Abstract
MicroRNA-375 (miR-375) is expressed at low levels in many types of solid tumor, particularly in gastrointestinal tumors. It is considered to be important in the development of cancer and certain diseases. Thus, more detailed knowledge is required on the particular functions of miR-375. miRs function by regulating target genes. Therefore, in the current study, miRWalk (which includes the data from 10 prediction software programs) was used to predict the target genes of miR-375. The genes, which were co-predicted using five different software programs were further analyzed using Database for Annotation, Visualization and Integrated Discovery online software [including gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis]. Subsequently, the online tool, Search Tool for the Retrieval of Interacting Genes, was used to analyze the protein-protein interaction and construct modules using Cytoscape. The result demonstrated 6,574 predicted genes, 1,325 of which were co-predicted. The GO analysis result indicated that, in biological processes, the co-predicted genes were significantly enriched in the regulation of nervous system development and cell differentiation, and the highest enrichment of molecular function was ion binding. In KEGG analysis, the genes were enriched in the Hippo signaling pathway, glutamatergic synapse, circadian entrainment and the phosphoinositide 3-kinase (PI3K)-Akt signaling pathway. The top 10 hub proteins were mechanistic target of rapamycin, PH domain and leucine rich repeat protein phosphatase 1, ubiquitously transcribed tetratricopeptide repeat containing, Y-linked, histone deacetylase 2, F-box and leucine rich repeat protein 19, KIT proto-oncogene receptor tyrosine kinase, angiotensinogen, Janus kinase 2, fibroblast growth factor 2 and RNA polymerase II subunit A. These proteins predominantly regulate the development and progression of cancer, hypertension, essential thrombocythemia and inflammation. The genes in the top seven modules selected were identified to be primarily enriched in chemokines, extracellular matrix-receptor interaction, focal adhesion, the PI3K-Akt signaling pathway, amoebiasis and protein processing signaling pathway. Thus, the target genes and hub proteins that were predicted in the current study were identified to be important in regulating the development and progression of cancer and certain diseases. Furthermore, they present potential novel biomarkers for tumor diagnosis and candidate targets for treatment, and indicate that further research is required to establish the functions of miR-375.
Keywords: miR-375, biological information, target gene, regulation, disease
Introduction
The mature microRNAs (miRs) are approximately 20–22 nucleotide molecules and regulate approximately one-third of human genes. These miRs are termed non-coding RNAs. miRs function by complete or incomplete complementary combination to their target genes, which subsequently affects degradation, stability and translation (1). Subsequently, the target genes influence signaling pathways or other molecules to function (2). The primary (pri)-miRs (length, 300–1,000 mucleotides) transform into precursor (pre)-miRs (length, 70–90 mucleotides) and the pre-miRs transform into mature miRs (length, 20–22 mucleotides) via processing with Dicer enzyme (3).
miR-375 is expressed at low levels in many types of solid tumor, such as esophageal carcinoma (4,5), and gastric (6), liver (7), colon (8) and pancreatic (9) cancers, and has been recorded to be associated with the development and progression of cancer. In addition, it was significant in other diseases, including diabetes (10,11), and autoimmune thyroid (12) and allergic (13) diseases. As miR-375 performs pivotal roles in the above-mentioned diseases and, to the best of our knowledge, there are no detailed studies regarding the regulatory role of its target genes, miRWalk was used in the present study to predict the target genes and further analyze the gene functions using gene ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and protein-protein interaction (PPI) analysis. The aim of the study was to obtain further information regarding the function of miR-375 and provide candidate target genes for diagnosis and treatment of certain human diseases.
Materials and methods
Target gene predictions
The target genes were predicted using the miRWalk 1.0 online tool (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk/micrornapredictedtarget.html). This database incorporates the following software programs: DIANAmT (http://diana.imis.athena-innovation.gr/DianaTools), PicTar4 (http://pictar.mdc-berlin.de/cgi-bin/PicTar_vertebrate.cgi), PicTar 5 (http://pictar.mdc-berlin.de/cgi-bin/new_PicTar_vertebrate.cgi), miRanda (http://www.microrna.org), miRWalk (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk/micrornapredictedtarget.html), miRDB (http://www.mirdb.org/miRDB/), RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid), RNA22 (https://omictools.com/rna22-tool), PITA (https://genie.weizmann.ac.il/pubs/mir07/mir07_data.html) and TargetScan (http://www.targetscan.org). The target genes, which were co-predicted using five of the above software programs, were selected for further analysis.
