Abstract
Malaria, caused by Plasmodium parasites, is thought to be one of the strongest selective forces that has shaped the genome of modern humans and was endemic in Europe until recent times. Due to its eradication around mid-twentieth century, the potential selective history of malaria in European populations is largely unknown. Here, we screen 224 ancient European genomes from the Upper Palaeolithic to the post-Roman period for 22 malaria-resistant alleles in twelve genes described in the literature. None of the most specific mutations for malaria resistance, like those at G6PD, HBB or Duffy blood group, have been detected among the available samples, while many other malaria-resistant alleles existed well before the advent of agriculture. We detected statistically significant differences between ancient and modern populations for the ATP2B4, FCGR2B and ABO genes and we found evidence of selection at IL-10 and ATP2B4 genes. However it is unclear whether malaria is the causative agent, because these genes are also involved in other immunological challenges. These results suggest that the selective force represented by malaria was relatively weak in Europe, a fact that could be associated to a recent historical introduction of the severe malaria pathogen.
Introduction
The malaria parasite Plasmodium falciparum is one of the main causes of child mortality worldwide, although other species from the same genus, P. vivax, P. malariae and P. ovale are also causative agents of the disease. Therefore, it is not surprising that malaria is one of the strongest known selective pressures that have recently shaped the human genome. Malaria is the evolutionary driving force behind sickle-cell disease (HbS), thalassemia, glucose-6-phosphatase deficiency (G6PD) and other erythrocyte defects that together comprise the most common Mendelian diseases of humankind. Remarkably, populations from different geographical areas have developed different genetic mechanisms adapted to malaria resistance; for instance, the HbS allele at the HBB gene is common in Africa but rare in Southeast Asia, whereas the HbE alelle shows a reversed pattern. Resistance to malaria includes primarily genes involved in immunological response, but also some involved in inflammation and cell adhesion and even genes related to metabolic pathways. The fact that different malaria-resistance alleles have arisen in different places suggests that this adaptation occurred relatively recent in human history, at least well after the Out of Africa migration1.
P. falciparum may have rapidly spread from its African tropical origins to other tropical and subtropical regions of the world only within the last 6,000 years2. The recent origin of the worldwide P. falciparum populations, which are the most malignant of human malarial parasites, may account for its virulence3. Thus, haplotype analysis at the G6PD locus suggests that the African resistance allele to P. falciparum originated within the last 10,000 years4 while a similar analysis at the HbE alleles (variants of the HBB gene) in Southeast Asia yielded an even more recent date, around 5,000 years ago5. These and other estimates associate the resistance to P. falciparum with the onset of agriculture and the emergence of favourable environments for mosquito proliferation after the clearance of woodlands for farming. Falciparum-like illness (I.e. miasmas) appears to be very recent in Europe. It seems to have spread from India to the West, appearing in ancient Greece only by the 4th century BCE, where it was implicated in the decline of many city-state populations2. The retrieval of the eradicated European P. falciparum from 70-year-old slides from a malaria-endemic region in Spain confirms a recent, phylogenetic link with present-day Indian haplotypes6. Therefore, it seems unlikely that P. falciparum would have had a significant impact on prehistoric European genomes.
On the other hand, P. vivax (and probably P. ovale and P. malaria) is likely an ancient parasite that evolved in Africa and spread to the rest of the world with the Out of Africa migration, around 60,000 years ago2. Phylogenetic analyses suggest that P. vivax could have an African origin but has largely disappeared from this continent after the spread of Duffy negative resistance7. The biology of P. vivax that makes it highly adapted to maintain itself in small mobile groups of hosts and its mild effect also supports the idea that it is an ancient human parasite. The limited data available does show that P. vivax and P. falciparum may have had at least partially different effects on the human genome1.
Malaria was endemic in Europe until very recently. Historical data describe malaria as affecting people as north as England, Scandinavia or Russia2. However, while there is information of some malaria-resistance alleles from Europe, notably from the Mediterranean area (such as the HBB and the G6PD variants), little is known about the magnitude of selection due to malaria in the continent because of the eradication of the parasite around 1950. The recent retrieval of more than 250 ancient genomes from different moments of the prehistory of Europe8–11 and the publication of several Genome wide association studies (GWAS) on malaria12, 13 now allows for a genome-wide screening of malaria-resistance alleles along space and time. Using this approach, we aim to understand the role of this disease as a selective force in the shaping of the gene pool of modern Europeans.
Results
Sample size and allele imputation
It was possible to screen 224 ancient individuals (Fig. 1 and Table S1) for 20 single nucleotide polymorphisms (SNPs) and two single nucleotide-deletions described to play a role in malaria resistance and distributed in twelve different genes: G6PD, HBB, ACKRQ, FCGR2B, TIRAP, ATP2B4, GRK5, IL-10, MARVELD3, CD36, CD40LG and ABO blood group system (Table 1). Due to low coverage on many ancient samples, complete genotypes are typically not available for all loci; therefore, we needed to rely on imputation methods to infer genotypes, using the 1,000 Genomes phased samples as a reference panel and Beagle-4 software14.
Figure 1.

Geographical distribution of ancient European samples included in the analysis of the malaria-resistant mutations. The map depicts the malaria-endemic zone57. The map was made using Worldmap package 1.3.1 of R software 3.2.2 (http://cran.r-project.org), and modified with Gimp 2.6 (https://www.gimp.org) and Pinta 1.6 (https://pinta-project.com).
Table 1.
