Abstract
Uterine leiomyoma is the most common female reproductive tract benign tumor. Little is known about protein composition and changes in the leiomyoma interstitial fluid (IF). The present study focused on changes in protein abundance in the IF of leiomyoma. Leiomyoma IFs and adjacent myometrial IFs were obtained and analyzed by two-dimensional electrophoresis (2-DE) coupled with mass spectrometry and western blotting for 2-DE data validation. A total of 25 unique proteins were observed to change significantly (P<0.05). Of these proteins with different abundance, 22 had not been previously identified in leiomyoma IF. In silico analysis predicted that three of these proteins were secreted via classical mechanisms, while 22 were secreted via non-classical mechanisms. Ingenuity Pathway Analysis identified 17 proteins associated with cellular migration and proliferation. Among these, phosphoglycerate mutase 1 had not been previously associated with leiomyoma. The abundance of seven proteins was further validated by western blotting. A comparative proteomic approach identified a number of proteins associated with cellular migration and proliferation, with changes in abundance in IF likely to be involved in tumor development. Further studies will be required to investigate the role of these proteins in leiomyoma IF and their possible association with tumor development and growth.
Keywords: leiomyoma, myometrium, 2-D electrophoresis, proteomics, interstitial fluid, mass spectrometry, secreted protein
Introduction
Uterine leiomyoma is the most common female reproductive tract benign tumor (1). This tumor is responsible for severe health problems, including pain, infertility, repetitive pregnancy loss and, most importantly, heavy uterine bleeding (2). No effective medical treatment is currently available, due to the poor understanding of the exact molecular pathways involved in its physiopathology (3). Ovarian hormones, growth factors, retinoic acid, vitamin D, transforming growth factor-β/Smad and genetic factors are associated with tumor development (4,5). Leiomyomas are characterized by a hyperproduction of extracellular matrix (ECM), which is constituted predominantly by collagens and other proteoglycans (6). The ECM components serve a key role in the regulation of migration, proliferation and differentiation of leiomyomas (6).
The interstitial fluid (IF) is an extracellular fluid bathing and surrounding the tissue cells, and is closely associated with pathological changes in the tissues. In addition to nutrients and oxygen, IF contains proteins secreted by tumors via classical and non-classical secretory pathways, as well as proteins released by extracellular vesicles (7). Several studies have shown that tumor growth is not only regulated by the malignancy of the tumor cells, but is also associated with several factors that are present in the tumor microenvironment, including cytokines, electrolytes and other secreted proteins (8). The IF flow movement may influence the degree of viscosity and elasticity of the ECM, thus modifying the intercellular communication and determining an increased mitotic activity (9). Collagen and other proteoglycans such as lumican (LUM) induce the activation of the integrin signaling pathway, which serves a key role in cytoskeleton reorganization, cell motility and proliferation (6). Early cytogenetic alterations are allegedly insufficient for tumor development; thus, additional alterations of the MAPK/ERK signaling pathway appear to be crucial (3).
For the reasons described above, the identification of changes in protein abundance in leiomyoma IF may be useful for the understanding of the tumor physiopathology. Proteomic studies on leiomyomas, carried out to identify dysregulated proteins, have been conducted in two-dimensional electrophoresis (2-DE) coupled with mass spectrometry (MS) (10) and gel electrophoresis liquid chromatography-MS (11). Our previous proteomic studies on leiomyoma tissue identified several dysregulated proteins involved in metabolic processes, oxidative stress and cell migration (12,13). In a previous study, seven proteins with changes in abundance were identified in the IF of leiomyomas (14). To date, no other proteomic study has been conducted on the IF of leiomyomas.
The aim of the present study was to analyze the proteome of leiomyoma and myometrium IF in order to identify changes in the abundance of proteins potentially associated with tumor growth. A comparative proteomic approach identified 17 proteins associated with cellular migration and proliferation with different abundance in leiomyoma IF.
Materials and methods
Samples
Tissue samples were obtained from eight premenopausal female patients who underwent hysterectomy for symptomatic uterine leiomyoma. Samples were collected between February 2015 and June 2015 at the Institute for Maternal and Child Health, IRCCS ‘Burlo Garofolo’ (Trieste, Italy). The procedures complied with the Declaration of Helsinki and were approved by the Technical and Scientific Committee of the Institute for Maternal and Child Health, IRCCS ‘Burlo Garofolo’ of Trieste, Italy (approval no. RC 19/08). All subjects signed a written informed consent. The patients did not receive any medication (including hormonal therapy) during the three months prior to surgery. All samples were obtained during the proliferative phase. Two samples were collected from each patient: One from the central area of the leiomyoma and one from the unaffected myometrium. The median age of the patients was 46 years (range, 42–52 years).
Preparation of IF
The IF was obtained as previously described (14,15). Two samples (300 mg each) were collected from each patient: One from the unaffected myometrium and one from the central area of the leiomyoma. All leiomyomas were confirmed histologically as benign ordinary leiomyomas. Leiomyoma and myometrium tissues were dissected into 0.1–0.3-cm3 pieces and rinsed three times with sterile PBS containing a protease inhibitor mixture (2 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 1 mM EDTA and 10 mM NaF). The samples were incubated with PBS in a humidified CO2 incubator for 1 h at 37°C. The supernatant was centrifuged at 5,000 × g for 20 min at 4°C, followed by centrifugation at 15,000 × g for 30 min at 4°C to remove cell debris. Approximately 5 ml of supernatant was desalted and concentrated using an Ultrafree-4 Centrifugal Filter Unit (EMD Millipore, Billerica, MA, USA) with a molecular mass cut-off of 10 kDa at 4,000 × g at 25°C until the remaining volume reached 200 µl. The obtained supernatant was designated as the IF, and the protein content of the supernatant was determined by Bradford assay.