GO and pathway enrichment analysis
The target genes were analyzed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) online tool [DAVID bioinformatics resources 6.8 (National Institute of Allergy and Infectious Diseases/National Institutes of Health); https://david.ncifcrf.gov/home.jsp]. From this database, GO enrichment and KEGG pathway were used for further analysis (14). The GO enrichment analysis included biological processes (BPs), cell component and molecular function (MF).
PPI network and module analysis
The database, Search Tool of Retrieval of Interacting Genes (STRING; version 10.0; http://string-db.org/) was used to perform the PPI analysis. This database is an effective online tool that overlays 9.6 million proteins from 2,031 organisms and provides 184 million PPIs. The PPI were constructed using Cytoscape software 3.4.0 and the proteins with a combined score >0.4 were selected for PPI analysis (the combined score of >0.4 was considered to be statistically significant). Finally, the Cytoscapeplug-in, Molecular Complex Detection (MCODE), was used to screen the modules of the PPI network and define the top modules as having MCODE scores >5 and node numbers >5.
Statistical analysis
For GO and pathway enrichment analysis, P<0.05 was considered to indicate a statistically significant difference. Furthermore, P-values were adjusted using the false discovery rate (FDR) method for multiple hypothesis testing. FDR<0.05 was established as the threshold value (15). For PPI network and module analysis, P<0.05 was considered to indicate a statistically significant difference.
Results
Target gene prediction
A total of 6,574 target genes of miR-375 were predicted using miRWalk. Of these, 1,325 were co-predicted using five of the software programs and selected for GO enrichment, KEGG pathway and PPI analysis.
GO enrichment and KEGG pathway analysis
The co-predicted target genes were analyzed using GO enrichment and KEGG pathway analysis. During the GO enrichment analysis, the genes were significantly enriched in the regulation of nervous system development and cell differentiation during BP; the highest enrichment of MF was regulating the ion binding (Table I). In KEGG pathway analysis, the genes were enriched in the Hippo signaling pathway, glutamatergic synapse, circadian entrainment and phosphoinositide 3-kinase (PI3K)-Akt signaling (P<0.05), while these enrichments were not significant according to the adjusted FDR (FDR>0.05).
Table I.
Gene ontology analysis of miR-375 target genes.
| Category | Term | Gene function | Gene no. (%) | P-value |
|---|---|---|---|---|
| GOTERM_BP_FAT | GO:0007399 | Nervous system development | 202 (17.66) | 5.62E-08 |