Summary of the genetic variants analysed in the study and the hypothetical functional explanation in relation to malaria resistance, when available.
| Reference SNP ID | Gene | Relation to malaria resistance | World distribution (% of resistant variant) | SNPs recovered from genome wide data samples (2 N) | SNPs recovered from targeted capture data (N) |
|---|---|---|---|---|---|
| rs1050828 | G6PD | G6PD A−, mutation of a Val to a Met in the residue 68 that causes instability | Europe: 0; Africa: 13; America: 1; Asia: 0 | 79,35% | 46,01% |
| rs1050829 | G6PD | G6PD A+ produces a substitution of a Asn to a Asp in the residue 126 | Europe: 0; Africa: 34; America: 3; Asia: 0 | 79,35% | 35,58% |
| rs5030868 | G6PD | G6PD Mediterranean, change from a Ser to a Phe in position 188 causing abnormality | Europe: 0; Africa: 0; America: 0; Asia: 0 | 23,91% | 3,067% |
| rs137852314 | G6PD | Mutation associated cause in enzyme derangement and associated with AluI site | Europe: 0; Africa: 0; America: 0; Asia: 0 | 23,91% | 3,067% |
| rs76723693 | G6PD | Substitution found in subjects with G6PD A− phenotype | Europe: 0; Africa: 1; America: 0; Asia: 0 | 80,34% | 1,84% |
| rs137852328 | G6PD | Substitution found in subjects with G6PD A− phenotype | Europe: 0; Africa: 0; America: 0; Asia: 0 | 26,087% | 1,84% |
| rs372091 | HBB | Identified in a GWAS study | Europe: 0; Africa: 7; America: 0; Asia: 0 | 93,48% | 41,72% |
| rs33930165 | HBB | Modification of erythrocyte structure, hemoglobine C | Europe: 0; Africa: 1; America: 0; Asia: 0 | 96,74% | 1,84% |
| rs33950507 | HBB | Modification of erythrocyte structure, hemoglobine E | Europe: 0; Africa: 0; America: 0; Asia: 1 | 96,74% | 1,84% |
| rs334 | HBB | Modification of erythrocyte structure, hemoglobine S | Europe: 0; Africa: 10; America: 1; Asia: 0 | 76,087% | 1,84% |
| rs2814778 | ACKR1 | Change in the structure of erytrochyte surface protein | Europe: 1; Africa: 96; America: 8; Asia: 0 | 93,48% | 40,91% |
| rs1050501 | FCGR2B | Enhancement of phacocytosis | Europe: 14; Africa: 25;America: 9; Asia: 20 | 53,26% | 2,45% |
| rs8177374 | TIRAP | Substitution of a Ser to a Leu in the residue 180 attenuating TLR2 signal transduction | Europe: 19; Africa: 1; America: 8; Asia: 9 | 59,78% | 3,68% |
| rs4951074 | ATP2B4 | Mutation in a Ca2+ pump in the erythrocyte affecting the P falciparum cycle | Europe: 10; Africa: 37; America: 6; Asia: 7 | 95,65% | 1,84% |
| rs10900585 | ATP2B4 | Mutation in a Ca2+ pump in the erythrocyte affecting the P falciparum cycle | Europe: 90; Africa: 58; America: 93; Asia: 92 | 90,22% | 26,99% |
| rs2230345 | GRK5 | Ser/Thr Kinase mutation that makes difficult the internalization of P falciparum in erythrocyte | Europe: 1; Africa: 30; America: 3; Asia: 5 | 72,83% | 6,13% |
| rs1800890 | IL-10 | IL-10 mutations decreases the level of this cytokines in the plasma | Europe: 37; Africa: 20; America: 24; Asia: 12 | 35,87% | 1,84% |
| rs2334880 | MARVELD3 | Identified in a GWAS study | Europe: 84; Africa: 60; America: 90; Asia: 98 | 85,87% | 11,65% |
| rs8176719 | ABO | Individuals homozygous classified as group O, associated with decreased risk of malaria. | Europe: 61; Africa: 71; America: 77; Asia:61 | 45,65% | 0% |
| rs8176746 | ABO | Determines the production of B antigens, associated with increased risk of malaria | Europe: 92; Africa: 83; America: 95; Asia:79 | 75% | 31,29% |
| rs3092945 | CD40LG | Mutation in a glycoprotein involved in B cell proliferation, antigen presenting cell activation is important in the immune response to infection | Europe: 1; Africa: 31; America: 4; Asia: 0 | 81,52% | 0% |
| rs201346212 | CD36 | Scavenger receptor CD36 plays important roles in malaria, including the sequestration of parasite-infected erythrocytes in microvascular capillaries | Europe: 0; Africa: 1; America: 0; Asia: 0 | 81,52% | 0% |
The allelic frequencies have been estimated from data from the 1,000 Genomes. The recovery statistics are also presented in this Table.
After imputation, the number of genotypes recovered for each nucleotide position was variable (Table 1 and Table S1). The proportion of recovered haplotypes in whole genome sequence data fluctuated from a maximum 96.74% in HBB SNP: rs33950507 and rs334 to a minimum 23.91% in ATP2B4 SNP rs5030868. The proportion of recovered alleles in targeted capture data samples fluctuated from a maximum of 40.91% in ACKRQ SNP: rs2814778 to zero observations for CD36 SNP: rs3092945, CD40LG SNP: rs201346212, and ABO SNP: rs817671. A dataset with genotypes from 92 genome-wide samples is presented in Table S2.
Even with such data limitations, we were able to detect a substantial number of carriers of the proposed malaria resistance alleles in our ancient genome dataset (Table 2). It is noteworthy that different derived genetic variants (at TIRAP, ATP2B4, MARVELD3, ABO, and IL-10) were already present prior to the arrival of farming, in Upper Palaeolithic and/or Mesolithic individuals. For other loci, the derived alleles are only seen in very recent periods. For instance, only one of the Iron Age individuals carries the T allele (rs2230345) at GRK5 gene, one individual carries the T allele (rs8176746) at ABO gene and only two individuals (one Bronze Age Russian and one Post-Roman British) carry the C allele (rs1050501) at the FCGR2B gene.
Table 2.
