Abstract
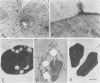
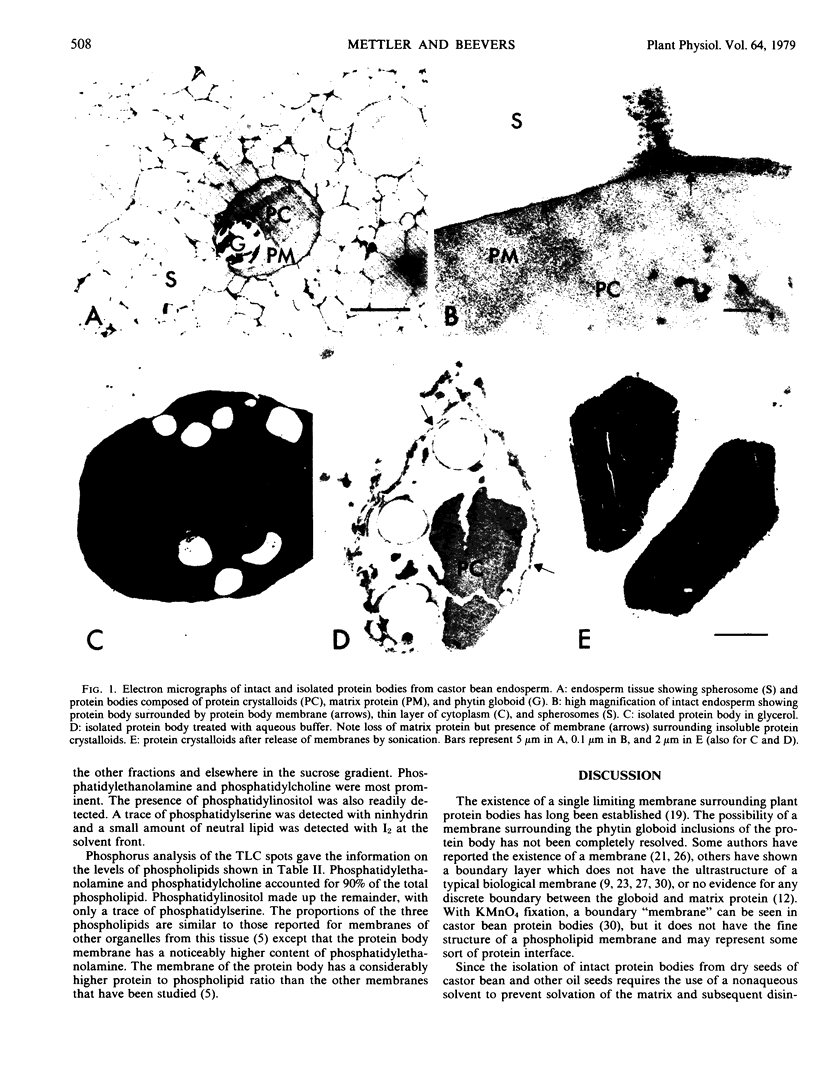
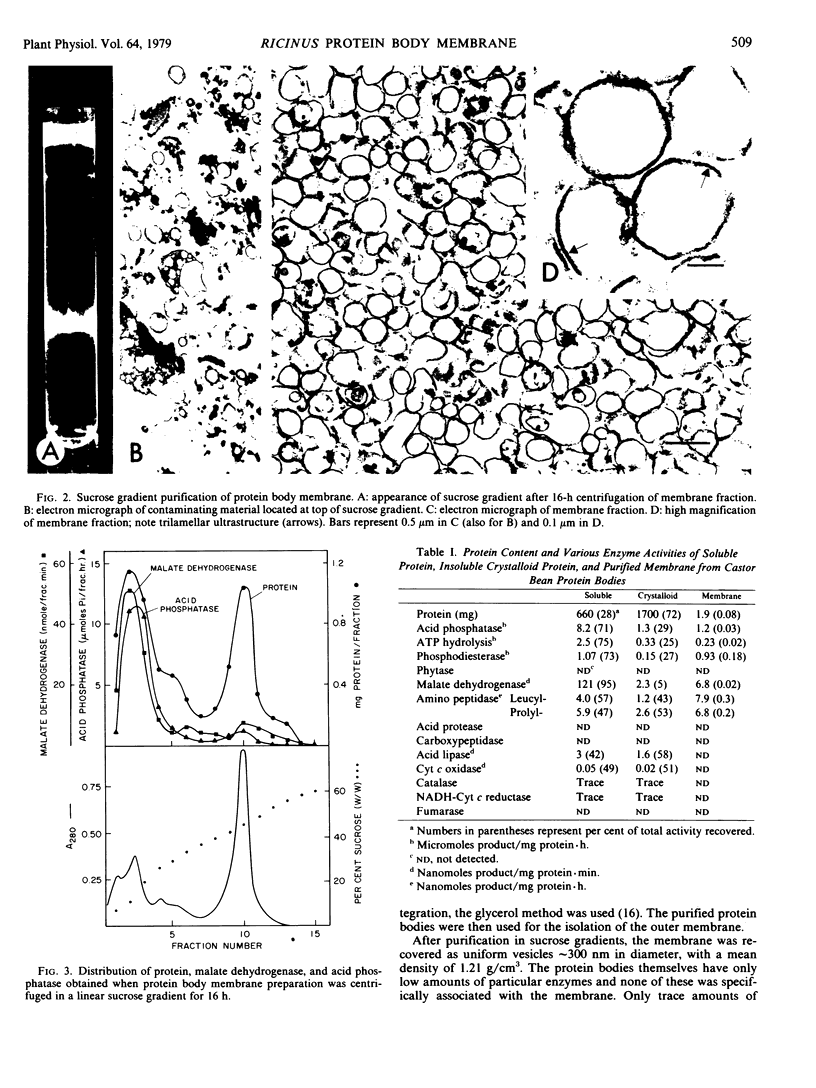
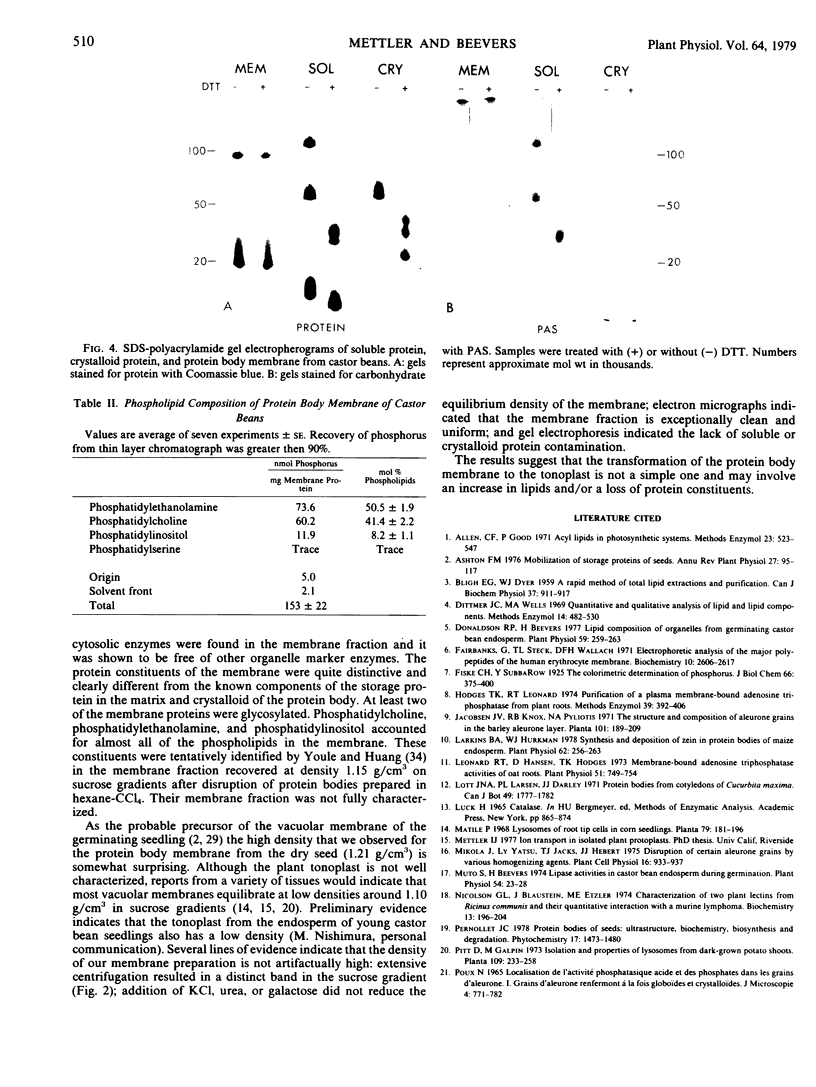
Intact protein bodies were isolated from dry castor bean seeds (Ricinus communis L.) after homogenization in nonaqueous medium. After repeated washing with glycerol to remove trapped lipid globules, the soluble matrix proteins were removed by the addition of aqueous buffer. The membrane remained attached to the insoluble protein crystalloids and was subsequently released by sonication. Purification of the membrane vesicles in a sucrose gradient produced a single band at a density of 1.21 grams per cubic centimeter. Treatment with 6 molar urea, 1 molar KCl, or 0.25 molar galactose had no effect on the equilibrium density of the membrane. Electron microscopy revealed a highly pure and uniform collection of membrane vesicles. No enzyme activity was specifically associated with the membrane. Sodium dodecyl sulfate gel electrophoresis of the protein body fractions showed that the membrane contained unique proteins, two of which were glycosylated. The membrane contained 153 nanomoles of phospholipid per milligram of protein. The composition of the