2-DE and image analysis
For 2-DE, 700 µg of proteins from each IF sample were denatured in 350 µl of dissolution buffer [7 M urea, 2 M thiourea, 4% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate, 40 mM Tris, 65 mM dithiothreitol (DTT) and 0.24% Bio-Lyte 3/10 (Bio-Rad Laboratories, Inc., Hercules, CA, USA)] and 7 µg bromophenol blue. ReadyStrip™ pH 3–10 non-linear (NL) 18-cm immobilized pH gradient (IPG) strips (Bio-Rad Laboratories, Inc.) were rehydrated in a dissolution buffer at 50 V for 12 h at 20°C, and isoelectric focusing (IEF) was performed in a PROTEAN IEF Cell (Bio-Rad Laboratories, Inc.) using the following protocol: 150 V for 1 h, 250 V for 2 h, increase from 250 V to 10,000 V in 2 h, 10,000 V for 10 h and 10,000 V for 6 h, for a total of 170,000 V/h. Serial incubations were performed following IEF. First, the IPG strips were equilibrated for 20 min in an equilibration buffer [6 M urea, 2% SDS, 50 mM Tris-HCl (pH 8.8), 30% glycerol and 1% DTT] and then in another equilibration buffer containing 4% iodoacetamide instead of DTT. For the second dimension, the equilibrated IPG strips were transferred to a 12% polyacrylamide gel (19×20 cm). The SDS gels were subjected to electrophoresis on a Protean II XL Cell (Bio-Rad Laboratories, Inc.) at 200 V constant voltage, until the bromophenol blue band reached the bottom of the gel. Upon electrophoresis, the gels were fixed in 40% methanol and 10% acetic acid, and then stained for 48 h with colloidal Coomassie Brilliant Blue. Triple experimental replicates were performed per sample. Molecular masses were determined by comparison with Precision Plus Protein™ Prestained Standards (Bio-Rad Laboratories, Inc.), covering a range between 10 and 250 kDa. 2-DE gels were scanned with a Molecular Imager PharosFX system and analyzed using the Proteomweaver software version 4.0 (both from Bio-Rad Laboratories, Inc.).
Quantification of spot levels
Spot quantification was performed as previously described (12). 2-DE image analysis was performed using the Proteomweaver 4 software. The analysis was performed by matching all gels from eight myometrial IFs and eight leiomyoma IFs. Differences were considered to be significant when the ratio of the mean percentage of relative volume (%V), determined as %V=Vsingle spot/Vtotal spot, was 1.5-fold for upregulated and 0.6-fold for downregulated proteins, and satisfied the non-parametric Wilcoxon signed-rank test for matched samples (P<0.05). Fold change was calculated as the ratio between the mean %V of the uterine leiomyoma and that of the normal myometrium.
Trypsin digestion and matrix-assisted laser desorption/ionization (MALDI)-time-of-flight (TOF)/TOF analysis
Spots from 2-DE gels were thoroughly washed with alternating changes of 50 mM NH4HCO3 and acetonitrile (ACN), and then dried under vacuum. A total of 5 µl of sequencing-grade modified trypsin (12.5 ng/ml in 50 mM NH4HCO3; Promega Corporation, Madison, WI, USA) were added to each gel spot, and samples were kept in ice for 30 min to allow the gel to completely rehydrate. A total of 50 mM NH4HCO3 was added to each sample until gel pieces were completely covered, and digestion was carried out overnight at 37°C. Peptides were extracted with three changes of 50% ACN/0.1% trifluoroacetic acid (TFA; Honeywell Riedel-de Haën, Seelze, Germany), dried under vacuum and then dissolved in 10 µl of 0.1% TFA. One µl of each sample was mixed with an equal volume of matrix [α-cyano-4-hydroxycinnamic acid (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 5 mg/ml in 70% ACN/0.1% TFA], and 0.8 µl of the resulting solution was spotted onto a stainless steel MALDI target plate (AB SCIEX, Framingham, MA, USA). Mass spectrometry (MS)/MS analysis was performed on a 4800 MALDI-TOF/TOF Analyzer (AB SCIEX) in a data-dependent mode: For each sample, a full MS scan was acquired, followed by MS/MS spectra of the 10 most intense signals.
Data were converted into Mascot generic format files and analyzed with Proteome Discoverer software (version 1.4; Thermo Fisher Scientific, Inc., Waltham, MA, USA) interfaced to a Mascot search engine (version 2.2.4; Matrix Science Ltd., London, UK). Data were searched against the human section of the UniProt database (www.uniprot.org; version 20,150,401; 90,411 sequences). Enzyme specificity was set to trypsin with ≤1 missed cleavage. Tolerance was set to 50 ppm and 0.3 Da for parent and fragment ions, respectively. Carbamidomethylation of cysteines was set as a fixed modification, while methionine oxidation was set as a variable modification. The software calculates the false discovery rate based on the search against a randomized database. Proteins were considered as positively identified if ≥2 unique peptides were identified for each protein with high confidence (q<0.01).
Prediction of secreted protein candidates
SecretomeP 2.0 (Center for Biological Sequence Analysis, Technical University of Denmark, Lyngby, Denmark) was used to classify proteins as following a classical (proteins with signal peptide) or non-classical (proteins without signal peptide but whose neural network score exceeded the normal threshold of 0.5) secretory pathway. To verify the possible release of proteins by exosomes and microvesicles, manual annotation on the Exocarta (exocarta.org) and Vesiclepedia (microvesicles.org/index.html) databases was used.