| GOTERM_BP_FAT | GO:0045595 | Regulation of cell differentiation | 153 (13.37) | 7.18E-08 |
| GOTERM_BP_FAT | GO:0051960 | Regulation of nervous system development | 89 (7.78) | 2.33E-07 |
| GOTERM_BP_FAT | GO:0048468 | Cell development | 181 (15.82) | 5.14E-07 |
| GOTERM_BP_FAT | GO:0010468 | Regulation of gene expression | 345 (30.16) | 7.66E-07 |
| GOTERM_BP_FAT | GO:200011 | Cellular macromolecule biosynthetic regulation | 319 (27.88) | 1.36E-06 |
| GOTERM_BP_FAT | GO:0010556 | Macromolecule biosynthetic regulation | 326 (28.5) | 1.95E-06 |
| GOTERM_BP_FAT | GO:0051171 | Nitrogen compound metabolic regulation | 346 (30.25) | 2.33E-06 |
| GOTERM_BP_FAT | GO:0072359 | Circulatory system development | 98 (8.57) | 2.51E-06 |
| GOTERM_BP_FAT | GO:0072358 | Cardiovascular system development | 98 (8.57) | 2.51E-06 |
| GOTERM_BP_FAT | GO:0050767 | Regulation of neurogenesis | 78 (6.82) | 2.60E-06 |
| GOTERM_BP_FAT | GO:0006366 | RNA polymerase II promoter transcription | 164 (14.34) | 3.33E-06 |
| GOTERM_BP_FAT | GO:006028 | Regulation of cell development | 91 (7.95) | 4.47E-06 |
| GOTERM_BP_FAT | GO:0045597 | Positive regulation of cell differentiation | 90 (7.86) | 5.03E-06 |
| GOTERM_BP_FAT | GO:0019219 | Nucleobase-containing compound metabolic | 323 (28.23) | 5.66E-06 |
| GOTERM_BP_FAT | GO:0010557 | Macromolecule biosynthetic process | 146 (12.76) | 6.02E-06 |
| GOTERM_BP_FAT | GO:0006355 | Regulation of transcription, DNA-templated | 288 (25.17) | 6.05E-06 |
| GOTERM_BP_FAT | GO:2000026 | Multicellular organismal development | 156 (13.64) | 9.06E-06 |
| GOTERM_BP_FAT | GO:1903506 | Nucleic acid-templated transcription | 288 (25.17) | 9.99E-06 |
| GOTERM_BP_FAT | GO:0022008 | Neurogenesis | 136 (11.89) | 1.08E-05 |
| GOTERM_BP_FAT | GO:0051252 | Reulation of RNA metabolic process | 297 (25.96) | 1.14E-05 |
| GOTERM_BP_FAT | GO:2001141 | Regulation of RNA biosynthetic process | 288 (25.17) | 1.55E-05 |
| GOTERM_BP_FAT | GO:0006357 | Transcription from RNA polymerase II promoter | 161 (14.07) | 1.91E-05 |
| GOTERM_BP_FAT | GO:0051128 | Regulation of cellular component organization | 196 (17.13) | 2.41E-05 |
| GOTERM_MF_FAT | GO:0043167 | Ion binding | 337 (0.18) | 1.47E-05 |
| GOTERM_MF_FAT | GO:0001071 | Nucleic acid binding transcription factor activity | 114 (0.06) | 2.02E-05 |
| GOTERM_MF_FAT | GO:0003700 | Transcription factor activity, DNA binding | 114 (0.06) | 2.02E-05 |
| GOTERM_MF_FAT | GO:0046872 | Metal ion binding | 320 (0.17) | 2.48E-05 |
PPI network and module construction
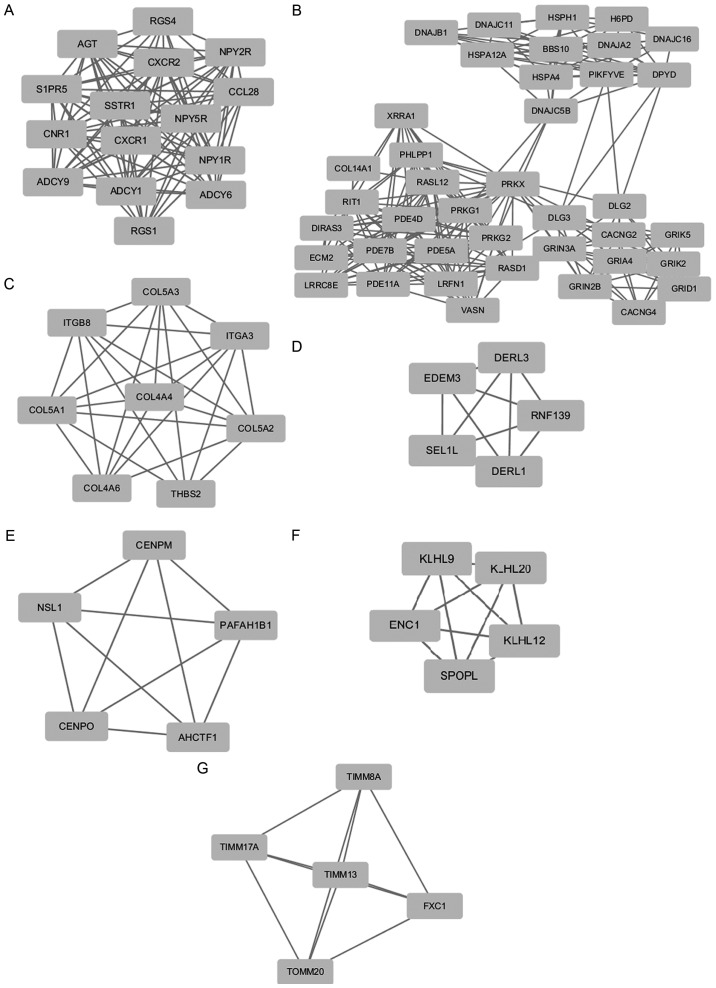
