Abstract
Abnormal expression levels of microRNA-204 (miR-204) have been identified in various types of human cancer. However, the expression and functions of miR-204, and the underlying molecular mechanism involved in the initiation and progression of hepatocellular carcinoma (HCC), require further investigation. The results of the present study demonstrated that miR-204 is downregulated in HCC tissues and cell lines. Notably, zinc finger E-box binding homeobox 2 (ZEB2) was identified as a direct target of miR-204 in HCC. In addition, miR-204 negatively regulates ZEB2 expression level in HCC cells at the post-transcriptional level. In functional studies, the overexpression of miR-204 inhibited the proliferation, migration and invasion of HCC cells. Furthermore, the knockdown of ZEB2 may mimic the functions of miR-204 in HCC cells, suggesting that ZEB2 is a direct functional target of miR-204. In conclusion, the results of the present study indicated that miR-204 suppresses the tumor growth, migration and invasion of HCC cells by directly targeting ZEB2, and may serve as a novel therapeutic target for HCC.
Keywords: microRNA-204, zinc finger E-box binding homeobox 2, proliferation, metastasis, motility, hepatocellular carcinoma
Introduction
Primary human hepatocellular carcinoma (HCC) is the most common form of liver cancer, the fifth most frequently diagnosed type of malignancy and the third leading cause of cancer-associated mortality, worldwide (1). According to statistical analysis, in 2015 an estimated 35,660 novel cases of HCC and 24,550 HCC-associated mortalities were recorded in the USA (2). Chronic hepatitis B virus (HBV) or hepatitis C virus infection, alcohol abuse, non-alcoholic fatty liver disease, autoimmune mediated hepatitis, primary biliary cirrhosis and exposure to carcinogens are the primary risk factors of HCC (3). Among these, infection with HBV is the most important risk factor associated with the disease worldwide and is responsible for the increasing incidence of HCC, particularly in China (4,5). Patients with HCC from China comprise 55% of all patients with HCC worldwide (6). Currently, liver resection, liver transplantation, radiotherapy, chemotherapy and targeted therapy are the standard therapeutic treatments used for patients with early stage HCC (7,8). However, the 5-year survival rate remains low, particularly for patients with HCC who are diagnosed at advanced stages, mainly due to the lack of effective therapeutic treatments (4,9). Therefore, it is important to investigate novel therapeutic approaches in order to improve the effectiveness of treatment and the prognosis for patients with HCC.
MicroRNAs (miRNAs/miR) belong to a group of evolutionarily conserved and non-coding small RNAs, 19–25 nucleotides in length (10). In humans, 1,881 miRNA precursors and 2,588 mature miRNA sequences have been deposited in miRBase since the first discovery of miRNA lin-4 in Caenorhabditis elegans (11–13). They have been demonstrated to negatively modulate the expression of targeted mRNAs in animals, plants and viruses by directly binding to the 3′untranslated region (3′UTR) of targeted mRNAs in base pairing, resulting in mRNA degradation or translational inhibition at the translational or post-transcriptional levels (14,15). Numerous previous studies have demonstrated that miRNAs are involved in a variety of cellular biological processes, including proliferation, the cell cycle, apoptosis, invasion, migration and metastasis (16–18). The abnormal expression levels of miRNAs have been identified in various types of human malignancies, including HCC, suggesting that miRNAs may contribute to the carcinogenesis and progression of these types of cancer (19–21). Previous studies have also indicated that miRNAs may be potential novel therapeutic targets for a number of cancer subtypes (22,23). Therefore, it is important to further study the abnormally expressed miRNAs and their roles in HCC, which may provide effective and novel therapeutic targets for HCC. The expression level and functions of miR-204 have been studied in several types of cancer; however, the role of miR-204 in HCC has yet to be elucidated. The present study aimed to investigate the expression patterns, biological functions and underlying molecular mechanisms of miR-204 in HCC.