Frequencies of malaria-resistant alleles by time period in nine selected genes and 224 available ancient European genomes.
| FCGR2B | TIRAP | GRK5 | IL-10 | ATP2B4 | MARVELD3 | ABO | |||
|---|---|---|---|---|---|---|---|---|---|
| rs1050501 | rs8177374 | rs2230345 | rs1800890 | rs4951074 | rs10900585 | rs2334880 | rs8176746 | rs8176719 | |
| UPPER PALEOLITHIC | TT = 7 | CC = 6 CT = 3 | AA = 10 A* = 1 | AA = 1 TA = 2 TT = 1 | GG = 12 GA = 2 | TT = 12 GT = 2 | GG = 5 AG = 9 G* = 7 | GG = 8 G* = 9 | – = 3 -G = 2 GG = 1 |
| EARLY NEOLITHIC | TT = 10 T* = 1 | CC = 3 CT = 8 TT = 1 T* = 2 | AA = 13 A* = 2 | AA = 2 TA = 2 TT = 1 | GG = 17 GA = 1 | TT = 13 T* = 2 | GA = 1 GG = 16 A* = 3 | GG = 24 G* = 12 | – = 2 GG = 3 -G = 3 |
| MIDDLE NEOLITHIC | TT = 1 T* = 1 | TT = 1 C* = 2 | AA = 3 A* = 2 | TA = 1 | GG = 5 GA = 1 | TT = 4 GT = 2 T* = 4 G* = 1 | GG = 4 G* = 2 A* = 1 | GG = 4 G* = 8 | – = 2 |
| LATE NEOLITHIC | TT = 3 T* = 1 | CC = 2 TT = 1 C* = 1 | AA = 5 A* = 2 | TA = 1 | GG = 6 | TT = 5 G* = 4 T* = 8 | GG = 6 G* = 1 | GG = 5 G* = 14 T* = 1 | – = 2 GG = 1 -G = 2 |
| BRONZE AGE | TT = 24 TC = 1 | CC = 15 CT = 3 TT = 1 C* = 1 | AA = 19 A* = 2 | AA = 1 TA = 4 TT = 4 | GG = 20 GA = 4 | TT = 23 GT = 1 T* = 10 G* = 5 | GG = 16 GA = 5 AA = 1 | GG = 21 GT = 1 G* = 6 | –* = 4 GG = 4 |
| IRON AGE | TT = 7 | CC = 5 | AA = 4 AT = 1 | AA = 2, TT = 3 | GG = 6 GA = 1 | TT = 5 G* = 1,T* = 1 | GG = 6 G* = 1 A* = 1 | GG = 6 G* = 1 | – = 3 GG = 1 -G = 1 |
| ROMAN | TT = 2 | CC = 2 CT = 1 | AA = 5 | TT = 3 | GG = 5 | TT = 5 | GG = 5 | GG = 5 | – = 1 |
| POST-ROMAN | TT = 2 CT = 1 | CC = 2 CT = 4 | AA = 6 | TA = 3 TT = 2 | GG = 8 | TT = 8 | GG = 7 GA = 1 | GG = 8 | – = 1 GG = 2 -G = 2 |
| ANCIENT POPULATIONS | T = 98% C = 2% | C = 76% T = 24% | A = 99% T = 1% | C = 40%, T = 60% | G = 95% A = 5% | G = 8% T = 92% | G = 90% A = 10% | G = 99% T = 1% | - = 57,5% G = 42,5% |
| GLOBAL EUROPEAN | EUR:T = 86%,T = 14% | EUR:C = 81%,T = 19% | EUR:A = 99%,T = 1% | EUR:A = 63%,T = 37% | EUR:G = 90%,A = 10% | EUR:T = 90%,G = 10% | EUR:G = 84%,A = 16% | EUR:G = 92%,T = 8% | EUR:- = 61%, G = 39% |
The frequencies of current European populations are displayed below the ancient populations. All genotypes included passed an imputation filter > 0.95. The * sign denotes that a specific allele is present, but we cannot confidently imputate the genotype. Only those SNPs showing at least one diverse allele have been included.
Ancient-modern population comparisons
For three genetic variants, we observed significant frequency differences between ancient and modern European populations from the 1,000 Genomes project, after adjusting for multiple tests: rs1050501 (FCGR2B) (OR = 7.86; CI = 2.07–66.66; p = 2.2e-16), rs4951074 (ATP2B4) (OR = 2.16; CI = 1.06–4.96; p = 0.02) and rs8176746 (ABO) (OR = 9.63; CI = 2.54–81.49; p = 1.19e-5). The three genes show lower frequencies than expected of the derived alleles in ancient populations as compared to present-day Europeans. Samples with data from these three statistically significant ancient vs modern variants are not geographically clustered, as can be seen in Fig. 2. Therefore, no clear climatic or latitudinal pattern that could be associated to the malaria distribution emerges.
Figure 2.
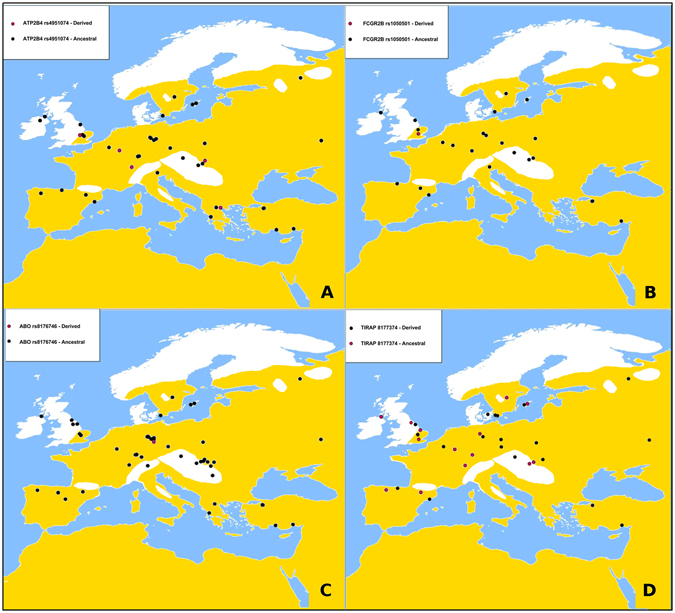
Geographical distribution of the rs8176746, rs4951074, rs1050501 and rs8177374 alleles in ancient European populations. The black circles represent those individuals with ancestral variants and red circles represent those individuals with derived variants presumably associated to malaria resistance. The map depicts the malaria-endemic zone57. The maps were made using Worldmap package 1.3.1 of R software 3.2.2 (http://cran.r-project.org), and modified with Gimp 2.6 (http://www.gimp.org) and Pinta 1.6 (http://pinta-project.com).
Population structure
Although malaria was prevalent along the continent, from Russia and Scandinavia to Southern Europe, some resistance mutations such as G6PD B- are nowadays restricted to the Mediterranean, which suggests that the impact of the disease may have been different between European regions. We therefore tested for population structure at these loci.