Western blotting
Western blot analysis was performed as previously described (16). An equal amount of protein to that used for 2-DE analysis (30 µg) was separated by 12% SDS-PAGE and then transferred to a nitrocellulose membrane. The residual binding sites on the membrane were blocked by treatment with 5% skim milk in TBS containing 0.05% Tween-20 (TBST) for 2 h at room temperature. Next, the membrane was incubated overnight at 4°C with 1:500-diluted primary rabbit polyclonal antibody against cellular retinoic acid binding protein 2 (CRABP2; cat. no. SAB2100477), 1:300-diluted primary rabbit polyclonal antibody against phosphoglycerate mutase 1 (PGAM1; cat. no. WH0005223M1), 1:800-diluted primary rabbit polyclonal antibody against aldolase A (ALDOA; cat. no. SAB1410385), 1:700-diluted primary rabbit polyclonal antibody against LUM (cat. no. SAB1401232), 1:500-diluted primary rabbit polyclonal antibody against annexin A2 (ANXA2; cat. no. SAB1410612), 1:1,000-diluted primary rabbit polyclonal antibody against calreticulin (CALR; cat. no. AV30114) and 1:200-diluted primary rabbit polyclonal antibody against peroxiredoxin (PRDX1; cat. no. HPA007730) (all antibodies were supplied by Sigma-Aldrich; Merck KGaA). The membrane was washed three times in TBST for 10 min, and then incubated for 2 h at 4°C with a horseradish peroxidase-conjugated anti-rabbit immunoglobulin G antibody (cat. no. A8275) (Sigma-Aldrich; Merck KGaA) at 1:3,000 dilution. Protein expression was visualized by chemiluminescence (SuperSignal West Pico Chemiluminescent Substrate; Thermo Fisher Scientific, Inc.), and the intensity of the signals was quantified by VersaDoc Imaging System (Bio-Rad Laboratories, Inc.). The intensities of the immunostained bands were normalized with the protein intensities measured by Red Ponceau S reagent (Sigma-Aldrich; Merck KGaA) from the same blot.
Ingenuity Pathway Analysis (IPA)
Changes in protein abundance identified by MS in leiomyoma IF were analyzed by IPA (Qiagen GmbH, Hilden, Germany) as previously described (16). Selected genes were incorporated in canonical pathways and bio-functions, and were used to generate biological networks. For the filter summary, only associations where confidence was high (predicted) or that had been experimentally observed were considered. As cut-off, and adjusted P<0.05 value was used. The network score was based on the hypergeometric distribution, and was calculated with a two-tailed Fisher's exact test. Since the majority of proteins identified participated in multiple processes, only the most relevant ones were reported.
Statistical analysis
Statistical analyses were carried out with the non-parametric Wilcoxon signed-rank test for matched samples for both 2-DE and western blot data. P<0.05 was considered to indicate a statistically significant difference. All analyses were conducted with Stata/IC 14.1 for Windows (StataCorp LP, College Station, TX, USA).
Results
2-DE and MS
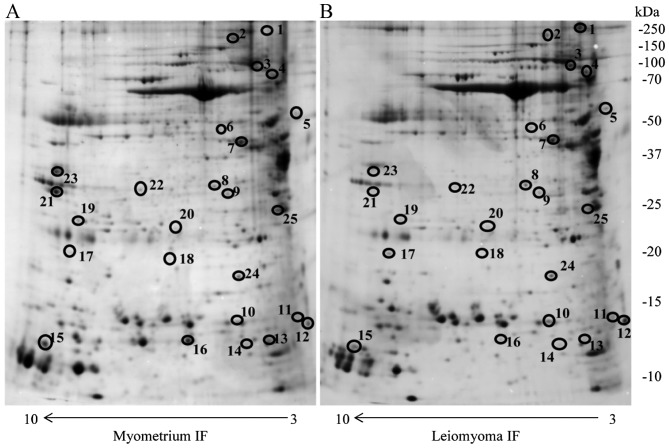
In the present study, the IF of eight leiomyomas and myometria from eight premenopausal women were resolved using 2-DE. Approximately 2,000 protein spots were detected in the 2-DE gels for the two types of IFs. Correlation of gel-pairs performed with an average matching efficiency of ~75%. The analysis revealed 25 protein spots with different abundance in the IF of leiomyoma compared with that of the IF of the myometrium. Only protein spots with fold change in %V ≥1.5 or ≤0.6 in intensity that were significant (P<0.05) were considered (Fig. 1). These proteins were identified by MALDI-TOF/TOF and database search against the human section of the UniProt database (version 20150401; 90,411 sequences) (Table I).
Figure 1.
Two-dimensional electrophoresis map of the (A) normal myometrium IF and (B) leiomyoma IF proteome. Immobilized pH gradient pH 3–10 non-linear strips were used for the first dimension and 12% polyacrylamide gels were used for the second dimension. IF, interstitial fluid. Circles indicate different proteins identified.
Table I.
List of proteins with significantly different abundance in the interstitial fluid of the leiomyoma compared with that of the normal myometrium, as identified by matrix-assisted laser desorption/ionization-TOF/TOF mass spectrometry, and classified by their corresponding molecular and cellular functions and subcellular localization. Only the most relevant molecular and cellular functions are reported.