Materials and methods
Clinical specimens and cell lines
A total of 82 HCC tissues and paired adjacent non-tumorous liver tissues were used in the present retrospective study. These were obtained from 82 HCC patients (including 34 male and 58 female patients; age range, 38–72 years) who were treated with surgical resection at The First Affiliated Hospital of Xiamen University (Xiamen, China) between May 2013 and March 2015. All of the patients enrolled in the study did not receive chemotherapy, radiotherapy or other treatment prior to surgery. Patients treated with chemotherapy or radiotherapy were excluded form the present study. HCC tissues and non-tumorous liver tissues were snap-frozen in liquid nitrogen following surgery and stored at −80°C. The present study was approved by the Research Ethics Committee of The First Affiliated Hospital of Xiamen University (Xiamen, China). Written informed consent was obtained from all patients prior to enrollment in the present study.
The human HCC cell lines (HepG2, PLC-5, SMMC-7721, HuH-7 and HLE), and the immortalized normal liver epithelial cell line (THLE-3) were purchased from the American Type Culture Collection (Manassas, VA, USA) The cells were cultured in high glucose Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% v/v fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
Cell transfection
miR-204 mimics and the corresponding negative control (NC) were purchased from GenePharma, Inc. (Sunnyvale, CA, USA). The pGL3-ZEB2-3′UTR wild-type (Wt) and pGL3-ZEB2-3′UTR mutant (Mut) were also obtained from GenePharma, Inc. For the knockdown of ZEB2 expression, small interfering (si) RNAs targeting ZEB2 or NC siRNA were purchased from Guangzhou Ribobio Co., Ltd. (Guangzhou, China). Oligonucleotide transfection or co-transfection was performed using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from HCC tissues, adjacent non-tumor liver tissues, HCC cell lines and THLE-3 immortalized normal liver epithelial cells using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.), following the manufacturer's protocol. In order to evaluate the expression profile of miR-204, complementary DNA (cDNA) was synthesized from 1 µg of total RNA, using the TaqMan® MicroRNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc.), followed by qPCR using the specific TaqMan MicroRNA assay (Applied Biosystems; Thermo Fisher Scientific, Inc.).
In order to determine the mRNA expression level of ZEB2, cDNA was synthesized using the GeneAmp RNA PCR kit (Thermo Fisher Scientific, Inc.), followed by qPCR using the Power SYBR® Green Master Mix (Thermo Fisher Scientific, Inc.). The RT-qPCR process was performed using the Applied Biosystems 7500 Real-time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). For both qPCR procedures, the thermocycling conditions were as follows: 95°C for 10 min, then 40 cycles of 95°C for 15 sec and 60°C for 1 min.
The primer sequences for miR-204 were as follows: Forward, 5′-GCGGCGCAAAGAATTCTCCT-3′, reverse, 5′-GTGCAGGGTCCGAGGT-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′, reverse, 5′-AACGCTTCACGAATTTGCGT-3′; ZEB2 forward, 5′-GCAATGTAGGTCTCTGCTGC-3′, reverse, 5′-CTCCCCTTTGCTCCTTCTCA-3′; GAPDH forward, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′, reverse, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. U6 and GADPH were used as internal controls for miR-204 and ZEB2 mRNA expression, respectively. The relative expression level of miR-294 and ZEB2 mRNA was determined with the 2−ΔΔCq method (24).
Bioinformatics analysis
In order to find putative targets of miR-204, a bioinformatics analysis was performed with TargetScan (http://www.targetscan.org/).
Dual-luciferase reporter assay
For the reporter assays, cells were seeded into 24-well plates at 50–60% confluence, and co-transfected with miR-204 mimics (20 pmol), NC (20 pmol) and pGL3-ZEB2-3′UTR Wt (1 µg) or pGL3-ZEB2-3′UTR Mut (1 µg) at room temperature. Following a 48-h transfection, cells were collected and the luciferase activities were determined by performing a dual-luciferase reporter assay (Promega Corporation, Madison, WI, USA), according to the manufacturer's protocol. The Renilla luciferase activities were measured using an xMark™ microplate absorbance spectrophotometer (Bio-Rad Laboratories, Inc.) and used as internal controls for firefly luciferase activity.