phosphoglycerides was 51% ethanolamine, 41% choline, 8% inositol, and a trace of serine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Donaldson R. P., Beevers H. Lipid composition of organelles from germinating castor bean endosperm. Plant Physiol. 1977 Feb;59(2):259–263. doi: 10.1104/pp.59.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Hurkman W. J. Synthesis and deposition of zein in protein bodies of maize endosperm. Plant Physiol. 1978 Aug;62(2):256–263. doi: 10.1104/pp.62.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Beevers H. Lipase Activities in Castor Bean Endosperm during Germination. Plant Physiol. 1974 Jul;54(1):23–28. doi: 10.1104/pp.54.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Swift J. G., Buttrose M. S. Freeze-etch studies of protein bodies in wheat scutellum. J Ultrastruct Res. 1972 Aug;40(3):378–390. doi: 10.1016/s0022-5320(72)90108-6. [DOI] [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Proteases and Peptidases of Castor Bean Endosperm: Enzyme Characterization and Changes during Germination. Plant Physiol. 1978 Nov;62(5):746–750. doi: 10.1104/pp.62.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Protein bodies of castor bean endosperm: isolation, fractionation, and the characterization of protein components. Plant Physiol. 1976 Dec;58(6):710–716. doi: 10.1104/pp.58.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B., Beevers H. Phosphatidic Acid synthesis in castor bean endosperm. Plant Physiol. 1977 Mar;59(3):459–463. doi: 10.1104/pp.59.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Association of lysosomal activity with aleurone grains in plant seeds. Arch Biochem Biophys. 1968 Mar 20;124(1):466–471. doi: 10.1016/0003-9861(68)90354-8. [DOI] [PubMed] [Google Scholar]
- Youle R. J., Huang A. H. Albumin storage proteins in the protein bodies of castor bean. Plant Physiol. 1978 Jan;61(1):13–16. doi: 10.1104/pp.61.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle R. J., Huang A. H. Evidence that the castor bean allergens are the albumin storage proteins in the protein bodies of castor bean. Plant Physiol. 1978 Jun;61(6):1040–1042. doi: 10.1104/pp.61.6.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle R. J., Huang A. H. Protein Bodies from the Endosperm of Castor Bean: Subfractionation, Protein Components, Lectins, and Changes during Germination. Plant Physiol. 1976 Dec;58(6):703–709. doi: 10.1104/pp.58.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]