The only SNP to show significant North-South differences in allele frequencies in modern Europeans was the rs8177374 SNP at TIRAP gene. The allelic frequency of the derived allele in South-Europe populations (IBS and TSI) was 34%, statistically significant higher than the 16% of the derived alleles in North-European populations (CEU, GBR and FIN) (codominant model: OR = 4.04; CI = 1.37–11.91; p = 1.428e-05 and log-additive model: OR = 2.20; CI = 1.57–3.08; p = 2.522e-06). This trend cannot be discerned in the ancient samples (see Fig. 2).
Although the problems of assessing population structure with a dataset of low-coverage, geographically and temporally disparate ancient genomes cannot be overlooked we sought to explore the genetic structure among ancient and modern European with an FST and G-test approach. We have grouped the ancient samples in eight periods (Upper Palaeolithic, Early Neolithic, Middle Neolithic, Late Neolithic, Bronze Age, Iron Age, Roman Age and Post-Roman Age) (Table S1). The FST pairwise comparisons did not reveal any structural differentiation in the established sub-groupings (Table S4). A G-test analysis detected a significant geographic differentiation of the variants associated with malaria resistance among the five present-day European populations (CEU, IBS, TSI, GBR, FIN) of the 1,000 Genomes database (G-statistic; 102.304, p-value: 0.01) (Table S5). This suggests that very fine differences in allele frequencies among these populations likely exist. We further explored this issue with Chi-squared test comparisons for each SNP between all pairs of populations; we found that all pairwise tests including Finns (FIN) are statistically significant, and also one North-South test (IBS vs CEU).
Evidence of selection
To further explore the possibility that changes in our time series data could be attributed to selection acting on the derived alleles, we used a Bayesian method that estimates the frequency trajectory of the alleles based on observed frequencies and sampling dates15. The “Selection” software estimates a posterior probability of selection coefficients for heterozygous haplotypes (alpha 1) and for homozygous derived haplotypes (alpha 2) for each SNP and also the allelic age. The mean values of the selection coefficients and the likelihood values are shown in Fig. 3, and the posterior distribution of selection coefficients (alpha1 and alpha2) are shown in Supplementary Figures S6–S13.
Figure 3.
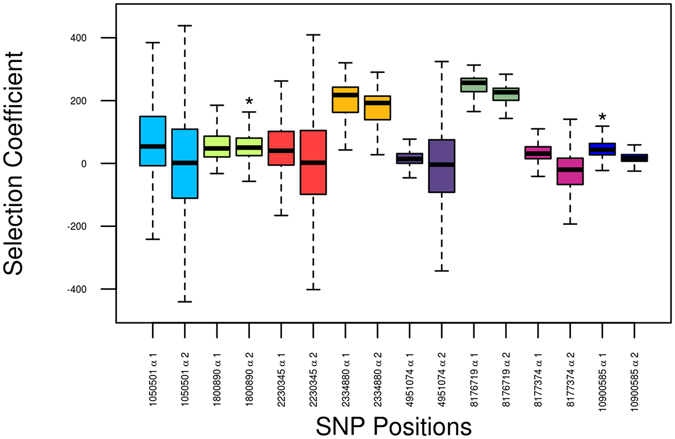
Mean values and standard deviations of the alpha 1 and alpha 2 selection coefficients for the malaria-resistant SNPs that exhibit variability (https://github.com/Schraiber/selection). *Statistically significant selection coefficients.
Two genetic variants showed statistically significant evidence of positive selection on the derived allele: rs1800890 SNP at IL-10 gene with a favoured derived-homozygous genotype (alpha 2: mean value = 57.7; p = 0.046) and rs10900585 SNP at ATP2B4 gene, with a favoured heterozygous genotype (alpha 1: mean value = 48.85; p = 0.015); p is the probability of the selection coefficient to be <0.
The allelic ages estimates showed that the oldest derived allele is rs8176719 at the ABO blood group gene. The age estimates of all alleles (Supplementary Table S6) predate our sampling period with the exception of rs1050501 at the FCGR2B gene that yielded an age of 9,500 years.
Discussion
Globally, the comparison of the allele frequencies between ancient and current European populations did not reveal strong variations in most of the analysed genes. In most cases, and despite limitations in sample size, the allele frequencies in the ancient samples were very similar to those of the modern populations (Table 2).
The fact that we can, in most cases, detect the derived allele in a dataset based on a handful of ancient genomes implies that these alleles had already reached relatively high frequencies in the ancient populations. Even with the uncertainties associated to the small sample size, we provide for first time information about the ages of the derived malaria resistance alleles. With the single exception of the FCGR2B gene, all of them predate the emergence of the Neolithic by thousands of years. However, the period when they became prevalent and potentially important from a phenotypical point of view, can be much more recent. It has been suggested that the spread of malaria was triggered by the deforestation associated with the farming expansion16. However, our results do not indicate any clear shift in the allelic frequencies of the malaria-resistant genes in response to the onset of farming.
P. vivax is thought to be a relatively ancient parasite that probably was common when Homo sapiens colonized Europe, about 45,000 years ago2. It is therefore not surprising that this parasite could have had a significant impact on pre-Neolithic European as well as African genomes. The three traits that have been clearly shown by functional studies to protect against P. vivax are: Duffy negativity (ACKR1 gene), Ovalocytosis (in South East Asian groups) and G6PD deficiency17–19. The first two have little if any effect on P. falciparum while G6PD seems to have more effect on P. vivax than on P. falciparum. So far, none of the ancient genomes available displays any mutation at the crucial G6PD or Duffy loci. In addition, we do not find a single carrier of the HBB mutation. We don’t know if this is attributable to the rather limited sample size or if their absence reflects a relatively recent origin of these mutations among European populations. The frequencies of these mutations are very low in current populations (for instance, there is not a single carrier among the European populations of the 1,000 Genomes project). However, the sample size for some of the SNPs, such as the rs372091 at the HBB locus, is not that small and currently includes 155 ancient genomes.