Western blot analysis
Transfected HepG2 and HuH-7 cells were washed with PBS, harvested with a cell scraper and homogenized using radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Haimen, China) supplemented with protease inhibitor (Pierce; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The protein concentration was detected using a bicinchoninic acid assay protein kit (Thermo Fisher Scientific, Inc.), and equal amounts of protein (20 µg) were separated using 10% SDS-PAGE and then transferred to a polyvinylidine fluoride membrane (Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, the membranes were blocked with TBS and Tween-20 (TBST) supplemented with 5% skimmed milk for 2 h at room temperature prior to incubation with primary antibodies overnight at 4°C. The primary antibodies used were mouse anti-human monoclonal ZEB2 (1:1,000 dilution; cat. no. sc-271984; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and mouse anti-human monoclonal β-actin (1:1,000 dilution; cat. no. sc-47778; Santa Cruz Biotechnology, Inc.). β-actin was used as the internal control for ZEB2. Following washing with TBST three times, the membranes were probed with goat anti-mouse IgG horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) at room temperature for 2 h and visualized using a FluorChem imaging system (Alpha Innotech Co., San Leandro, CA, USA).
Cell proliferation assay
Cell counting kit-8 (CCK8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was used to determine the effect of miR-204 on the proliferative ability of cells. Cells were seeded into 96-well plates at a density of 3,000 cells/well. Following overnight incubation at 37°C with 5% CO2, cells were transfected with miRNAs (50 pmol/ml) or siRNAs (50 pmol/ml). A CCK8 assay was performed every 24 h subsequent to transfection, up to a total of 96 h. In brief, cells were treated with 10 µl CCK8 solution for 2 h at 37°C. Absorbance was detected at 450 nm for each well using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). All experiments were performed with 5 replications.
Cell migration and invasion assays
Cell migration and invasion assays were performed with a Transwell chambers (8-µm pore size; Corning Incorporated, Corning, NY, USA) coated with (invasion assay) or without (migration assay) Matrigel (BD Biosciences, San Jose, CA, USA). For migration and invasion assays, 1×105 transfected cells in 300 µl FBS-free culture medium were seeded into the upper Transwell chambers. The lower Transwell chambers received 500 µl culture medium supplemented with 20% FBS. Following a 24-h incubation at 37°C with 5% CO2, non-migrated cells were removed using a cotton swab. Cells migrating or invading to the lower surface membranes of the Transwell chambers were fixed with methanol and stained with 0.5% crystal violet. Subsequent to washing with PBS three times, cells were counted in five randomly selected visual fields under an inverted microscope (magnification, ×200; Olympus Corporation, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± standard deviation, and were analyzed using Student's t-test or one-way analysis of variance, followed by Student Newman Keuls post hoc test, with SPSS 19.0 software (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
miR-204 is downregulated in HCC tissues and cell lines
In order to evaluate the expression levels of miR-204 in HCC tissues, RT-qPCR was performed to determine the differential expression levels of miR-204 in HCC tissues and paired adjacent non-tumorous liver tissues. The results demonstrated that miR-204 expression levels are decreased in HCC tissues compared with in adjacent non-tumorous liver tissues (Fig. 1A, P<0.05).
Figure 1.
