Abstract
Background
In 2011, Malawi launched Option B+, a program of universal ART treatment for pregnant and lactating women to optimize maternal health and prevent pediatric HIV infection. For optimal outcomes, women need to achieve HIVRNA suppression. We report 6 month HIVRNA suppression and HIV drug resistance in the PURE study.
Methods
PURE study was a cluster-randomized controlled trial evaluating three strategies for promoting uptake and retention; Arm 1: Standard of Care, Arm 2: Facility Peer Support and Arm 3: Community Peer support. Pregnant and breastfeeding mothers were enrolled and followed according to Malawi ART guidelines. Dried blood spots for HIVRNA testing were collected at 6 months. Samples with ART failure (HIVRNA ≥1000 copies/ml) had resistance testing. We calculated odds ratios for ART failure using generalized estimating equations with a logit link and binomial distribution.
Results
We enrolled 1269 women across 21 sites in Southern and Central Malawi. Most enrolled while pregnant(86%) and were WHO Stage 1(95%). At 6 months, 950/1269 (75%) were retained; 833/950 (88%) had HIVRNA testing conducted and 699/833(84%) were suppressed. Among those with HIVRNA ≥1000 copies/ml with successful amplification (N=55, 41% of all VL> 1000 copies/ml), confirmed HIV resistance was found in 35% (19/55), primarily to the Non-nucleoside reverse transcriptase inhibitors(NNRTI) class of drugs. ART failure was associated with treatment default but not study arm, age, WHO stage, or breastfeeding status.
Conclusions
Virologic suppression at 6 months was <90% targets, but the observed confirmed resistance rates suggest adherence support should be the primary approach for early failure in Option B+.
Keywords: HIV drug Resistance, Option B+
Background
In 2011, Malawi launched Option B+, a program of universal antiretroviral therapy (ART) for pregnant and lactating women regardless of CD4+ cell count and/or any clinical stage to optimize maternal health and prevent pediatric HIV infections1,2. The early results of the Malawi program have been dramatic and positive: by 2012, the number of HIV infected women receiving ART increased by 7 fold3, over 77% of women were retained in the program through 12 months post-initiation3,4, 96% of retained women had HIV RNA < 400 copies at 6 months post initiation5 and HIV infection rates among exposed children tested at < 2months were 2.4%4.
Alongside the Option B+ program, the Malawi ART program has advanced routine virological monitoring to assess treatment success. To date, results suggest high levels of suppression among patients retained in care.6 The current schedule for monitoring includes HIVRNA testing at 6 months post ART initiation, 2 years, and every 2 years thereafter. The 6 month collection time point, early in the treatment course, is designed to detect early adherence problems that may be addressed through appropriate counseling interventions. Alternatively, this early time point may detect individuals who are failing treatment due to pre-existing transmitted ART resistance.
The prevalence of ART drug resistance among Option B+ women in Malawi has not yet been described. A prior survey in Malawi found high virological suppression at 12 months (91%)7,8. However, the estimated prevalence of pre-treatment HIV resistance ranged from 2.8 and 6.5%7 which accounted for 37.5% of the detected resistance at 12 months. A more recent report from Malawi suggests that between 5-15% of pregnant women have transmitted HIV drug resistance using the WHO threshold survey (personal communication, Nellie Wadonda Kabondo), predominantly to the non-nucleoside reverse transcriptase inhibitor (NNRTI) class of drugs which forms the back bone of the most popular first line therapy worldwide 9.
In order to understand the relative contributions of pre-existing resistance and ART adherence to treatment failure, this study evaluated the HIV drug resistance at 6 months among women experiencing treatment failure in the PURE (PMTCT Uptake and REtention) study, a cluster randomized trial evaluating strategies for promoting uptake and retention in the Option B+ program in Malawi.
Methods
Study Design and Population
The PURE study was a cluster-randomized trial evaluating three strategies for promoting ART uptake and retention in care; Arm 1: Standard of Care, Arm 2: Facility Based peer support, and Arm 3: Community based peer support10,11. The PURE study was conducted in 21 sites in Central and Southern Malawi. Pregnant and Breastfeeding mothers were enrolled at the time of HIV diagnosis in the ANC or post-partum clinics and followed according to Malawi ART management protocols.
