Abstract
Interleukin-35 (IL-35) has been proposed as a novel immune-suppressing cytokine. However, the function of IL-35 in malignant diseases is yet to be elucidated. The present study investigated IL-35 expression levels in laryngeal squamous cell carcinoma (LSCC) tissues and the peripheral blood of patients to explore the potential involvement of IL-35 in LSCC progression. In the present study, IL-35 expression levels in tissues and peripheral blood were analyzed by reverse transcription-quantitative polymerase chain reaction and an enzyme-linked immunosorbent assay. The association between IL-35 expression levels and clinical characteristics was also evaluated. The present results demonstrated that IL-35 expression in tumor tissues was significantly higher than in adjacent normal tissues, and a significant association between IL-35 expression levels in tissues and the tumor site was detected. Furthermore, the expression of IL-35 in the peripheral blood of patients was significantly decreased subsequent to tumor resection. No correlation between peripheral blood IL-35 expression and clinical characteristics was detected. In conclusion, the present study demonstrated that IL-35 is highly expressed in LSCC tissues and in the peripheral blood of patients with LSCC. There was a notable, significant reduction of peripheral blood IL-35 expression following surgical resection of tumors. These results may be useful for diagnostic or therapeutic purposes in patients with LSCC.
Keywords: interleukin-35, Epstein Barr virus-induced gene, interleukin-12 p35, laryngeal squamous cell carcinoma, peripheral blood
Introduction
Laryngeal squamous cell carcinoma (LSCC) is one of the most common head and neck cancers, accounting annually for ~2.4% of new malignancies globally (1,2). Despite advances in the diagnosis and treatment of LSCC, patient prognosis has not improved over the last 20 years (2). It has been demonstrated that the immune system is involved in LSCC tumor progression (3). Within this system, regulatory T cells (Tregs) are associated with suppressive activities against tumor-specific T-cell responses (3,4). Several studies have demonstrated that Treg prevalence is higher in the peripheral blood and multiple human tumors, including LSCC (5–8).
Interleukin-35 (IL-35) has previously been proposed as a novel immune-suppressing cytokine and a key effector molecule of Treg function (9,10). IL-35 consists of the Epstein-Barr virus-induced gene 3 (EBI3) and interleukin-12 (IL-12) p35 subunits, and is secreted by forkhead box P3 (Foxp3+) cluster of differentiation (CD) 4+CD25+ Tregs or a Foxp3− Treg population induced by IL-35 (10,11). IL-35 suppresses the activity of T-helper (Th) 1, Th2 and Th17 cells, and expands Tregs (11,12). IL-35 is required for maximal Treg activity and it alone is sufficient to suppress T-cell proliferation (10). EBI3 or IL-12 p35-deficient (IL-35 deficient) Tregs have significantly reduced regulatory activity in vitro and fail to cure inflammatory bowel disease in vivo (10). However, the precise underlying mechanism behind the involvement of IL-35 in malignant diseases has yet to be elucidated. Previous studies have revealed that IL-35 is expressed in several tumor tissues, including lung and colon cancer, esophageal, hepatocellular and cervical carcinoma (13,14). IL-35 has been demonstrated to promote tumor angiogenesis and inhibit an antitumor cytotoxic lymphocyte (CTL) response (15). In addition, IL-35 expression is considered to be associated with colorectal cancer progression and prognosis (14). These results suggest that IL-35 may be involved in tumor progression. However, little is known about IL-35 expression in LSCC. Thus, the present study investigated IL-35 expression levels in LSCC tissues and the peripheral blood of patients with LSCC to explore the potential involvement of IL-35 in LSCC progression.
Materials and methods
Patients and specimens
The present study enrolled 19 male patients with LSCC. All patients were between stage I and IV of the disease and were diagnosed and treated with surgical management in the Department of Otolaryngology, the 2nd Affiliated Hospital of Zhejiang University (Zhejiang, China) between June 2012 and December 2012. No patients received chemotherapy or radiotherapy prior to surgery. Postoperative radiotherapy was performed in addition to surgical treatment for 7 patients. To assess the advancement of the disease, the 2010 International Union Against Cancer tumor node metastasis (TNM) classification scale was used. Tumor tissues, including 19 paired adjacent normal tissues, were obtained from operative specimens and stored at −80°C until use. Blood samples were collected from each patient prior to and one week subsequent to surgery, and stored at −80°C until RNA extraction. Informed consent was obtained from all participants, and the present study was approved by the Ethics Committee of the 2nd Affiliated Hospital, School of Medicine, Zhejiang University.