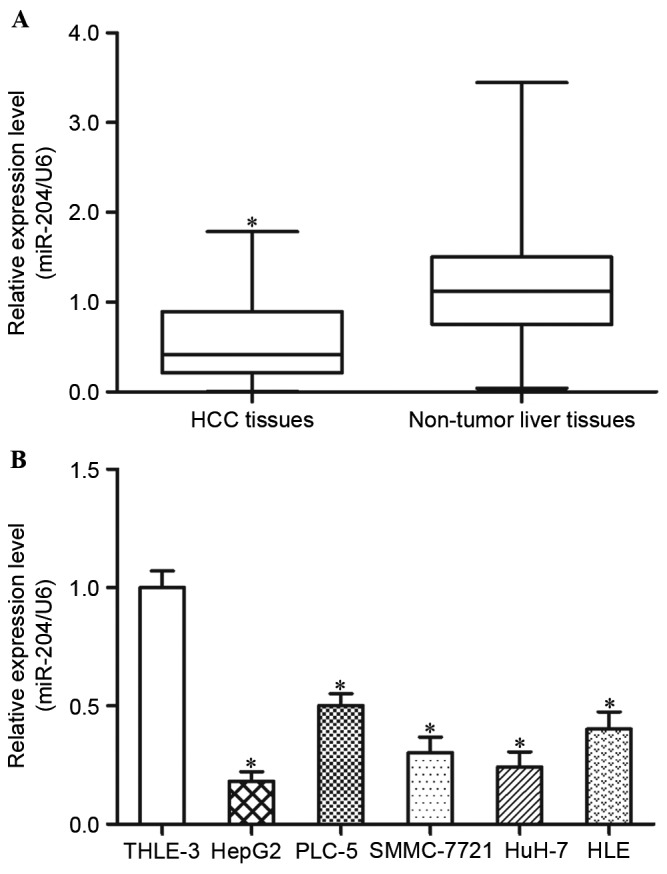
miR-204 is downregulated in HCC tissues and cell lines. (A) The expression levels of miR-204 in HCC tissues were significantly lower compared with those in paired adjacent non-tumorous liver tissues. Data are presented as box-and-whisker plots. A Student's t-test was used to assess significant differences between groups. (B) The results demonstrated that, compared with THLE-3 cells, the five HCC cell lines had significantly lower expression levels of miR-204. Data are presented as mean ± standard deviation. *P<0.05, compared with the respective controls. miR-204, microRNA-204; HCC, hepatocellular carcinoma.
In addition, the present study detected the expression levels of miR-204 in five HCC cell lines (HepG2, PLC-5, SMMC-7721, HuH-7 and HLE) and an immortalized normal liver epithelial cell line (THLE-3). The results demonstrated that, compared with THLE-3 cells, the five HCC cell lines exhibited lower expression levels of miR-204 (Fig. 1B, P<0.05). Within the five HCC cell lines, HepG2 and HuH-7 revealed the lowest miR-204 expression levels, and were selected for further functional studies. These findings indicated that miR-204 may be involved in the carcinogenesis and progression of HCC.
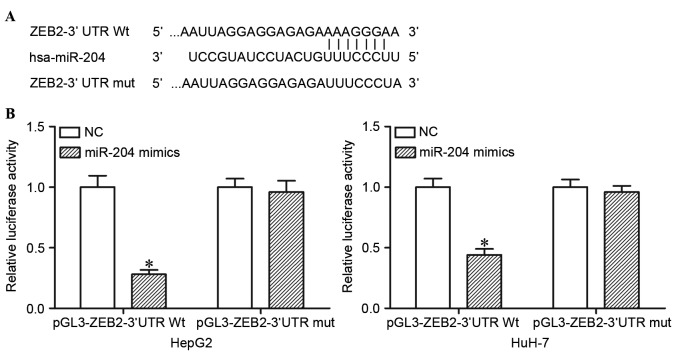
miR-204 directly targets the 3′UTR of ZEB2 in HCC cells
TargetScan was used to predict the potential target genes of miR-204. The analysis revealed that ZEB2 contained a miR-204 seed match at position 218–225 of ZEB2 3′UTR (Fig. 2A). In order to confirm whether miR-204 is a direct target of miR-204, dual luciferase reporter assays were performed. As presented in Fig. 2B, ectopic miR-204 expression in HepG2 and HuH-7 cells significantly suppressed the luciferase activities of pGL3-ZEB2-3′UTR Wt (P<0.05), but had no observable effect on the luciferase activities of pGL3- ZEB2-3′UTR Mut. miR-204 directly targeted the 3′UTR of ZEB2 in HCC cells.