The current shortage of ancient genomic data from the Mediterranean area (where severe malaria was most prevalent in historic times) represents a limitation of our survey. This is partially attributable to the warm environmental conditions in the area, which are unfavourable to DNA preservation20. At present, only one complete genome (a Cardial Early Neolithic specimen)21 and several Neolithic individuals from the oriental Mediterranean peninsulas (Greece and Anatolia)22, 23 derive from the thermo-Mediterranean zone21. However, a significant number of samples derive from regions that are close to the Mediterranean shores (Fig. 1).
While looking at the variation among modern Europeans with the G-statistic, the present-day frequencies of the SNPs related with malaria resistance denote the presence of substructure that should be further investigated with additional modern populations and neutral variation SNPs. The structure could be partly influenced by the distinctiveness of the Finnish sample but also by the SNP at TIRAP gene (rs8177374) (Table S5) that shows a clear latitudinal trend confirmed with the OR results. However, the same mutation has also been related to resistance to bacterial infections such as tuberculosis24. Therefore, it is unclear if the clinal pattern of variation for this allele is in fact associated to malaria infection or to other selective forces acting on a latitudinal basis.
Three genes (FCGR2B, ATP2B4 and ABO) show differences in the allelic frequency between ancient and modern Europeans. This could in principle be the signal of recent events of positive selection acting upon the associated SNPs.
The mutation at the FCGR2B gene (an ILE > THR change in the 232 residue of the protein) has been proposed to have a strong protective effect against severe malaria (OR = 0.56, p < 0.001) in African populations due to the enhancement of phagocythosis of P. falciparum infected-human erythrocytes25. This could explain the high frequency of this mutation in malaria endemic areas, although the same mutation has also been described as causative of Systemic Lupus Erythematosus25, 26. The only two individuals that carry this mutation are from periods and regions where malaria would be endemic in historical times: one is a Bronze Age individual from southern Russia and the other one an Anglo-Saxon from southern England. However, some older specimens (Table S1) also seem to carry the mutation, although the imputation test in those cases did not meet the 0.95 threshold. Polymorphisms at ATP2B4 gene, encoding for membrane calcium-transporter protect against malaria because of its function in homoeostasis control of the erythrocyte membrane27, although this association needs to be assessed with further functional evidence. The rs8176746 SNP at the ABO gene is in linkage disequilibrium with rs8176719 SNP that determines B antigens. The derived allele has been associated with increased risk of severe malaria28.
To evaluate if these genes could bear the footprints of recent positive selection (as opposed to neutral processes such as drift or migration), we consulted a genome browser with information about such signatures29, but none of them displayed any evidence of selective sweeps. An alternative to selection would be migration; it has been recently demonstrated that European populations were modelled by three genetic components that overlapped in the last thousands of years: the Mesolithic hunter-gatherers, the Early Neolithic farmers and the Late Neolithic steppe migrants11, 30. Although the right approach to distinguish between selection and migration would be the one followed by Mathieson et al.9, the lack of data in the original source populations for most of our SNPs precludes this approach. However, we noticed that differences in allelic frequencies detected in FCGR2B and ATP2B4 are restricted to very recent periods -after the Bronze Age- when the general composition of the European populations was already established. Therefore, the trend observed towards the increase of the derived allele is unlikely to be attributed to these ancient migrations.
We have modelled all the SNPs with an approach that rejects neutrality across a time series and found evidence for selection in SNPs rs10900585 at ATP2B4 and rs1800890 at IL-10 genes. This suggests that selection acted on these genes, an evidence that should be further explored from a functional point of view. The fact that no previous evidence of selective sweeps on these genes has been reported could be explained if the timing of the selective event was very recent or if the effect was regionally restricted. Nevertheless, it is reasonable to discard large-scale and dramatic selective events such as those seen for the lactase gene9.
In conclusion, it seems that most of the screened alleles were equally prevalent in past than in present time; in those alleles were differences are statistically significant, we cannot discard the effect of drift or other selective forces acting upon these immunity genes. This could indicate a weak adaptation to malaria, due to either the benignity of symptoms caused by P. vivax or to an undetectable adaptive response related to a very recent arrival to the European continent (which seems to be the case of severe malaria caused by P. falciparum). We have shown however that, despite the large geographic distribution, malaria was likely not an important selective pressure in ancient European populations and not nearly as important as it is in Africa today. Additionally we demonstrate that the study of the selective effect of malaria on ancient genomes is a feasible approach that will likely provide more information in the future with increasing sample sizes, especially from the Mediterranean area. With additional genomic data, and also the retrieval of DNA from the pathogen directly from ancient bones31, it would be possible to check if local selective events took place and to understand the role of malaria in the shaping of modern European genomes.
Methods
Ancient genomes
We have analysed all ancient European -sensu lato- genomes with a mean sequence coverage >1x published so far11, 21–23, 32–39. We have also included those genomes for which only targeted capture data is available9, 10, 30, 40–42. Our final dataset comprises genome-wide-data from 224 individuals (Fig. 1,Table S1) from which we have at least information for one of the malaria-resistant markers.
Screened genetic variants
We have screened all malaria-resistant alleles from the literature, even if most of them have been described as being selective in current African populations and involved in P. falciparum resistance. Of course this cannot be directly extrapolated to European populations but the limited information on the selective effect of P. vivax or P. falciparum on Europeans -especially outside the Mediterranean area- makes alternative approaches unfeasible. We have included 20 SNPs and two deletion belonging to twelve different genes (Table 1, Table S3). Some of the associations have been investigated from a functional perspective4 while others have only been recently detected on GWAS studies and their potential role in the protection against the disease is not yet well understood.
G6PD, the key enzyme in the oxidative pentose phosphate pathway is probably one of the most prevalent signs of malaria-resistance in European populations, at least in the Mediterranean area. The G6PD A- is the most prevalent resistant allele in African populations. The B- mutation, known as Mediterranean G6PD has a more severe effect and is commonly found around the Mediterranean islands, in places where malaria was endemic, in frequencies of 2–20%. We have analysed the SNPs associated to the G6PD A- (rs1050828, rs1050829) and Mediterranean G6PD (rs5030868) forms. Additional G6PD mutations associated to malaria resistance have also been included (rs137852314, rs76723693, rs137852328)43.