Based on the STRING database, the PPI network of the co-predicted target genes was constructed using Cytoscape software (Fig. 1). In this network, the nodes that were high degree, were identified as the hub proteins. The 10 included hub proteins were as follows: Mechanistic target of rapamycin (mTOR), PH domain and leucine rich repeat protein phosphatase 1 (PHLPP1), ubiquitously transcribed tetratricopeptide repeat containing, Y-linked (UTY), histone deacetylase 2 (HDAC2), F-box and leucine rich repeat protein 19 (FBXL19), KIT proto-oncogene receptor tyrosine kinase (KIT), angiotensinogen (AGT), Janus kinase 2 (JAK2), fibroblast growth factor 2 (FGF2), RNA polymerase II subunit A (POLR2A). The degree of mTOR was 70, which was the highest node. This network consisted of 1,107 nodes and 4,252 edges. There were seven top significant modules selected as the MCODE scores >5 and node numbers >5. The genes in the top seven nodes predominantly regulated chemokines, extracellular matrix (ECM)-receptor interaction, focal adhesion, PI3K-Akt, amoebiasis and protein processing pathways (Table II; P<0.05 and FDR<0.05).
Figure 1.
Top seven modules from the protein-protein interaction network analysis. (A) Module 1, (B) module 2, (C) module 3, (D) module 4, (E) module 5, (F) module 6 and (G) module 7.
Table II.
Enriched pathway of the top modules.
| Module | Pathway | P-value | False discovery rate | Nodes |
|---|---|---|---|---|
| 1 | Chemokine | 1.59E-07 | 9.35E-04 | ADCY1, ADCY9, ADCY6, CXCR1, CXCR2, CCL28 |
| 3 | Extracellular matrix-receptor interaction | 2.05E-14 | 1.46–11 | COL4A4, ITGB8, ITGA3, COL5A3, COL5A2, THBS2, COL4A6, COL5A1 |
| 3 | Focal adhesion | 1.06E-11 | 7.51–9 | COL4A4, ITGB8, ITGA3, COL5A3, COL5A2, THBS2, COL4A6, COL5A1 |
| 3 | PI3K-Akt | 3.96E-10 | 2.82–7 | COL4A4, ITGB8, ITGA3, COL5A3, COL5A2, THBS2, COL4A6, COL5A1 |
| 3 | Protein digestion and absorption | 4.78E-07 | 3.40E-04 | COL4A4, COL5A3, COL5A2, COL4A6, COL5A1 |
| 3 | Amoebiasis | 1.53E-06 | 0.001 | COL4A4, COL5A3, COL5A2, COL4A6, COL5A1 |
| 4 | Protein processing in endoplasmic reticulum | 1.14E-05 | 0.003 | DERL1, EDEM3, DERL3, SEL1L |
Discussion
miR-375 is important in certain diseases, including cancer, diabetes (10,11) and allergic diseases (4–13). According to previous studies, miR-375 is involved in the development and progression of cancer (16,17). As the functions of miRs are performed by target genes (1–3), further investigation into the regulatory role of the target genes is required. In the current study, the miR-375 target genes were predicted using the miRWalk online tool, which includes 10 software programs. This online tool predicted 6,574 genes and 1,325 of these were co-predicted using five of the software programs, which were further evaluated by GO enrichment, KEGG pathway and PPI analysis.