| Accession no.a | Spot no. | Protein description | Gene symbol | Protein score | Fold changeb | Molecular and cellular functions | Secretome P 2.0 | Exocarta | Veciclepedia | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| P51884 | 4 | Lumican | LUM | 122.78 | 6.0 | Cell migration | SignalP | 0.0139 | ||
| Q5HY54 | 1 | Filamin A | FLNA | 362.86 | 4.8 | Cellular assembly and organization | 0.45 | Yes | Yes | 0.0193 |
| P29373 | 14 | Cellular retinoic acid | CRABP2 | 142.69 | 3.0 | Cell proliferation | 0.59 | 0.0140 | ||
| binding protein 2 | ||||||||||
| Q06830 | 17 | Peroxiredoxin 1 | PRDX1 | 394.64 | 3.0 | Cell migration | 0.53 | 0.0173 | ||
| P05387 | 11 | Ribosomal protein lateral | RPLP2 | 288.99 | 2.7 | Not known | 0.27 | Yes | Yes | 0.0296 |
| stalk subunit P2 | ||||||||||
| P27797 | 5 | Calreticulin | CALR | 642.74 | 2.5 | Cell migration | SignalP | 0.0173 | ||
| P32119 | 24 | Peroxiredoxin 2 | PRDX2 | 161.45 | 2.0 | Cell proliferation | 0.52 | 0.0117 | ||
| A0A087WV45 | 10 | Transthyretin | TTR | 100.29 | 1.80 | Not known | SignalP | 0.0295 | ||
| P18669 | 20 | Phosphoglycerate mutase 1 | PGAM1 | 450.56 | 1.80 | Cell proliferation | 0.41 | Yes | Yes | 0.0251 |
| P55072 | 3 | Valosin-containing protein | VCP | 345.34 | 1.70 | Cell migration | 0.16 | Yes | Yes | 0.0296 |
| P60709 | 9 | β-actin | ACTB | 316.16 | 1.70 | Cell migration | 0.50 | Yes | Yes | 0.0418 |
| B4DXW1 | 6 | Actin-related protein 3 | ACTR3 | 400.00 | 1.60 | Cell migration | 0.44 | Yes | Yes | 0.0296 |
| P62158 | 12 | Calmodulin 1 | CALM1 | 283.75 | 1.60 | Cell migration | 0.45 | Yes | Yes | 0.0497 |
| P07195 | 8 | L-lactate dehydrogenase | LDHB | 457.75 | 1.50 | Cell proliferation | 0.57 | 0.0249 | ||
| B chain | ||||||||||
| K7EJ44 | 15 | Profilin 1 | PFN1 | 263.83 | 0.60 | Cellular assembly and | 0.47 | Yes | Yes | 0.0296 |
| organization | ||||||||||
| P04083 | 21 | Annexin 1 | ANXA1 | 671.52 | 0.60 | Cell proliferation | 0.51 | 0.0296 | ||
| P12277 | 7 | Creatine kinase B | CKB | 466.67 | 0.48 | Cellular assembly | 0.26 | Yes | Yes | 0.0357 |
| and organization | ||||||||||
| Q9Y490 | 2 | Talin 1 | TLN1 | 156.87 | 0.30 | Cellular assembly | 0.23 | Yes | Yes | 0.0193 |
| and organization | ||||||||||
| P07355 | 22 | Annexin 2 | ANXA2 | 442.58 | 0.19 | Cell proliferation | 0.75 | 0.0117 | ||
| E7EP94 | 25 | Heat shock protein family | HSPA1A | 60.00 | 0.16 | Cell migration | 0.28 | Yes | Yes | 0.0193 |
| A (Hsp70) member 1A | ||||||||||
| Q01995 | 16 | Transgelin | TAGLN | 280.64 | 0.16 | Cellular function | 0.56 | 0.0117 | ||
| and maintenance | ||||||||||
| H3BQN4 | 23 | Aldolase A | ALDOA | 370.58 | 0.10 | Cell migration | 0.36 | Yes | Yes | 0.0192 |
| Q5T0R7 | 19 | Adenylyl cyclase-associated | CAP1 | 374.82 | 0.06 | Cell migration | 0.44 | Yes | Yes | 0.0293 |
According to UniProt database (version 20150401; 90,411 sequences)
fold change was defined as the ratio of the mean %V according to the formula %V=Vsingle spot/Vtotal spot of uterine leiomyoma and normal myometrium. TOF, time.of.flight; %V, percentage of relative volume
In addition, proteins reported in our previous study, including desmin (DES), transgelin (TAGLN), serpin family A member 1 (SERPINA1), lamin A/C (LMNA), actinin α1 (ACTN1), PRDX2 and heat shock protein family A (Hsp70) member 1A (HSPA1A), were also identified (14). Of these proteins, TAGLN, PRDX2 and HSPA1A were significant, while DES, SERPINA1 (more abundant in the leiomyoma compared with in the myometrium), LMNA and ACTN1 (less abundant in the leiomyoma compared with in the myometrium) were not significant, and thus were not investigated further.
In total, 22 of the 25 proteins with different abundance reported in the present study have not been previously identified in the leiomyoma IF. Furthermore, among the identified proteins, one (namely PGAM1) has never been previously associated with leiomyoma.
Prediction of protein secretion and functional analysis of the leiomyoma proteome
To define if the 25 identified proteins were secreted by the classical or non-classical secretory pathway, the SignalIP version 4.1 (www.cbs.dtu.dk/services/SignalP) and SecretomeP 2.0 (www.cbs.dtu.dk/services/SecretomeP) prediction servers were used. In addition, the Vesiclepedia and Exocarta databases, which contain proteins identified in extracellular vesicles and exosomes released by non-classical secretory pathways respectively, were used. Out of the 25 proteins, three were assigned to the classical secretory pathway predicted by their signal peptide: LUM, CALR and transthyretin. The remaining proteins were predicted to be secreted by non-classical pathways to the extracellular space through various mechanisms (Table I).
IPA
Proteins with significantly different abundance in the IF of leiomyoma compared with that of the myometrium were used in the core analysis with IPA software. The analysis revealed that the identified proteins were associated with cellular migration, proliferation, assembly, organization, function and maintenance.
