Abstract
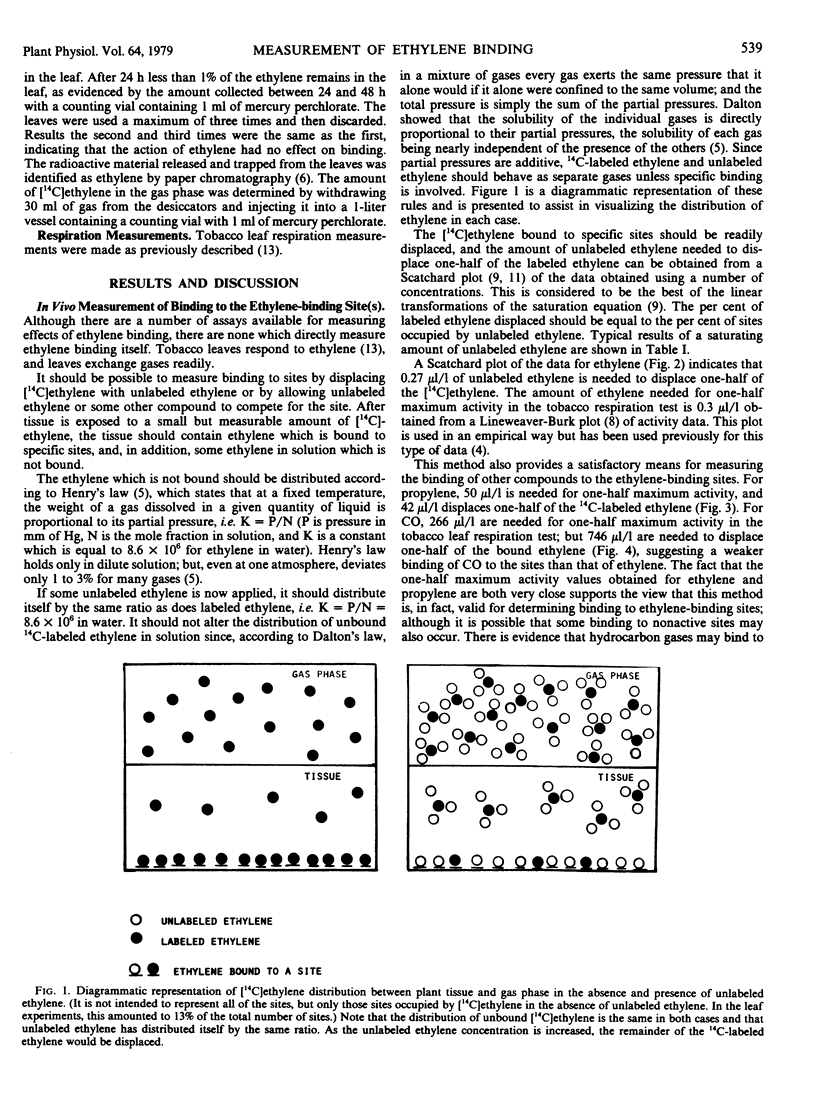
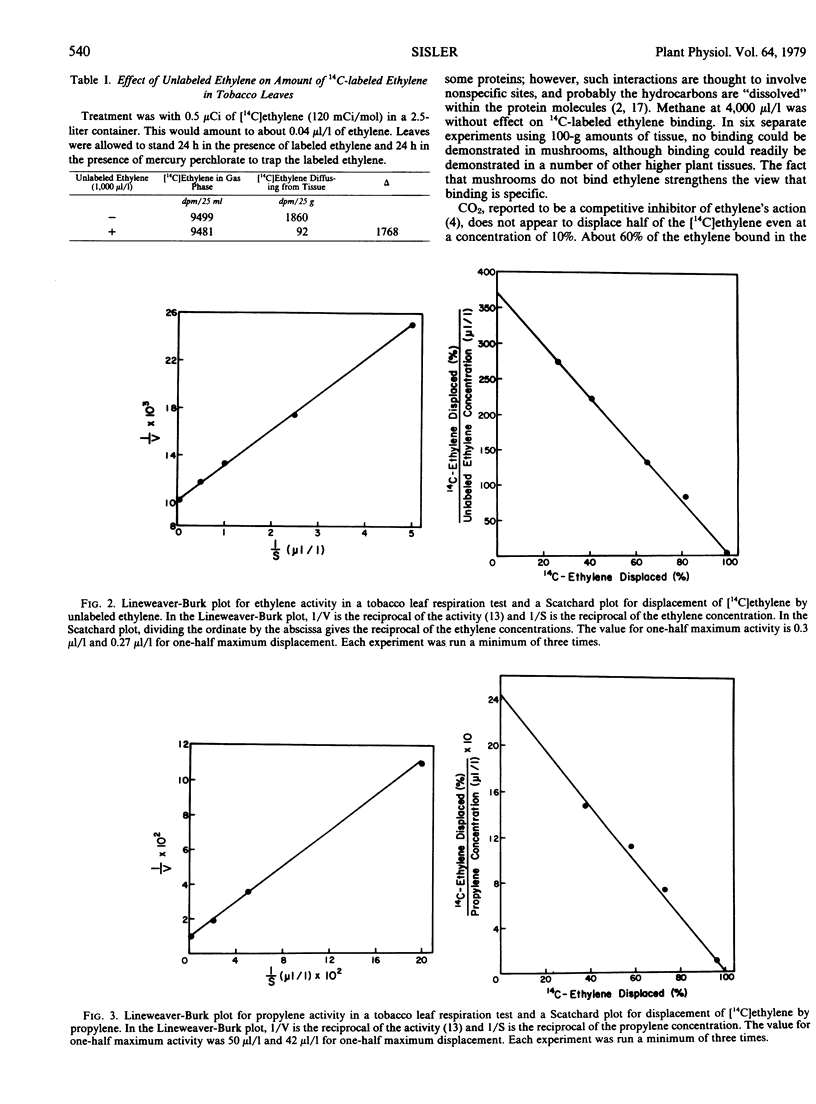
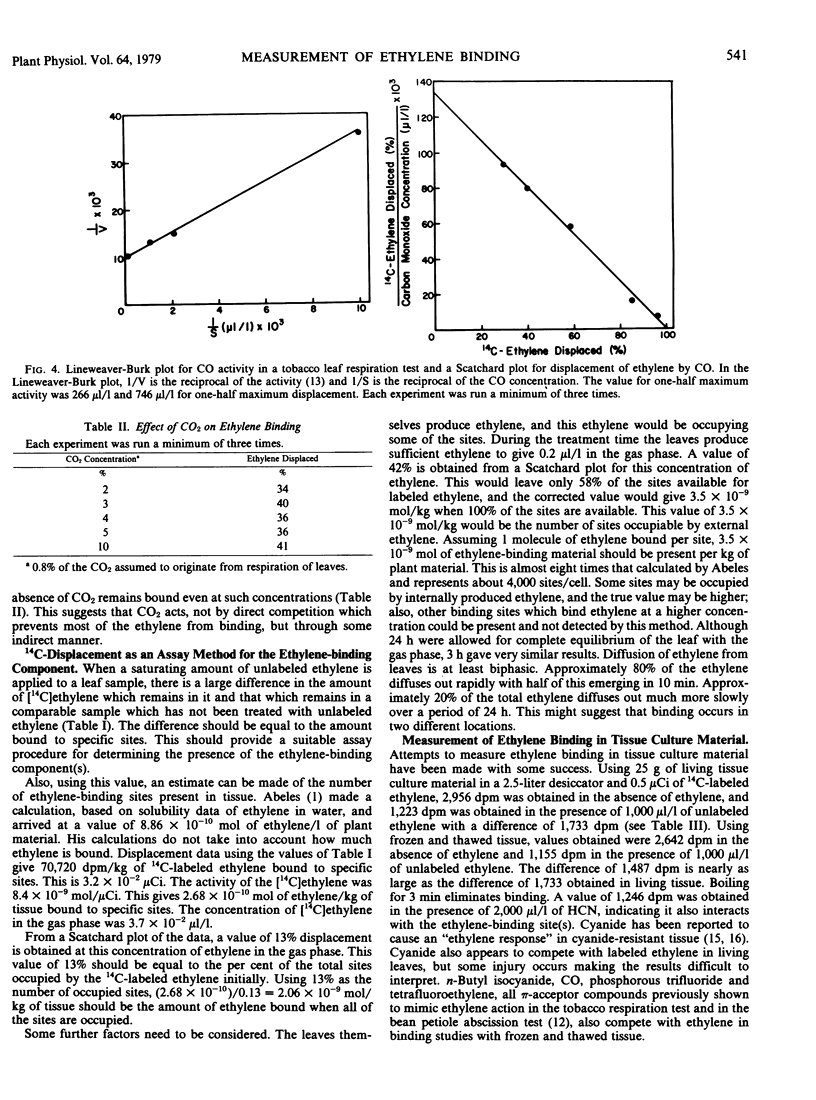
Tobacco leaves were exposed to 14C-labeled ethylene (3.7 × 10−2 microliters per liter) in the presence and absence of unlabeled ethylene and other compounds. Most of the [14C]ethylene appears to be bound to displaceable sites. Lineweaver-Burk plots for a one-half maximum response in a tobacco leaf respiration test gave a value of 0.3 microliter per liter for ethylene, 50 microliters per liter for propylene, and 266 microliters per liter for carbon monoxide. Scatchard plots for displacement of [14C]ethylene from the site gave 0.27 microliters per liter for ethylene, 42 microliters per liter for propylene, and 746 microliters per liter for carbon monoxide. At 2%, CO2 displaces about 35% of the bound ethylene, but increasing the concentration to 10% does not displace the remaining [14C]ethylene. A value of 3.5 nanomolar was calculated for the concentration of ethylene-binding sites available to exogenous ethylene. This does not account for the sites occupied by endogenous ethylene, and the total number of binding sites is probably somewhat higher. Using tissue culture material, the system was shown to be stable to freezing and thawing; and the π-acceptors, carbon monoxide, cyanide, n-butyl isocyanide, phosphorous trifluoride, and tetrafluoroethylene, were shown to compete with ethylene for binding.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. M. Effect of silver ion, carbon dioxide, and oxygen on ethylene action and metabolism. Plant Physiol. 1979 Jan;63(1):169–173. doi: 10.1104/pp.63.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. S., Crane F. L. Paper Chromatography Method for Identification of Ethylene. Plant Physiol. 1963 Nov;38(6):729–730. doi: 10.1104/pp.38.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Effects of Cyanide and Ethylene on the Respiration of Cyanide-sensitive and Cyanide-resistant Plant Tissues. Plant Physiol. 1976 Jul;58(1):47–50. doi: 10.1104/pp.58.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T., Laties G. G. Similarities between the Actions of Ethylene and Cyanide in Initiating the Climacteric and Ripening of Avocados. Plant Physiol. 1974 Oct;54(4):506–511. doi: 10.1104/pp.54.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISHNIA A. The solubility of hydrocarbon gases in protein solutions. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2200–2204. doi: 10.1073/pnas.48.12.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]