RNA extraction and reverse transcription
Total RNA from tissues and blood samples was isolated using TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and suspended in diethylpyrocarbonate-treated water. Random primers were used to reverse-transcribe the extracted total RNA with SuperScript III Reverse Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.). Briefly, 5 µl of total RNA, 1 µl of random primer (Invitrogen; Thermo Fisher Scientific, Inc.), 1 µl of 10 mM dNTP Mix (Invitrogen; Thermo Fisher Scientific, Inc.), and 5 µl of diethylpyrocarbonate-treated water were denatured at 65°C for 5 min and immediately placed on ice for 1 min. The following reagents were added: 4 µl of 5X First-Strand Buffer (Invitrogen; Thermo Fisher Scientific, Inc.), 2 µl of 0.1 M dithiothreitol (Invitrogen; Thermo Fisher Scientific, Inc.), 1 µl of 40 U/µl RNaseout (Invitrogen; Thermo Fisher Scientific, Inc.), and 1 µl of 200 U/µl SuperScript III Reverse Transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.). The contents were incubated for 5 min at 25°C, followed by incubation for 60 min at 50°C and 15 min at 70°C. The synthesized first-strand of cDNA was used for subsequent quantitative polymerase chain reaction analysis (qPCR).
qPCR
qPCR analysis was performed with the use of the ABI PRISM 7500 Fast Real-time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.) and the SYBR-Green labeling method. The primer sequences used were as follows: EBI3 forward 5′-GCCACGTCCTTCATCCTCAG-3′ and reverse 5′-GCCGCCACTTGGACGTAGTA-3′; IL-12 p35 forward 5′-GCAAAGCTTCTGATGGATCCT-3′ and reverse 5′-GCCTGCATCAGCTCATCAAT-3′. All samples were performed in triplicate. The PCR conditions were as follows: 95°C for 2 min, 40 cycles of 95°C for 10 sec, 60°C for 30 sec, 70°C for 30 sec. Quantification of expression of the target gene in the samples was accomplished by measuring the fractional cycle number at which the amount of expression reached a fixed threshold (Cq). The relative gene expression levels were calculated using the 2−∆∆Cq method (16), which was normalized to the endogenous β-actin as a control. The data are expressed as n-fold relative to the control.
ELISA assay
Tissues were homogenized with the aid of a Polytron homogenizer in ice-cold PBS. The homogenates were centrifuged for 20 min at 10,000 × g at 4°C to remove debris and insoluble material, and the supernatants were assayed for total protein content by bicinchoninic acid assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). IL-35 concentration was assayed using a human IL-35 ELISA kit (#88-7357; eBioscience, Inc., San Diego, CA, USA) according to the manufacturer's protocol. The optical density was measured at 450 nm on an iMark Microplate Absorbance Reader (Bio-Rad Laboratories, Inc.). The reported concentration of IL-35 was determined by subtracting the concentration of IL-35 in PBS alone and represented by relative units according to standard samples provided by the kit. All assays were performed in triplicate.
Statistical analysis
The Student's t-test was used for comparisons between groups. The associations between IL-35 expression and clinicopathological factors were analyzed using the χ2 test. Pearson's correlation test was used for identifying the correlations between groups. SPSS 19.0 statistical software (IBM SSPS, Armonk, NY, USA) was used to conduct all statistical analysis. P≤0.05 was considered to indicate a statistically significant difference.
Results
Clinical data
The clinicopathological features of the patients were summarized in Table I. All patients were male, with a mean age of 64.6±6.8 years (range, 55–74 years). Patients were followed for a median of 32 months (range, 28–34 months). During the follow-up, 3 patients relapsed. Out of the 3 relapses, 2 recurred at the primary site and 1 patient had neck and lung metastasis. A total of 2 patients succumbed during the follow-up period.
Table I.