Clinical Follow up
The Malawi national guidelines recommend that newly diagnosed pregnant and breast feeding women initiate Tenofovir/Lamivudine/Efavirenz (TDF/3TC/EFV) therapy as a single fixed dose combination tablet. Women were seen monthly for the first 6 months, and then quarterly. Dried blood spots (DBS) were collected at the time of ART initiation, 6 months and 2 years for HIVRNA testing and possible resistance testing. For the viral load (VL) and resistance analysis, DBS collected through month 11 were considered as “6 month” specimens and all women with VL data were included regardless of whether they had any default episodes during the follow-up period. Default within the national program is defined as missing an antiretroviral appointment by 60 days but such individuals can return to care. Demographics, WHO stage, and treatment outcomes were collected using the standard HIV monitoring tools, extracted and double entered into an Access database.
Sample Collection and Resistance Testing
HIVRNA ≥ 1000 copies/ml was considered ART failure and those samples were sent for additional resistance testing. Drug resistance testing was also conducted on pre-treatment samples of those with detected drug resistance at month 6.
All health care staff at the sites were trained in the collection of DBS for HIVRNA using DBS at the initial study training and received refresher training by the study coordinators prior to the 6 month follow-up time point. DBS cards were collected by ART clinic using fingerstick. Cards were dried, packaged with desiccant sachets, stored on-site, and transported at ambient temperature to the UNC Project laboratory in Lilongwe (15 minutes to 6 hours away depending on the study site). VL testing was conducted on DBS cards using Abbott RealTime HIV-1 Assay (Abbott Laboratories, Chicago, IL). After testing, cards were returned to plastic zip bags, desiccant was replaced as necessary, and cards were eventually transferred to a -20°C freezer. DBS cards where the VL was ≥1000 copies/ml and the corresponding baseline samples were shipped on dry ice to the United States for sequencing analysis.
Resistance testing
RNA was extracted from DBS cards using the Sample Preparation System for RNA on the Abbott m2000sp (Abbott Laboratories). One to three entire spots (depending on availability) were eluted in 1.7ml RNA Lysis Buffer (Promega) for 2 hours at ambient temperature and extracted using the Abbott 0.6 ml protocol. Previously described primers 12 did not produce any amplicons, so new nested primers for smaller regions of the RT gene (codons 41-116 and 135-230) were used. RNA was concentrated using RNA Clean and Concentrator columns (Zymo Research), eluting into 10ul water. The concentrated RNA was used to synthesize a single cDNA strand with 2 downstream primers and SuperScript III reverse transcriptase (Invitrogen). One-quarter of the reaction was used in a nested PCR using the Expand High Fidelity PCR System (Roche Life Science). The two RT regions were amplified and sequenced. Successful sequences were analyzed using Sequencher 5.3 software and the results were submitted to the Stanford University Drug Resistance Database (http://hivdb.stanford.edu) to determine resistance mutations. In some cases, one of the two RT regions did not amplify, so resistance was only scored for the region that was successfully amplified.
Statistical Analysis
Associations with ART failure (VL≥1000 copies/ml) were evaluated using generalized estimating equations (GEE) with a logit link and binomial distribution and exchangeable correlation structure, to account for clustering. Variables of interest included study arm, age (>=25 years vs <25 years), pregnancy vs lactating at ART initiation, and WHO stage (Stage 1 vs. other). Resistance patterns were described (any NRTI resistance, any NNRTI resistance, and any detected reverse transcriptase resistance) among amplified specimens. All Analyses were conducted using Stata Version 14.0.
Ethics Statement
This study was approved by the National Health Sciences Research Committee, the University of North Carolina Institutional Review Board, and the WHO Ethics Review Committee. All participants provided written informed consent.
Results
This study enrolled 1272 women across 21 sites in Southern and Central Malawi, of which 3 were deemed ineligible and not included in analysis. The majority enrolled while pregnant (86%), were WHO Stage 1(95%), and the median age was 26 years (IQR 22-31) (Table 1). Only 6 women (0.4%) had ever received any previous PMTCT intervention. At the 6 months evaluation time (512 person years), 950/1269 (75%) were retained in care, of whom 833/950 (88%) had viral load testing conducted, with Arm 1 (standard of care) having a lower proportion of viral load specimens collected (Table 2). Notably, ninety two individuals were documented to have defaulted from care but returned to receive HIVRNA during the evaluations period [Arm A: 23/447 (5.1%), Arm B: 33/428 (7.7%), and Arm C: 35/394 (8.9%), p=0.096]. The median duration of ART at the time of collection was 260 days (IQR 232, 295) and was similar across arms (p=0.326). At 6 months, 699/833 (84%) were suppressed with VL<1000 copies/ml. There was no difference between the arms (Table 2), but those with a default episode had lower rates of virologic suppression than those with continuous retention (56/92 (61%) vs. 643/742(87%), p<0.001).