The GO enrichment analysis indicated that the co-predicted genes were significantly enriched in nervous system development and cell differentiation regulation during BP. In addition, it revealed that the highest enrichment of MF was during the regulation of ion binding. In KEGG pathway analysis, the genes were enriched in glutamatergic synapse, circadian entrainment, and Hippo and PI3K-Akt signaling pathways. These indicated that the functions of the target genes were associated with the growth of nerve and cancer cells, as previously reported (16–19). Abdelmohsen et al (18) evaluated the functions of miR-375 in neuronal cells. The results demonstrated that miR-375 reduces the Hu antigen D levels of neurites and inhibits cell differentiation and, in turn, affects the abundance of dendrites. Osako et al (16) evaluated the role of miR-375 in esophageal squamous cell carcinoma. Thei results revealed that regulation of the miR-375 expression levels affects matrix metalloproteinase 13, and markedly inhibits cancer cell migration and invasion. Lian et al (17) identified that miR-375 may target the recepteur d'origine nantais receptor and function as a tumor-suppressor in gastric cancer. He et al (19) investigated the role of miR-375 as a prognostic biomarker in esophageal carcinoma and the results revealed that this miR may serve as a biomarker in regions of China with a high incidence of esophageal carcinoma. The above-mentioned studies indicated that miR-375 is important in the progression of cancer and certain diseases, and may present as a novel biomarker during cancer diagnosis.
In the PPI analysis, the results demonstrated that the top 10 hub proteins included mTOR, PHLPP1, UTY, HDAC2, FBXL19, KIT, AGT, JAK2, FGF2 and POLR2A. mTOR was the highest degree node and, as a hub protein, interacted with 70 genes in the regulatory network. This protein is a member of the phosphatidylinositol kinase-related kinases, which mediate cellular reactions, such as DNA injury and inanition. mTOR may be arrested during the cell-cycle signaling pathway and causes immunosuppressive effects (20). The PHLPP1 protein acts as a tumor inhibitor in certain types of cancer and accelerates apoptosis by dephosphorylating and inactivating Akt (21). The UTY gene is involved in PPI, as this gene encodes a protein that contains tetratricopeptide repeats (22). The HDAC2 protein has a significant role in cell cycle progression, transcriptional regulation and developmental events by forming transcription inhibitory factor complexes (23). The FBXL19 protein is a member of the E3 ubiquitin ligases. This protein combines with the transmembrane receptor, interleukin 1 receptor 1 and regulates lung inflammation and psoriasis (24). The KIT protein is a type 3 transmembrane receptor of mast cell growth factor. The gene mutations were reported to be associated with gastrointestinal stromal tumors, acute myeloid leukemia, peel spot disease and large cell disease (25). The AGT protein participates in the pathogenesis and progression of hypertension and pre-eclampsia. Furthermore, gene mutations were reported to be associated with a susceptibility to hypertension, the causing of nephric tubular dysgenesis and the disorder of nephric tubular development. The defects of this gene were proposed to be associated with atrial fibrillation and inflammatory bowel disease (26–28). The JAK2 gene has been implicated in cytokine receptor signaling pathways and was identified to be associated with the reactions to human interferon-γ (29). The FGF2 protein is a member of the FGF family and is involved in wound healing, limb and nervous system development, and carcinoma growth (30). POLR2A encodes the largest subunit of RNA polymerase II, the product of this gene includes a carboxy-terminal domain composed of heptapeptide repeats, which are required for polymerase activity (31). The functions of the above-mentioned hub proteins indicate that the hub proteins were particularly important in certain BPs, and primarily regulated the development and progression of cancer, hypertension, essential thrombocythemia and inflammation. Recent studies reveal that certain hub proteins may serve as novel biomarkers and targets in certain diseases (20,23,25,27,28).
The PPI module analysis demonstrated that the genes in the seven selected top modules were predominantly enriched in chemokines, ECM-receptor interactions, focal adhesion, the PI3K-Akt signaling pathway, amoebiasis and the protein processing signaling pathway. The present study indicates that miR-375 is important in immunologic disease and cancer progression, as previously reported (12,13,16). Furthermore, chemokine and ECM-receptor interactions influence the immune system. Lu et al (32) found that miR-375 was downregulated in esophageal squamous and bronchial columnar epithelial cells, using interleukin (IL)-13 stimulation. This indicated that miR-375 was involved in regulating and fine adjustment to IL-13-mediated reactions. The PI3K-Akt signaling pathway has been reported to be closely associated with the growth of cells, such as proliferation, migration, invasion and metastasis in cancer (33–35), and certain other diseases, including diabetic kidney (36) and traumatic brain injury (37).