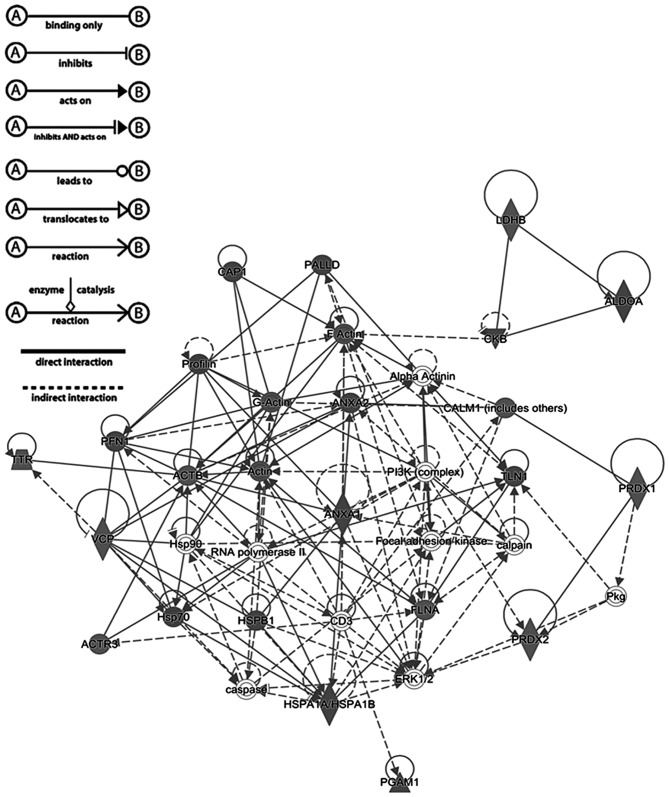
A total of 17 proteins were observed to be associated with cellular migration and proliferation according to the IPA prediction (Table I). Fig. 2 shows a graphical representation of the predicted interaction between the proteins with significantly different abundance identified in the present study. This network (score, 56) includes 25 identified proteins involved in neurological diseases, cellular migration, and skeletal and muscular disorders. The representative interaction network suggests that the proteins with changes in abundance identified in the present study may influence the activity of other proteins within the network.
Figure 2.
Top-scored molecular networks involving proteins with different abundance in the leiomyoma interstitial fluid, according to the results of Ingenuity Pathway Analysis software. Proteins in the network are represented by their gene symbols.
Immunohistochemical study of protein expression
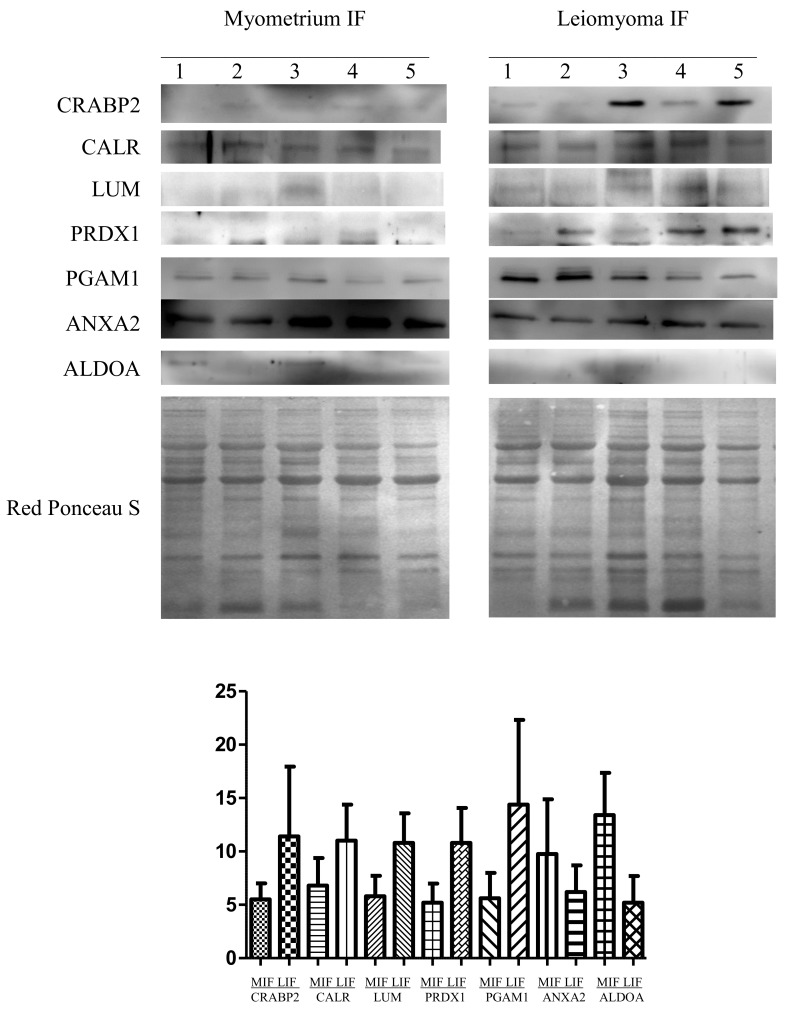
Proteins with different abundance in the leiomyoma IF (namely LUM, PRDX, CALR, CRABP2, ANXA2, ALDOA and PGAM1) were further validated by western blotting in five leiomyomas (IF) vs. normal myometrium (IF) (Fig. 3). The abundance of LUM, PRDX1, CALR, CRABP2 and PGAM1 was significantly higher in leiomyomas than in the myometrium, while for ANXA2 and ALDOA, their abundance was significantly lower in leiomyomas with respect to that in the myometrium, thus confirming the results obtained from the 2-DE analysis. To normalize the results of the western blot analysis, the total protein content of each sample, as determined by Red Ponceau S staining, was used. According to our previous results (12), proteins encoded by housekeeping genes (i.e., β-actin and tubulin) are usually upregulated in leiomyomas and, therefore, not adequate to be used as controls for normalization.
Figure 3.
Western blot analysis of CRABP2, CALR, LUM, PRDX1, PGAM1, ANXA2 and ALDOA in paired MIF and LIF. The intensity of the immunostained bands was normalized against the total protein intensities measured from the same blot stained with Red Ponceau S. The bar graph shows the relative expression (band density) of proteins in the MIF and LIF. The results are shown as a histogram (mean) with error bars representing the standard deviation. All differences were observed to be significant (Wilcoxon signed-rank test for matched samples, P<0.05). MIF, myometrium IF; LIF, leiomyoma IF; IF, interstitial fluid; CRABP2, cellular retinoic acid binding protein 2; CALR, calreticulin; LUM, lumican; PRDX, peroxiredoxin; PGAM1, phosphoglycerate mutase 1; ANXA2; annexin A2; ALDOA, aldolase A.