Table 1. Baseline Characteristics of 1269 Women participating in PURE study.
| Arm 1 | % | Arm 2 | % | Arm 3 | % | |
|---|---|---|---|---|---|---|
| N | 447 | 428 | 394 | |||
| Health Zone | ||||||
| Central West | 144 | 32.2% | 40 | 9.3% | 88 | 22.3% |
| South East | 179 | 40.0% | 177 | 41.4% | 229 | 58.1% |
| South West | 124 | 27.7% | 211 | 49.3% | 77 | 19.5% |
| Facility size | ||||||
| smallest (20) | 119 | 26.6% | 122 | 28.5% | 88 | 22.3% |
| small (50) | 126 | 28.2% | 103 | 24.1% | 104 | 26.4% |
| medium (75) | 75 | 16.8% | 78 | 18.2% | 77 | 19.5% |
| large(125) | 127 | 28.4% | 125 | 29.2% | 125 | 31.7% |
| Pregnant/Lactating | ||||||
| Pregnant | 401 | 89.7% | 360 | 84.1% | 333 | 84.5% |
| Lactating | 46 | 10.3% | 68 | 15.9% | 61 | 15.5% |
| Age | ||||||
| 15-21 | 79 | 17.7% | 100 | 23.4% | 87 | 22.2% |
| 22-28 | 174 | 38.9% | 175 | 40.9% | 139 | 35.3% |
| 29-35 | 157 | 35.1% | 120 | 28.0% | 129 | 32.7% |
| ≥36 | 35 | 7.8% | 31 | 7.2% | 37 | 9.4% |
| Missing | 2 | 0.4% | 2 | 0.5% | 2 | 0.5% |
| Marital Status | ||||||
| Never married | 7 | 1.5% | 5 | 1.2% | 9 | 2.3% |
| Married | 419 | 93.7% | 393 | 91.8% | 363 | 92.1% |
| Divorced | 12 | 2.7% | 18 | 4.2% | 19 | 4.8% |
| Widowed | 5 | 1.1% | 9 | 2.1% | 3 | 0.8% |
| Missing | 4 | 0.9% | 3 | 0.7% | ||
| Currently having a sexual partner | ||||||
| Yes | 426 | 95.3% | 400 | 93.4% | 364 | 92.4% |
| No | 13 | 2.9% | 20 | 4.7% | 27 | 6.9% |
| Missing | 8 | 1.8% | 8 | 1.9% | 3 | 0.8% |
| Current sexual partner living in catchment area | ||||||
| Yes | 355 | 83.3% | 328 | 82.0% | 308 | 84.6% |
| No | 49 | 11.5% | 50 | 12.5% | 33 | 9.1% |
| Missing | 22 | 5.2% | 22 | 5.5% | 23 | 6% |
Table 2. Virologic and Resistance outcomes at 6 months according to study arm.
| Outcome | Arm 1 | Arm 2 | Arm 3 | p-value |
|---|---|---|---|---|
| Number enrolled (N) | 447 | 428 | 394 | N/A |
| Retained at 6 mo (n/N, %) | 312 (70%) | 323 (75%) | 315 (80%) | |
| Duration of ART use at time of sampling (median, IQR) | 263(232-294) | 252 (228-292) | 267(239-302) | 0.326 |
| HIVRNA collected (n/N, %) | 247 (55%) | 312 (72%) | 274 (70%) | <0.001 |
| < 1000 Copies/ml (n, %) | 208 (84%) | 254(81 %) | 237 (87%) | 0.20 |
| 1000< 5000 copies/ml (n, %) | 24 (10%) | 26 (8 %) | 21 (8 %) | |
| >5000 copies/ml (n, %) | 15 (6 %) | 32 (10%) | 16 (6%) | |
| Log10 HIVRNA (median, IQR) | 3.4 (3.2-4.4) | 3.9 (3.3-4.6) | 3.5 (3.3-4.6) | 0.723 |
| Eligible for Resistance testing | 39 | 58 | 37 | N/A |
| Conducted Resistance (n, %) | 34 (87%) | 56 (97%) | 27 (73%) | 0.003 |
| Failed to Amplify (n, %) | 20 (59%) | 27 (48%) | 15 (56%) | 0.226 |
| Amplified (n, %) | 14 (41%) | 29 (52%) | 12 (44%) | 0.226 |
| “Any Resistance”, (n, %) | 3 (8%) | 9(24%) | 7 (58%) | 0.198 |
| NRTI resistance | 1 (7%) | 1 (3%) | 3 (25%) | 0.130 |
| NNRTI resistance | 3 (8%) | 9 (24%) | 7 (58%) | 0.198 |
Overall, 134 individuals (16%) had VL>=1000 copies/ml were eligible for resistance testing (Figure 1). 117/134 (87%) specimens were evaluated for drug resistance with some variation across study arms (Table 2).