In conclusion, miR-375 is important in certain diseases, such as cancer, metabolic and immune disease. The target genes and hub proteins predicted during the current study may present as potential novel biomarkers in tumor diagnosis and as candidate targets during treatment of certain diseases. In addition, the current study provides a basis for further research into the function of miR-375.
Acknowledgements
The current study was funded by grants from the Technical New Star of Zhujiang, Pan Yu district, Guangzhou (grant nos. 2013-special-15-6.09 and 2013-special-15-6.10), the Traditional Chinese Medicine Bureau of Guangdong Province (grant no. 20161186) and the Science and technology program of Guangdong (grant no. 2016A020215012).
References
- 1.Piletič K, Kunej T. MicroRNA epigenetic signatures in human disease. Arch Toxicol. 2016;90:2405–2419. doi: 10.1007/s00204-016-1815-7. [DOI] [PubMed] [Google Scholar]
- 2.Sheikh AM, Small HY, Currie G, Delles C. Systematic review of micro-RNA expression in pre-eclampsia identifies a n number of common pathways associated with the disease. PLoS One. 2016;11:1–36. doi: 10.1371/journal.pone.0160808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shao Y, Geng Y, Gu W, Huang J, Ning Z, Pei H. Prognostic significance of microRNA-375 downregulation in solid tumors: A meta-analysis. Dis Markers. 2014;2014:626185. doi: 10.1155/2014/626185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isozaki Y, Hoshino I, Akutsu Y, Hanari N, Mori M, Nishimori T, Murakami K, Akanuma N, Toyozumi T, Takahashi M, et al. Screening of alternative drugs to the tumor suppressor miR-375 in esophageal squamous cell carcinoma using the connectivity map. Oncology. 2014;87:351–363. doi: 10.1159/000365592. [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Ajani JA, Gu J, Chang DW, Tan W, Hildebrandt MA, Huang M, Wang KK, Hawk E. MicroRNA expression signatures during malignant progression from Barrett's esophagus to esophageal adenocarcinoma. Cancer Prev Res (Phila) 2013;6:196–205. doi: 10.1158/1940-6207.CAPR-12-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsukamoto Y, Nakada C, Noguchi T, Tanigawa M, Nguyen LT, Uchida T, Hijiya N, Matsuura K, Fujioka T, Seto M, et al. MicroRNA-375 is downregulated in gastric carcinomas and regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer Res. 2010;70:2339–2349. doi: 10.1158/0008-5472.CAN-09-2777. [DOI] [PubMed] [Google Scholar]
- 7.Liu AM, Poon RT, Luk JM. MicroRNA-375 targets Hippo-signaling effector YAP in liver cancer and inhibits tumor properties. Biochem Biophys Res Commun. 2010;394:623–627. doi: 10.1016/j.bbrc.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 8.Zaharie F, Muresan MS, Petrushev B, Berce C, Gafencu GA, Selicean S, Jurj A, Cojocneanu-Petric R, Lisencu CI, Pop LA, et al. Exosome-Carried microRNA-375 Inhibits Cell Progression and Dissemination via Bcl-2 Blocking in Colon Cancer. J Gastrointestin Liver Dis. 2015;24:435–443. doi: 10.15403/jgld.2014.1121.244.375. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, Song S, Cen J, Zhu D, Li D, Zhang Z. MicroRNA-375 is downregulated in pancreatic cancer and inhibits cell proliferation in vitro. Oncol Res. 2012;20:197–203. doi: 10.3727/096504013X13589503482734. [DOI] [PubMed] [Google Scholar]