Discussion
In tumor diseases, secreted proteins create conditions favorable to the disorder, promoting tumor progression and growth (17). Proteomic studies are essential for the acquisition of knowledge about the qualitative and quantitative composition of the IF of the cell, and may be crucial to understand the biology of cell interaction, proliferation, differentiation, communication and migration. In a previous study, seven proteins that were differently expressed in the IF of leiomyomas compared with that of the normal myometrium were identified (13). The present study continued to investigate different abundant proteins likely to be associated with tumor development in the IF.
The adoption of 18-cm pH 3–10 NL strips with polyacrylamide gels of 19×20 cm in size instead of 7-cm pH 3–10 strips with polyacrylamide gels of 8×8 cm in size markedly increased the spot number. This allowed the identification of 22 proteins that had not been previously identified in the leiomyoma IF, while PGAM1 had never previously been associated with leiomyoma. IPA analysis predicted that 17 out of the 25 proteins are associated with cellular migration and proliferation. In addition, the abundance of the proteins identified in our previous study (DES, TAGLN, SERPINA1, LMNA, ACTN1, PRDX2 and HSPA1A) is in line with the results of the present study on the leiomyoma IF. Furthermore, the levels of CRABP2, CALM1, LDHB, SERPINA1 and DES were confirmed to be upregulated, while those of TAGLN, LMNA and ACTN1 were downregulated, both in the proteome (12,13) and in the IF. Seven of these proteins (LUM, PRDX1, CRABP2, CALR, ANXA2, ALDOA and PGAM1) were validated by western blotting, thus confirming the 2-DE data.
An inadequate vascularization during tumor growth results in microenvironmental stress, which induces the activation of a range of stress response pathways, including the unfolding protein response (UPR) (18). UPR activation is required for tumor cell growth under hypoxic conditions (18). CALR is a protein involved in the UPR pathway. It participates in several biological processes, including cell adhesion, gene expression, RNA stability and regulation of Ca2+ homeostasis (19,20). Cell stress increases the concentration of nitric oxide, inducing the release of CALR from cells in the extracellular space (21). This may be associated with alterations in the viscosity and elasticity of the ECM.
The presence of adenylyl cyclase-associated protein 1 (CAP1) in the extracellular environment was documented in the urine of patients with systemic autoimmune diseases, and was identified as an efficient substrate of gelatinase B/matrix metallopeptidase (MMP)-9 (22). Degradation of CAP1 by MMP-9 is important for tumor proliferation (22), as it may accelerate ECM accumulation, thereby promoting tumor growth.
Inflammatory and pro-inflammatory factors serve a key role in cancer development with the involvement of the transcription factor nuclear factor (NF)-κB, which also has a crucial role in cell growth, tumorigenesis and apoptosis (23). Several pro-inflammatory factors, including NF-κB, interleukin-1β, tumor necrosis factor-α and cyclooxygenase-2, are involved in the cellular proliferation and growth of leiomyomas (23). PRDX1 and PRDX2 are released by cells in the presence of pro-inflammatory factors. Their release stimulates the production of inflammatory cytokines, thus increasing the proliferative effect, which serves a role in matrix production (24,25).
CRABP2 is a key protein for the binding and transport of retinoic acid (26). Increasing levels of retinoic acid induce the overexpression of MMP-11, which degrades insulin-like growth factor-binding proteins, and may result in cell proliferation, decreased apoptosis and acceleration of ECM accumulation, thereby promoting the growth of uterine leiomyomas (27).
The present study identified two glycolytic enzymes (ALDOA and PGAM1) with different abundance in the leiomyoma IF. This further confirms that glycolytic enzymes can be secreted by cells, attest metabolic dysregulation in leiomyomas (12) and perform non-enzymatic activities such as structural or regulatory functions at their extracellular localization (28).
ANXA2 is a Ca2+-dependent phospholipid binding protein able to bind plasminogen and mediate the localized generation of plasmin (29). The present study has shown that the IF of leiomyomas is characterized by a lower abundance compared with the myometrium, of ALDOA and ANXA2, which could be associated with a decreased proteolytic degradation of the ECM in leiomyomas.
HSPA1A is a member of the Hsp70 family (30). In cooperation with other chaperones, HSPA1A mediates the folding of newly translated polypeptides in the cytosol, as well as within organelles (31). Once secreted, HSPA1A participates in paracrine or autocrine interactions with adjacent cell surfaces, controlling the expression of several genes associated with apoptosis and cell migration (32,33).
It is well known that leiomyomas are characterized by an abnormal ECM production (6). Proteoglycan components of the ECM such as LUM bind to integrin, activating the integrin signaling pathway, and leading to cytoskeletal rearrangement and cell motility (34). Physiologically, LUM is involved in several biological processes, including the glycosaminoglycan metabolic process, angiogenesis, cell proliferation, migration, adhesion and ECM organization (35). Leiomyoma growth may be closely associated with secreted LUM, which is in turn associated with cell motility and growth.
IPA revealed remarkable data on the interaction among proteins. Several proteins identified in the present study (including PRDX2, ANXA1, ANXA2, HSPA1A, filamin Aα, CALR, calmodulin 1 and talin 1) interact substantially with extracellular signal-regulated kinase-1/2, platelet-derived growth factor-BB and calpain, which serve a key role in cell growth, adhesion, survival and differentiation (36,37).