Figure 1.
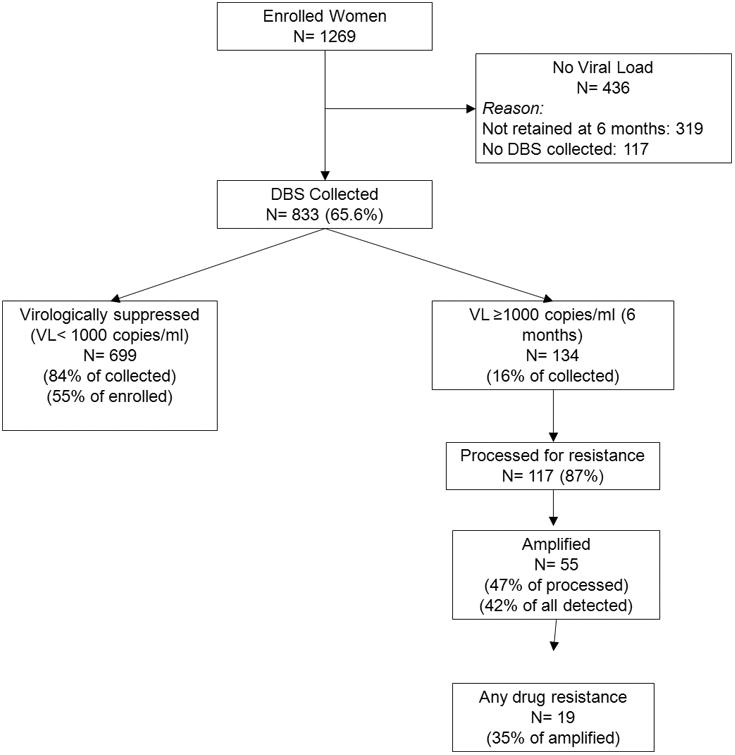
Flow Algorithm highlighting participant and sample flow to evaluate HIV drug resistance.
Overall, 62/117 (53%) failed to amplify either amplicon (RT codons 41-116 and 135-230), which did not vary across arms. Failure to amplify was strongly associated with having lower HIVRNA at the time of failure evaluation. Specifically, 50/62 (81%) of samples that failed to amplify had HIVRNA < 5000 copies/ml. Of those samples that amplified (55), 26 amplified for both primer domains, 17 for region 1 (RT codons 41-116), and 12 for region 2 (RT codons 135-230).
Among the specimens that amplified, 19/55 (35%) had resistance detected with some samples having multiple mutations. NNRTI resistance was more common [19/55 (35%) of amplified specimens] than NRTI resistance [4/55 (7.4%) of amplified specimens]; NRTI resistance was only seen in combination with NNRTI resistance. While not statistically significant, higher rates of confirmed resistance were seen in both of the intervention arms. Also, there was no difference in detected drug resistance patterns among those with default episodes. However, overall more women with default episodes [5/92 (5%)] had confirmed resistance than women with continuous ART use[14/741 (2%)] by virtue of the higher rate of detectable HIVRNA (see Figure 3, Supplementary Digital Content).
