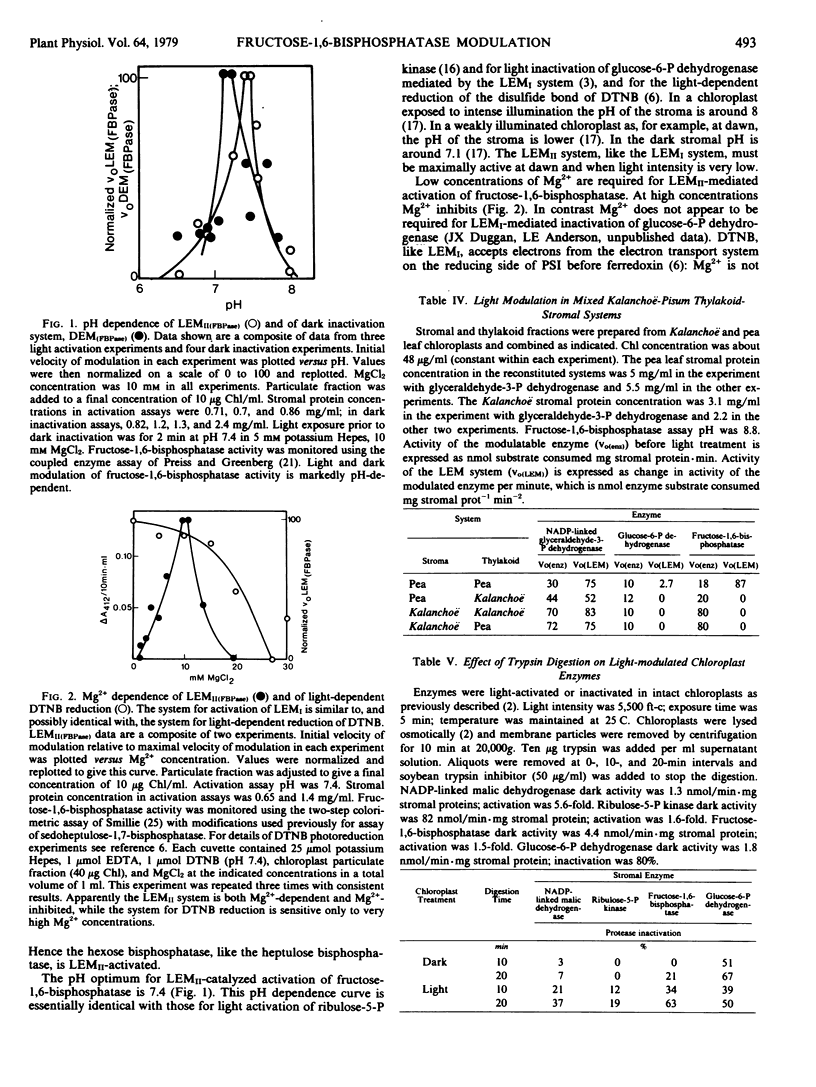
Inhibitor experiments indicate that light effect mediatorII which is reductively activated by transfer of electrons from the photosynthetic electron transport system at or beyond ferredoxin, is involved in activation by light of fructose-1,6-bisphosphatase in the pea plant. Activation proceeds optimally when the pH is low and Mg2+ is 10 millimolar. Modulation by light results in increases in maximal velocity, apparently as a result of changes in enzyme conformation. Pea leaf thylakoids are effective in modulating the activity of glyceraldehyde-3-phosphate dehydrogenase but not of fructose-1,6-bisphosphatase or glucose-6-phosphate dehydrogenase in Kalanchoë stromal extracts. There is apparently species specificity for modulation of some, but not all, of the modulatable enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E. Activation of pea leaf chloroplast sedoheptulose 1,7-diphosphate phosphatase by light and dithiothreitol. Biochem Biophys Res Commun. 1974 Aug 5;59(3):907–913. doi: 10.1016/s0006-291x(74)80065-3. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Avron M. Light Modulation of Enzyme Activity in Chloroplasts: Generation of Membrane-bound Vicinal-Dithiol Groups by Photosynthetic Electron Transport. Plant Physiol. 1976 Feb;57(2):209–213. doi: 10.1104/pp.57.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Duggan J. X. Light modulation of glucose-6-phosphate dehydrogenase: partial characterization of the light inactivation system and its effects on the properties of the chloroplastic and cytoplasmic forms of the enzyme. Plant Physiol. 1976 Aug;58(2):135–139. doi: 10.1104/pp.58.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Heinrikson R. L., Nyes C. Chloroplast and cytoplasmic enzymes 1, 2, 3. Subunit structure of pea leaf aldolases. Arch Biochem Biophys. 1975 Jul;169(1):262–268. doi: 10.1016/0003-9861(75)90340-9. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Lim T. C. Chloroplast glyceraldehyde 3-phosphate dehydrogenase: light-dependent change in the enzyme. FEBS Lett. 1972 Nov 1;27(2):189–191. doi: 10.1016/0014-5793(72)80616-1. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Nehrlich S. C., Champigny M. L. Light modulation of enzyme activity: activation of the light effect mediators by reduction and modulation of enzyme activity by thiol-disulfide exchange. Plant Physiol. 1978 Apr;61(4):601–605. doi: 10.1104/pp.61.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Pacold I. Chloroplast and Cytoplasmic Enzymes: IV. Pea Leaf Fructose 1,6-Diphosphate Aldolases. Plant Physiol. 1972 Mar;49(3):393–397. doi: 10.1104/pp.49.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier D., Latzko E. Properties and regulation of C-1-fructose-1,6-diphosphatase from spinach chloroplasts. Biochim Biophys Acta. 1975 Jul 8;396(1):141–148. doi: 10.1016/0005-2728(75)90197-8. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Gupta V. K., Anderson L. E. Light modulation of the activity of carbon metabolism enzymes in the crassulacean Acid metabolism plant kalanchoë. Plant Physiol. 1978 Mar;61(3):469–471. doi: 10.1104/pp.61.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Kelly G. J., Zimmermann G., Latzko E. Light induced activation of fructose-1, 6-bisphosphatase in isolated intact chloroplasts. Biochem Biophys Res Commun. 1976 May 3;70(1):193–199. doi: 10.1016/0006-291x(76)91127-x. [DOI] [PubMed] [Google Scholar]
- Melandri B. A., Pupillo P., Baccarini-Melandri A. D-glyceraldehyde-3-phosphate dehydrogenase in photosynthetic cells. I. The reversible light-induced activation in vivo of NADP-dependent enzyme and its relationship to NAD-dependent activities. Biochim Biophys Acta. 1970 Nov 11;220(2):178–189. doi: 10.1016/0005-2744(70)90004-5. [DOI] [PubMed] [Google Scholar]
- Pacold I., Anderson L. E. Chloroplast and Cytoplasmic Enzymes: VI. Pea Leaf 3-Phosphoglycerate Kinases. Plant Physiol. 1975 Feb;55(2):168–171. doi: 10.1104/pp.55.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann P., Buchanan B. B. Role of ferredoxin in the activation of sedoheptulose diphosphatase in isolated chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):189–192. doi: 10.1016/0005-2728(75)90217-0. [DOI] [PubMed] [Google Scholar]
- Schürmann P., Wolosiuk R. A. Studies on the regulatory properties of chloroplast fructose-1,6-bisphosphatase. Biochim Biophys Acta. 1978 Jan 12;522(1):130–138. doi: 10.1016/0005-2744(78)90329-7. [DOI] [PubMed] [Google Scholar]