In conclusion, the comparative proteomic approach conducted in the present study identified a number of proteins associated with cellular migration and proliferation with different abundance in the IF. Secreted proteins may be involved in mechanisms associated with leiomyoma development. Further studies will be required to investigate the role of these proteins in the leiomyoma IF and their association with tumor development, in order to identify novel therapeutic targets and develop new pharmacological approaches for the treatment of leiomyoma.
Acknowledgements
The authors acknowledge the help of Dr Emmanouil Athanasakis (Medical Genetics Department, Institute for Maternal and Child Health-IRCCS ‘Burlo Garofolo’, Trieste, Italy) for data acquisition. The present study was internally funded by the Institute for Maternal and Child Health-IRCCS ‘Burlo Garofolo’ of Trieste, Italy (grant no. RC 19/08).
References
- 1.Hashimoto K, Azuma C, Kamiura S, Kimura T, Nobunaga T, Kanai T, Sawada M, Noguchi S, Saji F. Clonal determination of uterine leiomyomas by analyzing differential inactivation of the X-chromosome-linked phosphoglycerokinase gene. Gynecol Obstet Invest. 1995;40:204–208. doi: 10.1159/000292336. [DOI] [PubMed] [Google Scholar]
- 2.Haney AF. Clinical decision making regarding leiomyomata: What we need in the next millennium. Environ Health Perspect. 2000;108:S835–S839. doi: 10.1289/ehp.00108s5835. (Suppl 5) [DOI] [PubMed] [Google Scholar]
- 3.Borahay MA, Al-Hendy A, Kilic GS, Boehning D. Signaling pathways in leiomyoma: Understanding pathobiology and implications for therapy. Mol Med. 2015;21:242–256. doi: 10.2119/molmed.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catherino WH, Malik M. Uterine leiomyomas express a molecular pattern that lowers retinoic acid exposure. Fertil Steril. 2007;87:1388–1398. doi: 10.1016/j.fertnstert.2006.11.107. [DOI] [PubMed] [Google Scholar]
- 5.Arslan AA, Gold LI, Mittal K, Suen TC, Belitskaya-Levy I, Tang MS, Toniolo P. Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: New evidence and a systematic review. Hum Reprod. 2005;20:852–863. doi: 10.1093/humrep/deh698. [DOI] [PubMed] [Google Scholar]
- 6.Malik M, Catherino WH. Novel method to characterize primary cultures of leiomyoma and myometrium with the use of confirmatory biomarker gene arrays. Fertil Steril. 2007;87:1166–1172. doi: 10.1016/j.fertnstert.2006.08.111. [DOI] [PubMed] [Google Scholar]
- 7.Gromov P, Gromova I, Olsen CJ, Timmermans-Wielenga V, Talman ML, Serizawa RR, Moreira JM. Tumor interstitial fluid-a treasure trove of cancer biomarkers. Biochim Biophys Acta. 2103;1834:2259–2270. doi: 10.1016/j.bbapap.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Spano D, Zollo M. Tumor microenvironment: A main actor in the metastasis process. Clin Exp Metastasis. 2012;29:381–395. doi: 10.1007/s10585-012-9457-5. [DOI] [PubMed] [Google Scholar]
- 9.Norian JM, Owen CM, Taboas J, Korecki C, Tuan R, Malik M, Catherino WH, Segars JH. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31:57–65. doi: 10.1016/j.matbio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lv J, Zhu X, Dong K, Lin Y, Hu Y, Zhu C. Reduced expression of 14-3-3 gamma in uterine leiomyoma as identified by proteomics. Fertil Steril. 2008;90:1892–1898. doi: 10.1016/j.fertnstert.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 11.Lemeer S, Gholami AM, Wu Z, Kuster B. Quantitative proteome profiling of human myoma and myometrium tissue reveals kinase expression signatures with potential for therapeutic intervention. Proteomics. 2015;15:356–364. doi: 10.1002/pmic.201400213. [DOI] [PubMed] [Google Scholar]
- 12.Ura B, Scrimin F, Arrigoni G, Franchin C, Monasta L, Ricci G. A proteomic approach for the identification of up-regulated proteins involved in the metabolic process of the leiomyoma. Int J Mol Sci. 2016;17:540. doi: 10.3390/ijms17040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ura B, Scrimin F, Arrigoni G, Athanasakis E, Aloisio M, Monasta L, Ricci G. Abnormal expression of leiomyoma cytoskeletal proteins involved in cell migration. Oncol Rep. 2016;35:3094–3100. doi: 10.3892/or.2016.4688. [DOI] [PubMed] [Google Scholar]
- 14.Ura B, Scrimin F, Zanconati F, Arrigoni G, Monasta L, Romano A, Banco R, Zweyer M, Milani D, Ricci G. Two-dimensional gel electrophoresis analysis of the leiomyoma interstitial fluid reveals altered protein expression with a possible involvement in pathogenesis. Oncol Rep. 2015;33:2219–2226. doi: 10.3892/or.2015.3827. [DOI] [PubMed] [Google Scholar]
- 15.Gromov P, Gromova I, Bunkenborg J, Cabezon T, Moreira JM, Timmermans-Wielenga V, Roepstorff P, Rank F, Celis JE. Up-regulated proteins in the fluid bathing the tumour cell microenvironment as potential serological markers for early detection of cancer of the breast. Mol Oncol. 2010;4:65–89. doi: 10.1016/j.molonc.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mischiati C, Ura B, Roncoroni L, Elli L, Cervellati C, Squerzanti M, Conte D, Doneda L, de Polverino Laureto P, de Franceschi G, et al. Changes in protein expression in two cholangiocarcinoma cell lines undergoing formation of multicellular tumor spheroids in vitro. PLoS One. 2015;10:e0118906. doi: 10.1371/journal.pone.0118906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, McLachlan JA. Estrogen-associated genes in uterine leiomyoma. Ann N Y Acad Sci. 2001;948:112–120. doi: 10.1111/j.1749-6632.2001.tb03992.x. [DOI] [PubMed] [Google Scholar]