Clinicopathological features of 19 male patients with laryngeal squamous cell carcinoma.
| Age (years) | Tumor site | Tumor node metastasis stage | Histological grade |
|---|---|---|---|
| 58 | Glottic | II | G2 |
| 57 | Supraglottic | IV | G2 |
| 65 | Glottic | I | G1 |
| 74 | Glottic | I | G2 |
| 60 | Supraglottic | III | G2 |
| 69 | Glottic | I | G2 |
| 62 | Supraglottic | IV | G2 |
| 55 | Supraglottic | II | G2 |
| 74 | Supraglottic | IV | G2 |
| 68 | Glottic | III | G3 |
| 74 | Glottic | II | G3 |
| 74 | Glottic | II | G1 |
| 58 | Glottic | I | G1 |
| 70 | Glottic | IV | G1 |
| 57 | Supraglottic | III | G3 |
| 59 | Glottic | III | G2 |
| 71 | Glottic | I | G1 |
| 63 | Glottic | III | G1 |
| 59 | Glottic | II | G1 |
G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated.
Expression of IL-35 in LSCC tissues
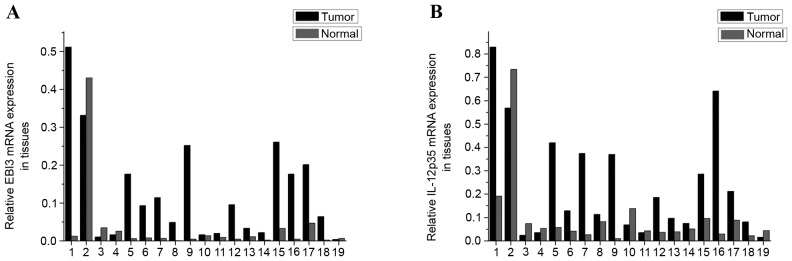
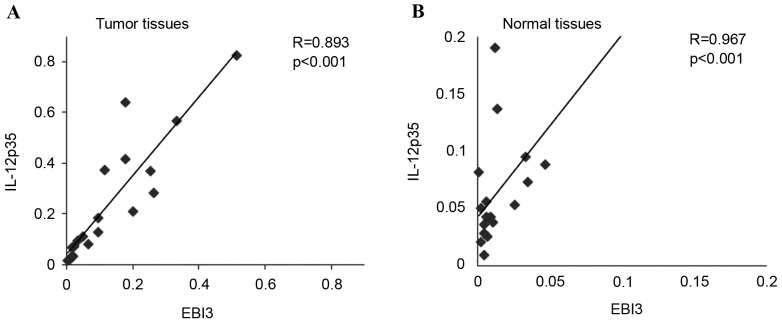
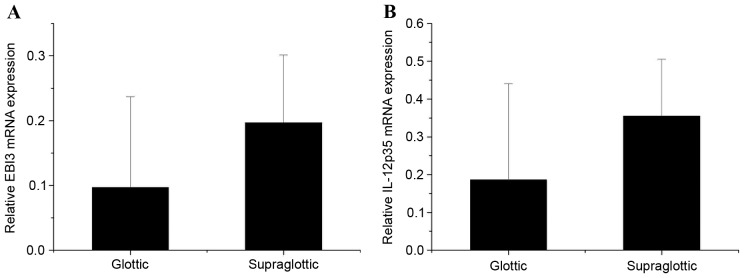
The present study revealed that EBI3 and IL-12p35 gene expression levels in LSCC tumor samples were significantly higher than that in normal tissues (P=0.004 and P=0.016, respectively; Fig. 1A and B, respectively). In addition, expression levels of the EBI3 gene were positively correlated with the IL-12p35 gene in tumor tissues (Fig. 2A) and normal tissues (Fig. 2B). These results revealed that the expression levels of the IL-35 gene in LSCC tissues was higher than that in normal tissues, which prompted the exploration of the concentration of IL-35 in tissue lysates. The ELISA analysis results revealed that the expression of IL-35 in LSCC tissues was also significantly higher than that in normal tissues (P=0.001; Fig. 3). Significantly higher expression levels of the EBI3 and IL-12p35 genes were detected in supraglottic LSCC compared with that in glottic LSCC (P=0.028 and P=0.035, respectively; Fig. 4A and B, respectively). The ELISA results also indicated such differences, although the differences were not statistically significant (P=0.106; Fig. 5). No correlation was detected in terms of TNM stage and histology grade with either EBI3 or IL-12p35 gene expression determined by qPCR, or IL-35 expression determined by ELISA.