Haemoglobin beta locus (HBB) determines the sequence of two types of polypeptidic chains in adult haemoglobin, HbA. Variants in the HBB gene such as HbC and HbS change the structure of the erythrocytes and confer resistance to P. falciparum in Asian and African populations44. The mutations that cause both HbS (rs334) and HbC (rs33930165) were screened1, 45. Recent studies based on GWAS have found an additional mutation in the HBB gene related to malaria resistance (rs372091)12. HbE caused by rs33950507 mutation) is a mainly Asiatic variant of HBB gene that also confers resistance to the infection of P. falciparum 46.
Duffy blood group system, Fy (a-b-) (ACKR1 gene) confers resistance to P. vivax and P. knowlesi through the mutation rs2814778 that changes the structure of the protein, which is a receptor for Plasmodium in the erythrocytes47. This phenotype is especially present in West African populations were P. vivax originated13.
Additional mutations have been detected by GWAS analysis at MARVELD3 (rs2334880), ATP2B4 (rs4951074 and rs10900585) and ABO (rs81767199)12. The MARVELD3 gene encodes for a tight junction-associated transmembrane protein of endothelial cells, a key point in the pathology of malaria infection. This mutation is supposed to confer resistance to P. falciparum malaria although it is not confirmed12. The ABO gene encodes for a glycosyltransferase enzyme. Mutations in this enzyme determine the ABO blood group. The individuals who are homozygous for a single nucleotide deletion (rs8176719) are classified as blood group O48. O blood group is linked with a reduced risk of severe malaria28, 49. However, we note that, besides malaria, the O blood group has been associated to susceptibility to other pathogens such as Helicobacter pylori 50 and severe cholera51. We have also analyzed the rs8176746 SNP. This mutation, which is in LD with the rs8176719 variant, determines the production of B antigens48. The presence of the derived allele in this locus is associated with an increased risk of severe malaria28. Evidence of malaria protection has been also described at CD40LG gene in Gambian populations: homozygotes for the derived rs3092945 allele show a reduced risk of suffering severe malaria. However, a significant increase of severe malaria risk was found for the same allele in Kenyan populations28. In the same study, a severe malaria protective effect of the SNP rs201346212 heterozygous genotype (CD36 gene) was described. Other possible evidences on P. falciparum malaria resistance include additional genes such as GRK5, a G protein receptor (mutation rs2230345)52, IL-10 (rs1800890), FCGR2B (rs105050) and TIRAP (rs8177374).
Imputation
Due to the low coverage in many ancient samples and gaps associated to the targeted capture data, we performed imputations of SNPs not covered by any DNA read using Beagle-4 software14 and the Tuscans (TSI) population of the 1,000 Genomes project53 as a reference panel.
Some of the alleles screened involve C to T or G to A changes that could be affected by post-mortem DNA degradation54. To control for this confounding factor we have screened the genetic background in which the SNP is located, following the same approach as in ref. 9. In brief, the problematic SNP was removed on each ancient genome, genotype likelihoods were estimated and the SNP was imputed using Beagle-4 and the phased information of the 1,000 Genomes. This approach provides us with a probability estimate that a specific allele in a given sample is likely to be there or not, even in the absence of reads covering that nucleotide position. We have considered genotypes that yielded a probability higher than 0.95.
The genotypes obtained from genome sequence data and validated with Beagle-4 software were used to generate genotype frequencies. The allelic frequencies were estimated using both validated genome sequence data and targeted capture data. Because of the impossibility to infer genotypes in the case of targeted capture data only one random allele per position was taken into account.
Statistical analysis
The allelic frequencies obtained with validated genotypes and targeted capture data were stratified by geographic position of the samples (North Europe and South Europe -the latter including Iberia, Italy, the Balkan peninsula and Anatolia-) and period (Upper Palaeolithic, Early Neolithic, Middle Neolithic, Late Neolithic, Bronze Age, Iron Age, Roman Age, Post-Roman Age).
To explore potential differences in the genotypic composition between modern South (Iberians and Tuscans) and North Europeans (Finnish, British and CEU) populations, we tested two different genetic models (codominant and logistic additive models) for each SNP, with a case-control approach. A likelihood ratio test was estimated for each SNP and both models using a Bonferroni correction. A Fisher’s exact test with a case-control approach was used for comparing allele frequencies in ancient versus modern European populations in those SNPs with more than one detected case of derived allele. Due to the assumption that ancient frequencies are directly comparable to the modern ones, the Fisher’s exact test is anti-conservative here; however we have included the p-value in the cases were we found an>1 OR, just as an additional, supporting evidence.
Population structure analysis
To determine the level of differentiation and substructure among subpopulations the statistic FST55 was calculated. The fixation index was determined performing all the possible pairwise calculations comparing North-South present-day Europeans, the five European populations of 1000 genomes, North-South ancient Europeans and Period-stratified ancient Europeans (the populations were clustered in three supra populations: Upper Paleolithic; Pre-Neolithic, Middle-Neolithic, Past-Neolithic; Bronze Age, Iron Age, Roman, Post-Roman). Nine SNPs that exhibited variability (Table 2) were used to perform the comparisons. To test the divergence and the stratification within the population the statistic FSI (where I stands for individual and S for sub-population), was also used to estimate the departure from panmixia at the sub-population level.
Additionally the G-test was calculated as it is described in Goudet et al.56. The authors showed that the likelihood ratio G-test, is the most powerful statistic to detect population differentiation, particularly when samples are unbalanced. These authors also showed that for diploids, the appropriate unit to randomize is the diploid genotype rather than the allele (of course, the contingency tables are based on alleles, not genotypes). The likelihood ratio G-statistic was performed on a contingency table of alleles at one locus x sampling unit; the individual genotypes were randomly permutated among samples and the G-statistic obtained from the original dataset is compared to the G-statistics obtained from the permuted datasets, yielding a P-value of the test.