- 10.Marchand L, Jalabert A, Meugnier E, Van den Hende K, Fabien N, Nicolino M, Madec AM, Thivolet C, Rome S. miRNA-375 a Sensor of Glucotoxicity Is Altered in the Serum of Children with Newly Diagnosed Type 1 Diabetes. J Diabetes Res. 2016;2016:1869082. doi: 10.1155/2016/1869082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deiuliis JA. MicroRNAs as regulators of metabolic disease: Pathophysiologic significance and emerging role as biomarkers and therapeutics. Int J Obes. 2016;40:88–101. doi: 10.1038/ijo.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada H, Itoh M, Hiratsuka I, Hashimoto S. Circulating microRNAs in autoimmune thyroid diseases. Clin Endocrinol (Oxf) 2014;81:276–281. doi: 10.1111/cen.12432. [DOI] [PubMed] [Google Scholar]
- 13.Lu TX, Rothenberg ME. Diagnostic, functional, and therapeutic roles of microRNA in allergic diseases. J Allergy Clin Immunol. 2013;132:3–13. doi: 10.1016/j.jaci.2013.04.039. quiz 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torto-Alalibo T, Purwantini E, Lomax J, Setubal JC, Mukhopadhyay B, Tyler BM. Genetic resources for advanced biofuel production described with the Gene Ontology. Front Microbiol. 2014;5:528. doi: 10.3389/fmicb.2014.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du J, Yuan Z, Ma Z, Song J, Xie X, Chen Y. KEGG-PATH: Kyoto encyclopedia of genes and genomes-based pathway analysis using a path analysis model. Mol Biosyst. 2014;10:2441–2447. doi: 10.1039/C4MB00287C. [DOI] [PubMed] [Google Scholar]
- 16.Osako Y, Seki N, Kita Y, Yonemori K, Koshizuka K, Kurozumi A, Omoto I, Sasaki K, Uchikado Y, Kurahara H, et al. Regulation of MMP13 by antitumor microRNA-375 markedly inhibits cancer cell migration and invasion in esophageal squamous cell carcinoma. Int J Oncol. 2016;49:2255–2264. doi: 10.3892/ijo.2016.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lian S, Park JS, Xia Y, Nguyen TT, Joo YE, Kim KK, Kim HK, Jung YD. MicroRNA-375 functions as a tumor-suppressor gene in gastric cancer by targeting recepteur d'origine nantais. Int J Mol Sci. 2016;17:E1633. doi: 10.3390/ijms17101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelmohsen K, Hutchison ER, Lee EK, Kuwano Y, Kim MM, Masuda K, Srikantan S, Subaran SS, Marasa BS, Mattson MP, et al. miR-375 inhibits differentiation of neurites by lowering HuD levels. Mol Cell Biol. 2010;30:4197–4210. doi: 10.1128/MCB.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Jin J, Wang L, Hu Y, Liang D, Yang H, Liu Y, Shan B. Evaluation of miR-21 and miR-375 as prognostic biomarkers in oesophageal cancer in high-risk areas in China. Clin Exp Metastasis. 2017;34:73–84. doi: 10.1007/s10585-016-9828-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lew S, Chamberlain RS. Risk of metabolic complications in patients with solid tumors treated with mTOR inhibitors: Meta-analysis. Anticancer Res. 2016;36:1711–1718. [PubMed] [Google Scholar]
- 21.Chen H, Zhang K, Wu G, Song D, Chen K, Yang H. Low expression of PHLPP1 in sacral chordoma and its association with poor prognosis. Int J Clin Exp Pathol. 2015;8:14741–14748. [PMC free article] [PubMed] [Google Scholar]
- 22.Walport LJ, Hopkinson RJ, Vollmar M, Madden SK, Gileadi C, Oppermann U, Schofield CJ, Johansson C. Human UTY(KDM6C) is a male-specific Nε-methyl lysyl demethylase. J Biol Chem. 2014;289:18302–18313. doi: 10.1074/jbc.M114.555052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang R, Langdon SP, Tse M, Mullen P, Um IH, Faratian D, Harrison DJ. The role of HDAC2 in chromatin remodelling and response to chemotherapy in ovarian cancer. Oncotarget. 2016;7:4695–4711. doi: 10.18632/oncotarget.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katoh M, Katoh M. Identification and characterization of FBXL19 gene in silico. Int J Mol Med. 2004;14:1109–1114. [PubMed] [Google Scholar]
- 25.Garcia-Montero AC, Jara-Acevedo M, Alvarez-Twose I, Teodosio C, Sanchez-Muñoz L, Muñiz C, Muñoz-Gonzalez JI, Mayado A, Matito A, Caldas C, et al. KIT D816V-mutated bone marrow mesenchymal stem cells in indolent systemic mastocytosis are associated with disease progression. Blood. 2016;127:761–768. doi: 10.1182/blood-2015-07-655100. [DOI] [PubMed] [Google Scholar]