Figure 1.
mRNA expression levels of (A) EBI3 and (B) IL-12 p35 in laryngeal squamous cell carcinoma tumor samples and normal tissues.
Figure 2.
Correlation analysis between the expression of EBI3 and IL-12 p35 in (A) tumor tissues and (B) normal tissues. EBI3, Epstein-Barr virus-induced gene 3; IL-12, interleukin 12.
Figure 3.
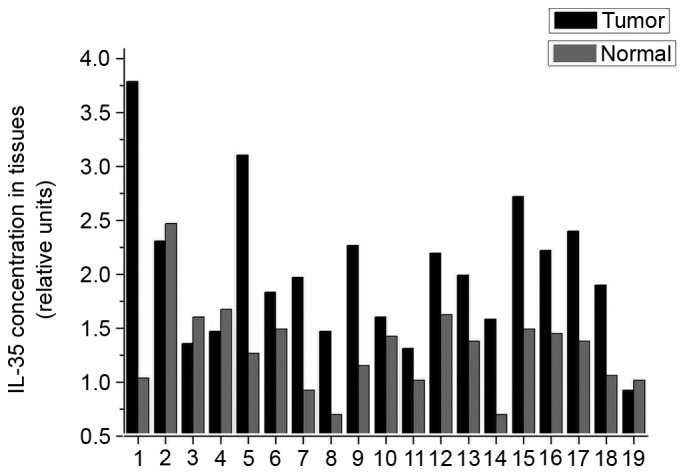
ELISA analysis of IL-35 protein expression in tumor tissues and normal tissues. IL-35, interleukin 35.
Figure 4.
mRNA expression levels of (A) EBI3 and (B) IL-12 p53 in supraglottic and glottic laryngeal squamous cell carcinoma. EBI3, Epstein-Barr virus-induced gene 3; IL-12, interleukin 12.
Figure 5.
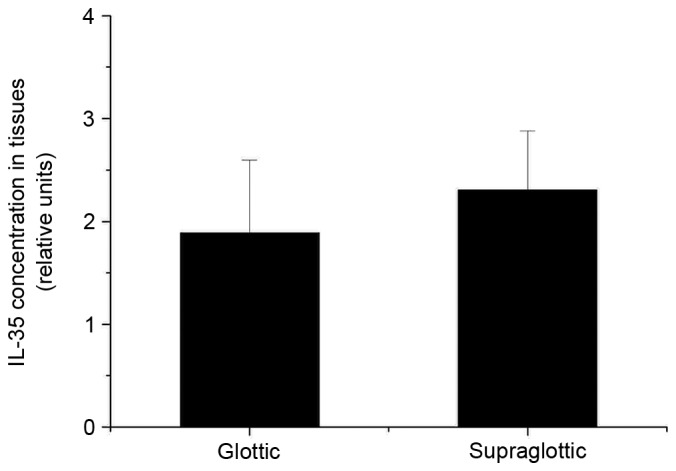
ELISA analysis of IL-35 protein expression levels in supraglottic and glottic laryngeal squamous cell carcinoma. IL-35, interleukin 35.