The pattern of NNRTI resistance included the K103N (6), G190 (4), e138 (4), Y181C (3), v106 (3), v90 (3), K101E (2), P225H (2), A98G (1), V179D (1), Y188l (1), L100i (1) while the NRTI resistance included the K65R (3), M184V (3), A62 (2), K70 (1), Y115(1)and T69N (1) (see table, Supplementary Digital Content 1). Overall, 13 individuals had major mutations that would confer reduced susceptibility to current first line and an additional 6 had minor mutations not expected to reduce susceptibility. Among those with detected ART resistance (19), 10 baseline samples were successfully amplified, of which 2 demonstrated baseline resistance. For one individual (Arm 1), both NNRTI (A98G, K101E, Y181C) and NRTI (M184V A62V) was present at baseline and this individual added the K65R mutation by month 6. No previous exposure to ART was reported. The other individual (Arm 2) had NNRTI resistance only and had no new mutations identified at month 6. The pre-treatment ART exposure of this individual was unknown.
Overall, according to arm, Arm 2 and 3 had a higher proportion of suppressed VL representing resistance prevention (Figure 2) with Arm 1 have 46% (95% CI 42-51%) suppression while Arm 2 and Arm 3 had 59% (95% CI 55-64) and 60% (95% CI 55-65) suppression, respectively. Confirmed resistance represented an extremely small fraction of the population.
Figure 2.
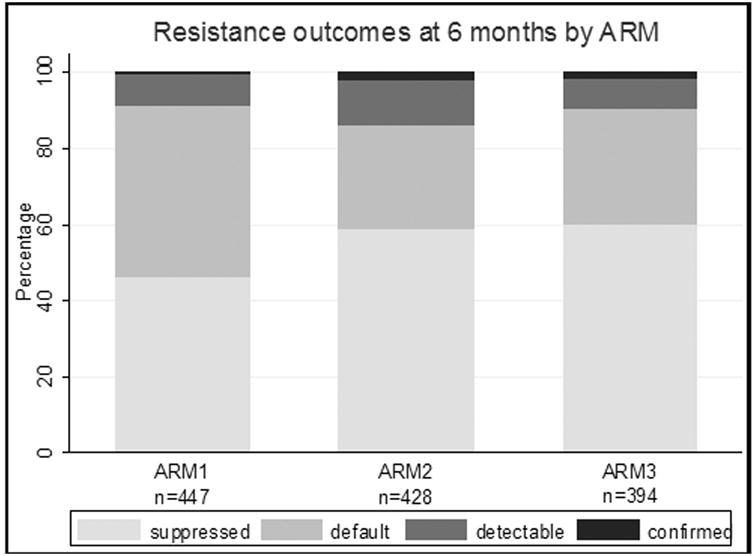
HIV drug resistance prevention outcomes at 6 months according to study arm. HIV viral suppression (Resistance prevention) was higher in Arm 2 and Arm 3.
Additionally, using VL<1000 copies as the failure definition, we found no association with ART failure rate according to age (< 25 vs. 25 and above), WHO stage (Stage 1 vs. other), or pregnant vs. breastfeeding status or treatment arm on unadjusted or adjusted analysis (Table 3). However, those without any default episodes were more likely to have achieved virological suppression (OR 0.23 95% CI 0.14-0.38).
Table 3. Adjusted and Unadjusted Logistic Regression showing factors associated with Virologic Suppression at 6 months.
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
|
| ||
| Study Arm | ||
| Arm 1 (Referent) | 1.0 | 1.0 |
| Arm 2 | 0.81 (0.45- 1.40) | 0.76 (0.43-1.30) |
| Arm 3 | 1.13 (0.61 - 2.12) | 1.17 (0.65-2.10) |
|
| ||
| Age | ||
| <25 (Referent) | 1.0 | 1.0 |
| >= 25 | 0.91 (0.63-1.33) | 1.01 (0.68-1.49) |
|
| ||
| WHO stage | ||
| Stage 1 (Referent) | 1.0 | 1.0 |
| Stage 2, 3 or 4 | 1.87 (0.82-4.27) | 2.01 (0.84-4.81) |
|
| ||
| Pregnancy status† | ||
| Pregnant (Ref) | 1.0 | 1.0 |
| Lactating | 0.83 (0.49-1.40) | 0.71 (0.41-1.22) |
|
| ||
| Default Event | ||
| Yes (Referent) | 1.0 | 1.0 |
| No | 0.25 (0.15-0.42) | 0.23 (0.14-0.38) |
Refers to status at the time of enrolment into the study, not at the time of HIVRNA collection.