- 26.Ferrario CM, VonCannon J, Jiao Y, Ahmad S, Bader M, Dell'Italia LJ, Groban L, Varagic J. Cardiac angiotensin-(1–12) expression and systemic hypertension in rats expressing the human angiotensinogen gene. Am J Physiol Heart Circ Physiol. 2016;310:H995–H1002. doi: 10.1152/ajpheart.00833.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao W, Jin L, Zhou Z, Yang M, Wu C, Wu L, Cui S. Overexpression of Intrarenal Renin-Angiotensin System in Human Acute Tubular Necrosis. Kidney Blood Press Res. 2016;41:746–756. doi: 10.1159/000450564. [DOI] [PubMed] [Google Scholar]
- 28.Hume GE, Doecke JD, Huang N, Fowler EV, Brown IS, Simms LA, Radford-Smith GL. Altered Expression of Angiotensinogen and Mediators of Angiogenesis in Ileal Crohn's Disease. J Gastrointestin Liver Dis. 2016;25:39–48. doi: 10.15403/jgld.2014.1121.251.chr. [DOI] [PubMed] [Google Scholar]
- 29.Zhao S, Zhang X, Xu Y, Feng Y, Sheng W, Cen J, Wu D, Han Y. Impact of JAK2V617F mutation burden on disease phenotype in chinese patients with JAK2V617F-positive polycythemia vera (PV) and essential thrombocythemia (ET) Int J Med Sci. 2016;13:85–91. doi: 10.7150/ijms.10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Twaroski K, Mallanna SK, Jing R, DiFurio F, Urick A, Duncan SA. FGF2 mediates hepatic progenitor cell formation during human pluripotent stem cell differentiation by inducing the WNT antagonist NKD1. Genes Dev. 2015;29:2463–2474. doi: 10.1101/gad.268961.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pineda G, Shen Z, de Albuquerque CP, Reynoso E, Chen J, Tu CC, Tang W, Briggs S, Zhou H, Wang JY. Proteomics studies of the interactome of RNA polymerase II C-terminal repeated domain. BMC Res Notes. 2015;8:616. doi: 10.1186/s13104-015-1569-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu TX, Lim EJ, Wen T, Plassard AJ, Hogan SP, Martin LJ, Aronow BJ, Rothenberg ME. MiR-375 is downregulated in epithelial cells after IL-13 stimulation and regulates an IL-13-induced epithelial transcriptome. Mucosal Immunol. 2012;5:388–396. doi: 10.1038/mi.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang W, Lai Y, Zhu M, Huang S, Feng W, Gu X. Combretastatin A4 Regulates Proliferation, Migration, Invasion, and Apoptosis of Thyroid Cancer Cells via PI3K/Akt Signaling Pathway. Med Sci Monit. 2016;22:4911–4917. doi: 10.12659/MSM.898545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su W, Li S, Chen X, Yin L, Ma P, Ma Y, Su B. GABARAPL1 suppresses metastasis by counteracting PI3K/Akt pathway in prostate cancer. Oncotarget. 2017;8:4449–4459. doi: 10.18632/oncotarget.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, Huang Y, Huang J, Lin L, Wei G. Gigantol attenuates the proliferation of human liver cancer HepG2 cells through the PI3K/Akt/NF-κB signaling pathway. Oncol. 2017;37:865–870. doi: 10.3892/or.2016.5299. [DOI] [PubMed] [Google Scholar]
- 36.Li F, Li L, Cheng M, Wang X, Hao J, Liu S, Duan H. SHIP, a novel factor to ameliorate extracellular matrix accumulation via suppressing PI3K/Akt/CTGF signaling in diabetic kidney disease. Biochem Biophys Res Commun. 2017;482:1477–1483. doi: 10.1016/j.bbrc.2016.12.060. [DOI] [PubMed] [Google Scholar]
- 37.Wang ZF, Pan ZY, Xu CS, Li ZQ. Activation of G-protein coupled estrogen receptor 1 improves early-onset cognitive impairment via PI3K/Akt pathway in rats with traumatic brain injury. Biochem Biophys Res Commun. 2017;482:948–953. doi: 10.1016/j.bbrc.2016.11.138. [DOI] [PubMed] [Google Scholar]