Expression of EBI3 and IL-12p35 mRNA in the peripheral blood of surgical LSCC patients
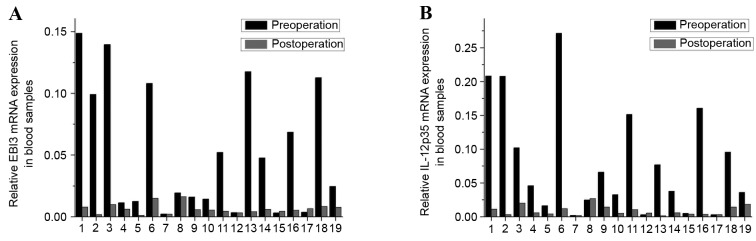
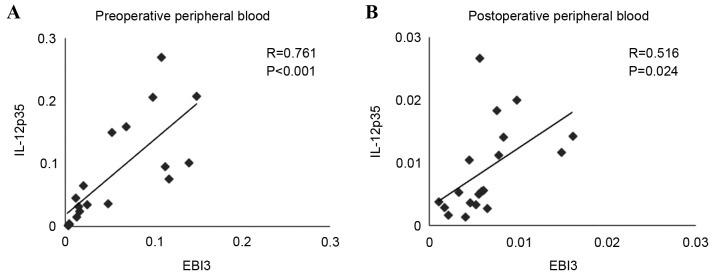
The expression levels of EBI3 and IL-12p35 mRNA were significantly reduced in the peripheral blood of postoperative patients compared with preoperative (P<0.001 and P=0.001, respectively; Fig. 6A and B, respectively). Similarly, the expression levels of the EBI3 gene were positively correlated with the IL-12p35 gene in preoperative (Fig. 7A) and postoperative blood (Fig. 7B). These results indicated that the expression of IL-35 in the peripheral blood of patients with LSCC was decreased following tumor resection. However, associations between the expression of EBI3 or IL-12p35 mRNA in the peripheral blood and clinicopathological parameters, including tumor site, TNM stage and histological grade, were not observed.
Figure 6.
mRNA expression levels of (A) EBI3 and (B) IL-12 p53 in the peripheral blood of preoperative and postoperative patients. EBI3, Epstein-Barr virus-induced gene 3; IL-12, interleukin 12.
Figure 7.
Correlation analysis between the expression of EBI3 and IL-12 p35 in (A) preoperative and (B) postoperative peripheral blood. EBI3, Epstein-Barr virus-induced gene 3; IL-12, interleukin 12.
The association between IL-35 expression and the prognosis of patients with LSCC
The present study analyzed the differences of IL-35 expression in tumor tissues or preoperative peripheral blood between relapsed and nonrelapsed patients. No significant difference in IL-35 expression was identified between relapsed and nonrelapsed patients.
Discussion
IL-35 is a novel member of the IL-12 family, which also includes IL-12, IL-23 and IL-27 (10). The IL-12 cytokine family members are well documented as important regulators of pro- and anti-inflammatory immune responses (10,17). The IL-12 family is unique in having the only heterodimeric cytokines, each member of which shares a and b chains as well as receptor subunits with other members of the family (17).
IL-35 shares the IL-12 p35 and EBI3 subunits with IL-12 and IL-27, respectively (10). The IL-35 receptor is composed of IL-12Rβ2 and glycoprotein 130 (gp130) subunits, which are shared with IL-12 and IL-27, respectively (18). IL-12 and IL-27 induce immune responses against tumors through their direct effects on tumors, via angiogenesis and lymphocytes (17,19). However, distinct from other members of the IL-12 family, IL-35 is a novel inhibitory cytokine and suppresses T cell proliferation through the unconventional signal transducer and activator of transcription (STAT) 1-STAT4 heterodimer signaling pathway (18). Previous studies have focused on the involvement of IL-35 in tumor immunity, and revealed that IL-35 is involved in tumor development and progression and is associated with poor prognosis (14,15,20–22).
The present report analyzed the expression of IL-35 in patients with LSCC. To the best of our knowledge, this is the first study to explore the function of this novel cytokine in LSCC. The present results revealed that the expression of IL-35 in LSCC tumor tissues is higher than in adjacent normal tissues, which is consistent with the data obtained from other tumors (14,21). In addition, IL-35 expression levels were revealed to be higher in supraglottic tumors than glottic tumors, which have been demonstrated to have improved prognosis compared with supraglottic tumors. These results indicated that IL-35 may be involved in the pathogenesis of LSCC. In the tumor microenvironment, cancer cells, Tregs and tumor-infiltrating dendritic cells were considered to be sources of IL-35 (15,23,24). It has been revealed that tumor-derived IL-35 increases tumorigenesis with a pro-tumor effect, and IL-35 production in the tumor microenvironment increases CD11b+Grl+ myeloid-derived suppressor cell (MDSC) accumulation and tumor angiogenesis (15). MDSCs induce immune suppression and inhibit CTL responses (25,26).