- 18.Desiniotis A, Kyprianou N. Significance of talin in cancer progression and metastasis. Int Rev Cell Mol Biol. 2011;289:117–147. doi: 10.1016/B978-0-12-386039-2.00004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandewynckel YP, Laukens D, Geerts A, Bogaerts E, Paridaens A, Verhelst X, Janssens S, Heindryckx F, Van Vlierberghe H. The paradox of the unfolded protein response in cancer. Anticancer Res. 2013;33:4683–4694. [PubMed] [Google Scholar]
- 20.Totary-Jain H, Naveh-Many T, Riahi Y, Kaiser N, Eckel J, Sasson S. Calreticulin destabilizes glucose transporter-1 mRNA in vascular endothelial and smooth muscle cells under high-glucose conditions. Circ Res. 2005;97:1001–1008. doi: 10.1161/01.RES.0000189260.46084.e5. [DOI] [PubMed] [Google Scholar]
- 21.Tarr JM, Young PJ, Morse R, Shaw DJ, Haigh R, Petrov PG, Johnson SJ, Winyard PG, Eggleton P. A mechanism of release of calreticulin from cells during apoptosis. J Mol Biol. 2010;401:799–812. doi: 10.1016/j.jmb.2010.06.064. [DOI] [PubMed] [Google Scholar]
- 22.Cauwe B, Martens E, Van den Steen PE, Proost P, Van Aelst I, Blockmans D, Opdenakker G. Adenylyl cyclase-associated protein-1/CAP1 as a biological target substrate of gelatinase B/MMP-9. Exp Cell Res. 2008;314:2739–2749. doi: 10.1016/j.yexcr.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Plewka A, Madej P, Plewka D, Kowalczyk A, Miskiewicz A, Wittek P, Leks T, Bilski R. Immunohistochemical localization of selected pro-inflammatory factors in uterine myomas and myometrium in women of various ages. Folia Histochem Cytobiol. 2013;51:73–83. doi: 10.5603/FHC.2013.0011. [DOI] [PubMed] [Google Scholar]
- 24.Mullen L, Hanschmann EM, Lillig CH, Herzenberg LA, Ghezzi P. Cysteine oxidation targets peroxiredoxins 1 and 2 for exosomal release through a novel mechanism of redox-dependent secretion. Mol Med. 2015;13:98–108. doi: 10.2119/molmed.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubota D, Mukaihara K, Yoshida A, Tsuda H, Kawai A, Kondo T. Proteomics study of open biopsy samples identifies peroxiredoxin 2 as a predictive biomarker of response to induction chemotherapy in osteosarcoma. J Proteomics. 2013;91:393–404. doi: 10.1016/j.jprot.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Aström A, Pettersson U, Voorhees JJ. Structure of the human cellular retinoic acid-binding protein II gene. Early transcriptional regulation by retinoic acid. J Biol Chem. 1992;267:25251–25255. [PubMed] [Google Scholar]
- 27.Zaitseva M, Vollenhoven BJ, Rogers PA. Retinoic acid pathway genes show significantly altered expression in uterine fibroids when compared with normal myometrium. Mol Hum Reprod. 2007;13:577–585. doi: 10.1093/molehr/gam040. [DOI] [PubMed] [Google Scholar]
- 28.Capello M, Ferri-Borgogno S, Cappello P, Novelli F. α-Enolase: A promising therapeutic and diagnostic tumor target. FEBS J. 2011;278:1064–1074. doi: 10.1111/j.1742-4658.2011.08025.x. [DOI] [PubMed] [Google Scholar]
- 29.Valapala M, Maji S, Borejdo J, Vishwanatha JK. Cell surface translocation of annexin A2 facilitates glutamate-induced extracellular proteolysis. J Biol Chem. 2014;289:15915–15926. doi: 10.1074/jbc.M113.511550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hageman J, Vos MJ, van Waarde MA, Kampinga HH. Comparison of intra-organellar chaperone capacity for dealing with stress-induced protein unfolding. J Biol Chem. 2007;282:34334–34345. doi: 10.1074/jbc.M703876200. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Barbi J, Bu S, Yang HY, Li Z, Gao Y, Jinasena D, Fu J, Lin F, Chen C, et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity. 2013;39:272–285. doi: 10.1016/j.immuni.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mambula SS, Stevenson MA, Ogawa K, Calderwood SK. Mechanisms for Hsp70 secretion: Crossing membranes without a leader. Methods. 2007;43:168–175. doi: 10.1016/j.ymeth.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniels GA, Sanchez-Perez L, Diaz RM, Kottke T, Thompson J, Lai M, Gough M, Karim M, Bushell A, Chong H. A simple method to cure established tumors by inflammatory killing of normal cells. Nat Biotechnol. 2004;22:1125–1132. doi: 10.1038/nbt1007. [DOI] [PubMed] [Google Scholar]
- 34.Martin San S, Soto-Suazo M, De Oliveira SF, Aplin JD, Abrahamsohn P, Zorn TM. Small leucine-rich proteoglycans (SLRPs) in uterine tissues during pregnancy in mice. Reproduction. 2003;125:585–595. doi: 10.1530/rep.0.1250585. [DOI] [PubMed] [Google Scholar]
- 35.Nikitovic D, Papoutsidakis A, Karamanos NK, Tzanakakis GN. Lumican affects tumor cell functions, tumor-ECM interactions, angiogenesis and inflammatory response. Matrix Biol. 2014;35:206–214. doi: 10.1016/j.matbio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med. 2009;15:75–83. doi: 10.1038/nm.1893. [DOI] [PubMed] [Google Scholar]
- 37.Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N, Brenguier L, Devaud C, Pasturaud P, Roudaut C, et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell. 1995;81:27–40. doi: 10.1016/0092-8674(95)90368-2. [DOI] [PubMed] [Google Scholar]