Also, to further understand the potential of previous unreported ART exposure including single dose NVP on virologic suppression and resistance, we evaluated the results according to gravidity status of the women. 864/1269 (68%) of the women had recorded data on gravidity. There was no difference in virologic suppression rates among primagravida women vs. multiparous women (84% (61/73) vs 84% (429/511), p=0.932). With respect to confirmed resistance, 1/7 (14.3%) primigravida women had resistance versus 9/32 (28.1%) multi-gravida women, p=0.448.
Discussion
In this description of HIV viral load suppression and drug resistance among HIV infected women in Option B+ program, HIV virological suppression was below the 90% suppression desirable target and did not differ according to treatment support arm, age, WHO stage, or lactation status. However, among those with elevated HIVRNA, relatively low rates of confirmed resistance were observed and the majority of detected resistance was acquired during treatment, suggesting the potential for intervention to promote adherence prior to the development of HIV drug resistance.
Compared to other cohorts in Malawi6 and elsewhere, suppression rates were lower than UNAIDS targets of 90% among individuals on ART thereby potentially compromising health gains for the mother and increasing transmission risk to the infant. In part, this higher rate was due to our failure definition of < 1000 copies/ml that was defined by Malawi guidelines and the appropriate threshold for DBS testing, but is not directly comparable to other surveys that have reported at lower VL thresholds. Additionally, our current evaluation occurred at 6 months. It is possible that individuals with very high baseline viral loads may not yet have fully suppressed. This is supported by the association between defaulting from care and increased likelihood of virological failure. While we did not collect exact dates for resuming treatment, women returning from default may not have been resumed on ART for sufficient time to suppress.
Some women were in care but viral load testing was missed at the clinic. These retained but not-tested women were similar in demographics but more likely to have been seen at a standard of care clinic rather than the peer-supported intervention arms. The study arm interventions focused on retention more than adherence such that virological suppression may not be different in this retained but non-tested group. In contrast, our finding of lower rates of suppression in those with defaulting episodes suggests those not retained in care are more likely to have non-suppression. Hence, at a population level, the suppression rate including women lost to follow-up is likely to be lower than we found in the VL tested population. Overall, this underscores the importance of effective interventions that promote retention, as seen in the PURE study11 to achieve the 90-90-90 targets.
Among patients with elevated viral loads and with successful amplification for resistance sequencing, NRTI and/or NNRTI resistance was detected in 35% of the women. This confirmed resistance rate is relatively low compared to previous studies evaluating drug resistance at 6 months13 and markedly contrasts the higher prevalence of confirmed HIV drug resistance among populations tested at or after 12 months of treatment or at the time of ART switch. 9,14-16 This lower rate may be falsely low due to the approach for drug resistance evaluation, whereby we included samples for evaluation even if only 1 of the 2 amplicons amplified and a large number of samples failed to amplify. We conservatively included any mutation identified, including minor mutations that may not affect drug susceptibility such that this may have overestimated resistance. Regardless, in a study conducted in Malawi using the same resistance testing strategy among ART experienced patients retained in care, high rates (>90%) of confirmed resistance were detected17 suggesting there is a notable distinction between the resistance patterns of Option B+ women at 6 months versus established patients on longer term treatment. Also, given we found defaulters had not necessarily yet developed an increased rate of resistance, programs designed to return these women to care as demonstrated in the PURE study can result in successful suppression. While some studies suggest that resuppression may occur in the presence of existing resistance18, generally the finding of HIV resistance suggests the regimen should be modified as eventual failure is more likely. For this population, the relatively low rate of resistance supports Malawi's current advice for management of detectable viral load which includes enhancing adherence counseling and rechecking viral load in 3 months to confirm continued viral replication19.
The pattern of ART resistance observed in our study was primarily due to NNRTI resistance. This may represent a combination of transmitted HIV drug resistance or acquired resistance from either current or previous ART use, including potentially previously single dose NVP use. Reported previous ART use for PMTCT or general health was extremely low in our cohort. Among the 2 individuals we identified with pre-treatment drug resistance, there was no reported ART exposure. We saw no difference in suppression according to gravidity status suggesting previous unreported PMTCT exposure was less likely. However, we acknowledge the need to further evaluate pre-treatment drug resistance in this cohort, particularly according to primigravida status, to better understand the contribution of transmitted drug resistance toward early treatment failure.