Wang et al (15) revealed that IL-35 does not directly inhibit CTL proliferation, differentiation or effector functions. The inhibition of CTL responses in tumors overexpressing IL-35 may be caused by the accumulation of MDSCs, which subsequently inhibits CTL responses (15). In addition, the authors identified that IL-35 upregulates gp130 to mediate cancer cell resistance to CTL destruction (15). Nicholl et al (20) stated that IL-35, an autocrine growth factor for pancreatic adenocarcinoma, promotes proliferation and inhibits apoptosis. In a study by Fan et al (22) IL-35 was considered to be associated with the genesis of gastric cancer. However, there are still contradictory results. Long et al (13) demonstrated that over-expression of IL-35 in human cancer cells inhibits cancer cell growth in vitro, and that IL-35 suppresses tumor growth via cell cycle arrest at the G1 phase and substantially sensitized cells to serum starvation-induced apoptosis through downregulation of cyclin D1 and survivin expression.
In addition to the results in LSCC tissues, the present study analyzed peripheral blood IL-35 levels. IL-35 expression levels in the peripheral blood of patients with LSCC were reduced significantly following surgical resection of tumor tissues. Similar results have been reported in patients with colorectal cancer (14). Hamidinia et al (27) demonstrated a significant upregulation of IL-35 expression in the peripheral blood of breast cancer patients compared with healthy controls. These results may be useful for diagnostic or therapeutic purposes in patients with malignant diseases.
The present study also explored the association between IL-35 expression and the prognosis of patients with LSCC. While no significant association was identified in the present study, an insufficient number of cases may have contributed to this lack of significance. In previous studies, over-expression of IL-35 in peripheral blood has been recognized as an unfavorable prognostic factor for malignant tumors, including nasopharyngeal carcinoma, pancreatic ductal adenocarcinoma, colorectal and non-small cell lung cancer (14,21,28,29). Nevertheless, another study detected increased IL-35 expression in early stage breast cancer and non-metastatic cases (27).
In conclusion, the present study demonstrated that IL-35 expression was increased in LSCC tissues and the peripheral blood of patients with LSCC. Notably, there was a significant reduction of IL-35 expression in the peripheral blood following surgical resection of LSCC tumors. Due to controversial results regarding the involvement of IL-35 in tumor progression and prognosis, further investigations are required to determine the exact biological involvement of this novel cytokine in the genesis and development of LSCC.
Acknowledgements
The present study was supported by grants from the Department of Education of Zhejiang Province (grant no. Y201225814), the Department of Public Health of Zhejiang Province (grant no. 2012KYB100), the Natural Science Foundation of Zhejiang Province (grant no. LY15H130002) and the Department of Science and Technology of Zhejiang Province (grant no. 2015C33115).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Marioni G, Marchese-Ragona R, Cartei G, Marchese F, Staffieri A. Current opinion in diagnosis and treatment of laryngeal carcinoma. Cancer Treat Rev. 2006;32:504–515. doi: 10.1016/j.ctrv.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Alhamarneh O, Amarnath SM, Stafford ND, Greenman J. Regulatory T cells: What role do they play in antitumor immunity in patients with head and neck cancer? Head Neck. 2008;30:251–261. doi: 10.1002/hed.20739. [DOI] [PubMed] [Google Scholar]
- 4.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 5.Chen B, Zhang D, Zhou J, Li Q, Zhou L, Li SM, Zhu L, Chou KY, Zhou L, Tao L, Lu LM. High CCR6/CCR7 expression and Foxp3+ Treg cell number are positively related to the progression of laryngeal squamous cell carcinoma. Oncol Rep. 2013;30:1380–1390. doi: 10.3892/or.2013.2603. [DOI] [PubMed] [Google Scholar]