Neutral selection testing
To test for the presence of selection driving the genotypic frequencies in past-populations and reject neutrality we used a specific software (https://github.com/Schraiber/selection) as described in ref. 15. This analysis based on Bayesian probabilities was performed for each allele that presented diversity among ancient individuals (Fig. 3). The 92 ancient samples with complete genotypic information were grouped in eight periods (Upper Palaeolithic, Early Neolithic, Middle Neolithic, Late Neolithic, Bronze Age, Iron Age, Roman and Post-Roman) to create a time series. The number of derived alleles was counted in each period, and selection coefficients were inferred from allele frequencies along time. The analysis was performed assuming a constant population size and 10,000 individuals of effective population size. The dates used in the simulations were: −0.04, −0.018, −0.015, −0.008, −0.006, −0.004, −0.0004 and 0 relatively to the most recent analysed age (post-Roman period). Two SNPs (rs951074 and rs2334880) presented high frequencies of the derived allele in all the sampling times. Because of the software models the allele arising from a new mutation, a new mutation is much more likely to rise to high frequency if it is under selection. In the analysis of rs951074 and rs2334880 SNPs the origin of the derived allele was not modelled because it was already extended in the initial periods. The value distributions of selection coefficients over time were plotted with R 3.2.2 (http://cran.r-project.org) software.
Electronic supplementary material
Acknowledgements
This research was supported by a grant to C.L.-F. from FEDER and Ministry of Economy and Competitiveness (BFU2015–64699-P) of Spain and by a grant 2014 SGR 464 (GRBIO) from the Departament d’Economia i Coneixement de la Generalitat de Catalunya (Spain) to S.C. We are grateful to Joshua Schraiber and Agnar Helgason for helpful comments and suggestions.
Author Contributions
C.L.-F. and P.G. conceived the study; P.G., I.O., T.d.-D. and S.C. analyzed data; C.L.-F. and P.G. wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-01534-5
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kwiatkowski DP. How Malaria Has Affected the Human Genome and What Human Genetics Can Teach Us about Malaria. Am. J. Hum. Genet. 2005;77:171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter R. Speculations on the origins of Plasmodium vivax malaria. Trends Parasitol. 2003;19:214–219. doi: 10.1016/S1471-4922(03)00070-9. [DOI] [PubMed] [Google Scholar]
- 3.Rich SM, Licht MC, Hudson RR, Ayala FJ. Malaria’s Eve: Evidence of a rcent population bottleneck throughout the world populations of Plasmodium falciparum. Proc. NAtl. Acad. Sci. 1998;95:4425–4430. doi: 10.1073/pnas.95.8.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tishkoff SA, et al. Haplotype diversity and linkage disequilibrium at human G6PD: recent origin of alleles that confer malarial resistance. Science. 2001;293:455–462. doi: 10.1126/science.1061573. [DOI] [PubMed] [Google Scholar]
- 5.Ohashi J, et al. Extended linkage disequilibrium surrounding the hemoglobin E variant due to malarial selection. Am. J. Hum. Genet. 2004;74:1198–1208. doi: 10.1086/421330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelabert P, et al. Mitochondrial DNA from the eradicated European Plasmodium vivax and P. falciparum from 70-year-old slides from the Ebro Delta in Spain. Proc. Natl. Acad. Sci 113. 2016;41:11495–11500. doi: 10.1073/pnas.1611017113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W, et al. African origin of the malaria parasite Plasmodium vivax. Nat. Commun. 2014;5:3346. doi: 10.1038/ncomms4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olalde I, Lalueza-Fox C. Modern humans’ paleogenomics and the new evidences on the European prehistory. Sci. Technol. Archaeol. Res. 2015;1:1–9. doi: 10.1179/2054892315Y.0000000002. [DOI] [Google Scholar]
- 9.Mathieson I, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499–503. doi: 10.1038/nature16152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haak W, et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature. 2015;522:207–211. doi: 10.1038/nature14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allentoft ME, et al. Population genomics of Bronze Age Eurasia. Nature. 2015;522:167–172. doi: 10.1038/nature14507. [DOI] [PubMed] [Google Scholar]
- 12.Timmann C, et al. Genome-wide association study indicates two novel resistance loci for severe malaria. Nature. 2012;489:443–446. doi: 10.1038/nature11334. [DOI] [PubMed] [Google Scholar]
- 13.Jallow M, et al. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nat. Genet. 2009;41:657–665. doi: 10.1038/ng.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Browning BL, Browning SR. Genotype imputation with millions of reference samples. Am. J. Hum. Genet. 2016;98:116–126. doi: 10.1016/j.ajhg.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schraiber JG, Evans SN, Slatkin M. Bayesian Inference of Natural Selection from Allele Frequency Time Series. Genetics. 2016;203:493–511. doi: 10.1534/genetics.116.187278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rich SM, et al. The origin of malignant malaria. Proc. Natl. Acad. Sci. USA. 2009;106:14902–14907. doi: 10.1073/pnas.0907740106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagao E, Seydel KB, Dvorak JA. Detergent-resistant erythrocyte membrane rafts are modified by a Plasmodium falciparum infection. Exp Parasitol. 2002;102:57–59. doi: 10.1016/S0014-4894(02)00143-1. [DOI] [PubMed] [Google Scholar]
- 18.Miller LH, Mason SJ, Clyde DF, McGinniss MH. The Resistance Factor to Plasmodium vivax in Blacks. N. Engl. J. Med. 1976;295:302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- 19.Luzzatto L, Usanga FA, Reddy S. Glucose-6-phosphate dehydrogenase deficient red cells: resistance to infection by malarial parasites. Science. 1969;164:839–842. doi: 10.1126/science.164.3881.839. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Garcera M, et al. Fragmentation of contaminant and endogenous DNA in ancient samples determined by shotgun sequencing; prospects for human palaeogenomics. PLoS One. 2011;6:e24161. doi: 10.1371/journal.pone.0024161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olalde I, et al. A Common Genetic Origin for Early Farmers from Mediterranean Cardial and Central European LBK Cultures. Mol. Biol. Evol. 2015;32:3132–3142. doi: 10.1093/molbev/msv181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kılınç GM, et al. The Demographic Development of the First Farmers in Anatolia. Curr. Biol. 2016;26:1–8. doi: 10.1016/j.cub.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmanová Z, et al. Early farmers from across Europe directly descended from Neolithic Aegeans. Proc. Natl. Acad. Sci. USA. 2016;113:6886–6891. doi: 10.1073/pnas.1523951113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khor CC, et al. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat. Genet. 2007;39:523–528. doi: 10.1038/ng1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willcocks LC, et al. A defunctioning polymorphism in FCGR2B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA. 2010;107:7881–7885. doi: 10.1073/pnas.0915133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clatworthy MR, et al. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proc. Natl. Acad. Sci. USA. 2007;104:7169–7174. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gazarini ML, Thomas AP, Pozzan T, Garcia CRS. Calcium signaling in a low calcium environment: how the intracellular malaria parasite solves the problem. J. Cell Biol. 2003;161:103–110. doi: 10.1083/jcb.200212130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockett KA, et al. Reappraisal of known malaria resistance loci in a large multicenter study. Nat. Genet. 2014;46:1197–1204. doi: 10.1038/ng.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pybus M, et al. 1000 Genomes Selection Browser 1.0: a genome browser dedicated to signatures of natural selection in modern humans. Nucleic Acids Res. 2014;42:D903–D909. doi: 10.1093/nar/gkt1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazaridis I, et al. Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature. 2014;513:409–413. doi: 10.1038/nature13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llamas B, et al. Ancient mitochondrial DNA provides high-resolution time scale of the peopling of the Americas. Sci. Adv. 2016;2:e1501385–e1501385. doi: 10.1126/sciadv.1501385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olalde I, et al. Derived immune and ancestral pigmentation alleles in a 7,000-year-old Mesolithic European. Nature. 2014;507:225–228. doi: 10.1038/nature12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skoglund P, et al. Genomic diversity and admixture differs for Stone-Age Scandinavian foragers and farmers. Science. 2014;344:747–750. doi: 10.1126/science.1253448. [DOI] [PubMed] [Google Scholar]
- 34.Gamba C, et al. Genome flux and stasis in a five millennium transect of European prehistory. Nat. Commun. 2014;5:5257. doi: 10.1038/ncomms6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seguin-Orlando A, et al. Paleogenomics. Genomic structure in Europeans dating back at least 36,200 years. Science. 2014;346:1113–1118. doi: 10.1126/science.aaa0114. [DOI] [PubMed] [Google Scholar]
- 36.Jones ER, et al. Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nat. Commun. 2015;6:8912. doi: 10.1038/ncomms9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassidy LM, et al. Neolithic and Bronze Age migration to Ireland and establishment of the insular Atlantic genome. Proc. Natl. Acad. Sci. USA. 2016;113:368–373. doi: 10.1073/pnas.1518445113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martiniano R, et al. Genomic signals of migration and continuity in Britain before the Anglo-Saxons. Nat. Commun. 2016;7:10326. doi: 10.1038/ncomms10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiffels S, et al. Iron Age and Anglo-Saxon genomes from East England reveal British migration history. Nat. Commun. 2016;7:10408. doi: 10.1038/ncomms10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu Q, et al. Genome sequence of a 45,000-year-old modern human from western Siberia. Nature. 2014;514:445–449. doi: 10.1038/nature13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu Q, et al. An early modern human from Romania with a recent Neanderthal ancestor. Nature. 2015;524:216–219. doi: 10.1038/nature14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu Q, et al. The genetic history of Ice Age Europe. Nature. 2016;534:200–205. doi: 10.1038/nature17993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirono A, Beutler E. Alternative splicing of human glucose-6-phosphate dehydrogenase messenger RNA in different tissues. J. Clin. Invest. 1989;83:343–346. doi: 10.1172/JCI113881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gouagna LC, et al. Genetic variation in human HBB is associated with Plasmodium falciparum transmission. Nat. Genet. 2010;42:328–331. doi: 10.1038/ng.554. [DOI] [PubMed] [Google Scholar]
- 45.Agarwal A, et al. Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood. 2000;96:2358–2363. [PubMed] [Google Scholar]
- 46.Zimmerman PA, et al. Emergence of FY*A(null) in a Plasmodium vivax-endemic region of Papua New Guinea. Proc. Natl. Acad. Sci. USA. 1999;96:13973–13977. doi: 10.1073/pnas.96.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tournamille C, Colin Y, Cartron JP, Le Van Kim C. Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat. Genet. 1995;10:224–228. doi: 10.1038/ng0695-224. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto F, Clausen H, White T, Marken J, Hakomori S. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990;345:229–233. doi: 10.1038/345229a0. [DOI] [PubMed] [Google Scholar]
- 49.Blumenfeld OO, Patnaik SK. Allelic genes of blood group antigens: a source of human mutations and cSNPs documented in the Blood Group Antigen Gene Mutation Database. Hum. Mutat. 2004;23:8–16. doi: 10.1002/humu.10296. [DOI] [PubMed] [Google Scholar]
- 50.Boren T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 51.Swerdlow DL, et al. Severe life-threatening cholera associated with blood group O in Peru: implications for the Latin American epidemic. J. Infect. Dis. 1994;170:468–472. doi: 10.1093/infdis/170.2.468. [DOI] [PubMed] [Google Scholar]
- 52.Gupta H, et al. Categorical complexities of Plasmodium falciparum malaria in individuals is associated with genetic variations in ADORA2A and GRK5 genes. Infect. Genet. Evol. 2015;34:188–199. doi: 10.1016/j.meegid.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Sudmant PH, et al. An integrated map of structural variation in 2,504 human genomes. Nature. 2015;526:75–81. doi: 10.1038/nature15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilbert MTP, et al. Distribution patterns of postmortem damage in human mitochondrial DNA. Am. J. Hum. Genet. 2003;72:32–47. doi: 10.1086/345378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weir BS, Hill WG. Estimating F-statistics. Annu. Rev. Genet. 2002;36:721–750. doi: 10.1146/annurev.genet.36.050802.093940. [DOI] [PubMed] [Google Scholar]
- 56.Goudet J, Roussett F. Testing Differentiation in Diploid Populations. Genetics. 1996;144:1933–1940. doi: 10.1093/genetics/144.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao X, Smith DL, Tatem AJ. Exploring the spatiotemporal drivers of malaria elimination in Europe. Malar. J. 2016;15:122. doi: 10.1186/s12936-016-1175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