We did not detect any difference between virological suppression and overall confirmed presence of drug resistance according to arm. However, noting that the uptake and retention of ART and HIVRNA collection at 6 months were improved in the peer supported arms11, likely there are significant gains in resistance prevention overall.
Our study includes the large population of Option B+ mothers attending various sized clinics with the highest HIV prevalence within existing operations of ART clinics in the Malawi government program, thereby representing a highly generalizable population. Yet, our complete resistance evaluation is limited by challenges of working within these settings. These include failure to collect all specimens among retained women at the appropriate timepoints and challenges with maintaining appropriate storage conditions for DBS. DBS can be sensitive to RNA degradation and hence we found using the two primer approach described resulted in more potential amplification of expected drug resistance mutations than traditional sequencing. Low level viremia (<5000 copies/ml) was also less likely to amplify thereby limiting our conclusions to individuals with higher VL. Likewise, as with most resistance testing, our methods would not detect minority resistant variants and non-adherent patients can revert to wild-type in the absence of selective drug pressure. Overall, drug resistance may be somewhat underestimated in our study.
In summary, by 6 months, participants in the PURE study had not yet achieved 90% suppression. Relatively low rates of confirmed HIV resistance coupled with lower suppression rates among those with defaulting episodes suggest supporting retention and adherence interventions for women engaged in the Option B+ program in Malawi can achieve treatment and suppression targets.
Supplementary Material
Supplemental Table. NRTI and NNRTI resistance mutations frequency
Supplemental Figure. Flow Algorithm highlighting participant and sample flow to evaluate HIV drug resistance according to defaulter status.
Acknowledgments
The PURE Malawi consortium is grateful to all study participants for their participation and to all clinic and intervention staff for their dedicated contributions to the PURE Malawi trial. We also recognize the contributions of the UNC project laboratory including Robert Krysiak, Gerald Tegha, and Severiano Phakati.Vote of thanks are given to Ellen Thom (WHO Country Office - Lilongwe), Dr Morkor Newman (WHO AFRO Office - Harare), Dr Nigel Rollins, Dr Nathan Shaffer, Dr Shaffiq Essajee, Nita Bellare and Dr. April Baller (WHO Headquarters - Geneva) for their support in the original design of the trial, implementation oversight and review of this manuscript.
Sources of Funding: The study was funded by the World Health Organization through an award for the INtegrating and Scaling up Pmtct through Implementation REsearch (INSPIRE) initiative from Global Affairs Canada and Aidsfondsprovided support for virologic and resistance assessment. This research was supported by the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program P30 AI50410.
Footnotes
Disclaimers (WHO/GAC/Aidsfonds): The opinions expressed in this article do not necessarily reflect the views of policies of the World Health Organization, Global Affairs Canada or Aidsfonds.
Conflict of Interest: The authors declare no conflict of interest
Trial registration: The trial was registered with ClinicalTrials.gov: ID Number NCT02005835.
References
- 1.Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011 Jul 16;378(9787):282–284. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- 2.Ministry of Health Malawi. Clinical Management of HIV in Adults and Children: Malawi Integrated Guidelines for Providing Service in: Antenatal Care, Maternity Care, Under Five Clinics, Family Planning Clinics, Exposed Infant/Pre-ART clinics, ART clinics. 1st. Lilongwe: Ministry of Health, Malawi; 2011. [accessed 9, 2017]. https://www.hiv.health.gov.mw. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Impact of an Innovative Approach to Prevent Mother-to-Child Transmission of HIV — Malawi, July 2011–September 2012. MMWR. 2013 Mar 1;62(08):148–151. 2013. [PMC free article] [PubMed] [Google Scholar]
- 4.Health MMo, editor. Department of HIV and AIDS Ministry of Health. Malawi Integrated HIV Program Report for Oct-Dec 2013. Lilongwe: 2013. [Google Scholar]