- 6.Sun W, Li WJ, Fu QL, Wu CY, Lin JZ, Zhu XL, Hou WJ, Wei Y, Wen YH, Wang YJ, Wen WP. Functionally distinct subsets of CD4+ regulatory T cells in patients with laryngeal squamous cell carcinoma are indicative of immune deregulation and disease progression. Oncol Rep. 2015;33:354–362. doi: 10.3892/or.2014.3553. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A, Hirohashi S. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 8.Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292. doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banchereau J, Pascual V, O'Garra A. From IL-2 to IL-37: The expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012;13:925–931. doi: 10.1038/ni.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 11.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37:3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 13.Long J, Zhang X, Wen M, Kong Q, Lv Z, An Y, Wei XQ. IL-35 over-expression increases apoptosis sensitivity and suppresses cell growth in human cancer cells. Biochem Biophys Res Commun. 2013;430:364–369. doi: 10.1016/j.bbrc.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Zeng JC, Zhang Z, Li TY, Liang YF, Wang HM, Bao JJ, Zhang JA, Wang WD, Xiang WY, Kong B, et al. Assessing the role of IL-35 in colorectal cancer progression and prognosis. Int J Clin Exp Pathol. 2013;6:1806–1816. [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Liu JQ, Liu Z, Shen R, Zhang G, Xu J, Basu S, Feng Y, Bai XF. Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J Immunol. 2013;190:2415–2423. doi: 10.4049/jimmunol.1202535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Vignali DA, Kuchroo VK. IL-12 family cytokines: Immunological playmakers. Nat Immunol. 2012;13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, Satoskar AR, Garcia KC, Hunter CA, Drake CG, et al. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol. 2012;13:290–299. doi: 10.1038/ni.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsui M, Kishida T, Nakano H, Yoshimoto K, Shin-Ya M, Shimada T, Nakai S, Imanishi J, Yoshimoto T, Hisa Y, Mazda O. Interleukin-27 activates natural killer cells and suppresses NK-resistant head and neck squamous cell carcinoma through inducing antibody-dependent cellular cytotoxicity. Cancer Res. 2009;69:2523–2530. doi: 10.1158/0008-5472.CAN-08-2793. [DOI] [PubMed] [Google Scholar]
- 20.Nicholl MB, Ledgewood CL, Chen X, Bai Q, Qin C, Cook KM, Herrick EJ, Diaz-Arias A, Moore BJ, Fang Y. IL-35 promotes pancreas cancer growth through enhancement of proliferation and inhibition of apoptosis: Evidence for a role as an autocrine growth factor. Cytokine. 2014;70:126–133. doi: 10.1016/j.cyto.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Sun H, Wu H, Tan Q, Xiang K. Interleukin 35 is an independent prognostic factor and a therapeutic target for nasopharyngeal carcinoma. Contemp Oncol (Pozn) 2015;19:120–124. doi: 10.5114/wo.2014.44754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan YG, Zhai JM, Wang W, Feng B, Yao GL, An YH, Zeng C. IL-35 over-expression is associated with genesis of gastric cancer. Asian Pac J Cancer Prev. 2015;16:2845–2849. doi: 10.7314/APJCP.2015.16.7.2845. [DOI] [PubMed] [Google Scholar]
- 23.Niedobitek G, Päzolt D, Teichmann M, Devergne O. Frequent expression of the Epstein-Barr virus (EBV)-induced gene, EBI3, an IL-12 p40-related cytokine, in Hodgkin and Reed-Sternberg cells. J Pathol. 2002;198:310–316. doi: 10.1002/path.1217. [DOI] [PubMed] [Google Scholar]
- 24.Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–8331. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 25.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–2563. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 26.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamidinia M, Boroujerdnia Ghafourian M, Talaiezadeh A, Solgi G, Roshani R, Iranprast S, Khodadadi A. Increased P-35, EBI3 transcripts and other treg markers in peripheral blood mononuclear cells of breast cancer patients with different clinical Stages. Adv Pharm Bull. 2015;5:261–267. doi: 10.15171/apb.2015.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu X, Tian T, Zhang B, Liu Y, Yuan C, Shao L, Guo Y, Fan K. Elevated plasma interleukin-35 levels predict poor prognosis in patients with non-small cell lung cancer. Tumour Biol. 2015;36:2651–2656. doi: 10.1007/s13277-014-2887-8. [DOI] [PubMed] [Google Scholar]
- 29.Jin P, Ren H, Sun W, Xin W, Zhang H, Hao J. Circulating IL-35 in pancreatic ductal adenocarcinoma patients. Hum Immunol. 2014;75:29–33. doi: 10.1016/j.humimm.2013.09.018. [DOI] [PubMed] [Google Scholar]