- 5.Speight C, Phiri SPS, Hosseinipour M, Tweya H, Chimbwandira F, Chikonda J, Phoso M, Kalulu M, Chauma A. IAS 2013: 7th IAS conference on HIV pathogenesis, Treatment, and Prevention. Kuala Lumpur, Malaysia: 2013. [accessed 9, 2017]. Implementing Option B+ for prevention of mother to child transmission at Bwaila Maternity Unit, Lilongwe: the first 18 months. http://pag.ias2013.org/abstracts. Abstract Number WELBCO1. [Google Scholar]
- 6.Rutstein SE, Kamwendo D, Lugali L, et al. Measures of viral load using Abbott RealTime HIV-1 Assay on venous and fingerstick dried blood spots from provider-collected specimens in Malawian District Hospitals. J Clin Virol. 2014 Aug;60(4):392–398. doi: 10.1016/j.jcv.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wadonda-Kabondo N, Bennett D, van Oosterhout JJ, et al. Prevalence of HIV drug resistance before and 1 year after treatment initiation in 4 sites in the Malawi antiretroviral treatment program. Clin Infect Dis. 2012 May;54(4):S362–368. doi: 10.1093/cid/cir987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadonda-Kabondo N, Hedt BL, van Oosterhout JJ, et al. A retrospective survey of HIV drug resistance among patients 1 year after initiation of antiretroviral therapy at 4 clinics in Malawi. Clin Infect Dis. 2012 May;54(4):S355–361. doi: 10.1093/cid/cis004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. WHO HIV drug resistance report - 2012. Geneva: World Health Organization; 2012. [accessed feb 9 2017]. www.who.int. [Google Scholar]
- 10.Rosenberg NE, van Lettow M, Tweya H, et al. Improving PMTCT uptake and retention services through novel approaches in peer-based family-supported care in the clinic and community: a 3-arm cluster randomized trial (PURE Malawi) J Acquir Immune Defic Syndr. 2014 Nov 1;67(Suppl 2):S114–119. doi: 10.1097/QAI.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phiri S, Lettow Mv, Tweya H, et al. Impact of facility - based and community - based support on uptake and retention in HIV Prevention of Mother to Child Transmission (PMTCT) Option B+ services in Malawi: A cluster randomized controlled trial. JAIDS. 2017 doi: 10.1097/QAI.0000000000001357. [DOI] [PubMed] [Google Scholar]
- 12.Farr SL, Nelson JA, Ng'ombe TJ, et al. Addition of 7 days of zidovudine plus lamivudine to peripartum single-dose nevirapine effectively reduces nevirapine resistance postpartum in HIV-infected mothers in Malawi. J Acquir Immune Defic Syndr. 2010 Aug 15;54(5):515–523. doi: 10.1097/qai.0b013e3181e3a70e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kouanfack C, Montavon C, Laurent C, et al. Low levels of antiretroviral-resistant HIV infection in a routine clinic in Cameroon that uses the World Health Organization (WHO) public health approach to monitor antiretroviral treatment and adequacy with the WHO recommendation for second-line treatment. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009 May 1;48(9):1318–1322. doi: 10.1086/597779. [DOI] [PubMed] [Google Scholar]
- 14.Hosseinipour MC, Gupta RK, Van Zyl G, Eron JJ, Nachega JB. Emergence of HIV drug resistance during first- and second-line antiretroviral therapy in resource-limited settings. J Infect Dis. 2013 Jun 15;207(Suppl 2):S49–56. doi: 10.1093/infdis/jit107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosseinipour MC, van Oosterhout JJ, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009 Jun 1;23(9):1127–1134. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallis CL, Aga E, Ribaudo H, et al. Drug susceptibility and resistance mutations after first-line failure in resource limited settings. Clin Infect Dis. 2014 Sep 1;59(5):706–715. doi: 10.1093/cid/ciu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutstein SE, Compliment K, Nelson JAE, et al. Infectious Diseases Society of America IDWeek2016. New Orleans, LA: 2016. Treatment Switch Algorithms for ART Viral Load Monitoring: Implications of Highly-Prevalent Resistance Among Previously Unmonitored Cohort in Malawi. Abstract 1551. [Google Scholar]
- 18.Hoffmann CJ, Charalambous S, Sim J, et al. Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa. Clin Infect Dis. 2009 Dec 15;49(12):1928–1935. doi: 10.1086/648444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ministry of Health Malawi. 3rd. Lilongwe: Ministry of Health; 2016. [accessed 9, 2017]. Clinical Management of HIV in Adults and Children: Malawi Integrated Guidelines for Providing Service in: Antenatal Care, Maternity Care, Under Five Clinics, Family Planning Clinics, Exposed Infant/Pre-ART clinics, ART clinics. https://www.hiv.health.gov.mw. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table. NRTI and NNRTI resistance mutations frequency
Supplemental Figure. Flow Algorithm highlighting participant and sample flow to evaluate HIV drug resistance according to defaulter status.