Figure 2.
miR-204 directly targets the 3′UTR of ZEB2 in HCC. (A) The predicted binding sites of miR-204 on 3′UTR of ZEB2 and the mutant ZEB2 3′UTR sequence at binding site. (B) HepG2 and HuH-7 cells were co-transfected with pGL3-ZEB2-3′UTR Wt or pGL3-ZEB2-3′UTR Mut and miR-204 mimics or NC. miR-204 significantly decreased the luciferase activities of pGL3-ZEB2-3′UTR Wt, but had no effect on the luciferase activities of pGL3-ZEB2-3′UTR. Data are presented as mean ± standard deviation. *P<0.05, compared with the respective controls. miR-204, microRNA-204; 3′UTR, 3′untranslated region; ZEB2, zinc finger E-box binding homeobox 2; HCC, hepatocellular carcinoma; Wt, wild-type; Mut, mutant; NC, negative control.
miR-204 negatively regulates ZEB2 expression levels at the post-transcriptional level
The present study performed RT-qPCR and western blot analysis in order to determine whether miR-204 regulated ZEB2 expression. The RT-qPCR results indicated that high expression levels of miR-204 decreased ZEB2 mRNA expression levels in HepG2 and HuH-7 cells (Fig. 3A; P<0.05). Western blot analysis revealed that overexpression of miR-204 may reduce ZEB2 protein expression levels in HepG2 and HuH-7 cells (Fig. 3B; P<0.05). Collectively, miR-204 negatively modulated ZEB2 expression levels in HCC cells via an underlying mRNA cleavage mechanism at the post-transcriptional level.
Figure 3.
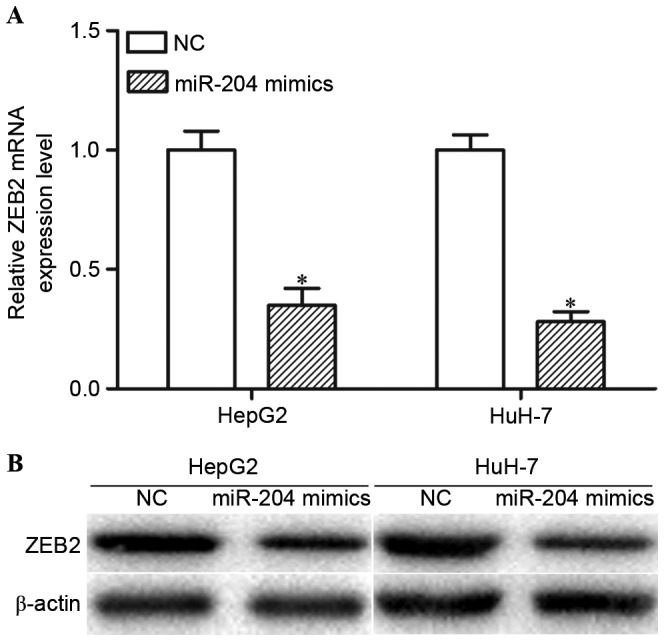
miR-204 negatively regulates ZEB2 expression at the post-transcriptional level. (A) RT-qPCR analysis demonstrated that ZEB2 mRNA expression was downregulated in miR-204 mimic-transfected HepG2 and HuH-7 cells. Data are presented as mean ± standard deviation. (B) Western blot analysis indicated that miR-204 decreased ZEB2 protein expression levels in HepG2 and HuH-7 cells. *P<0.05, compared with the respective controls. miR-204, microRNA-204; ZEB2, zinc finger E-box binding homeobox 2; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; NC, negative control.
miR-204 suppresses the proliferation, migration and invasion of HCC cells
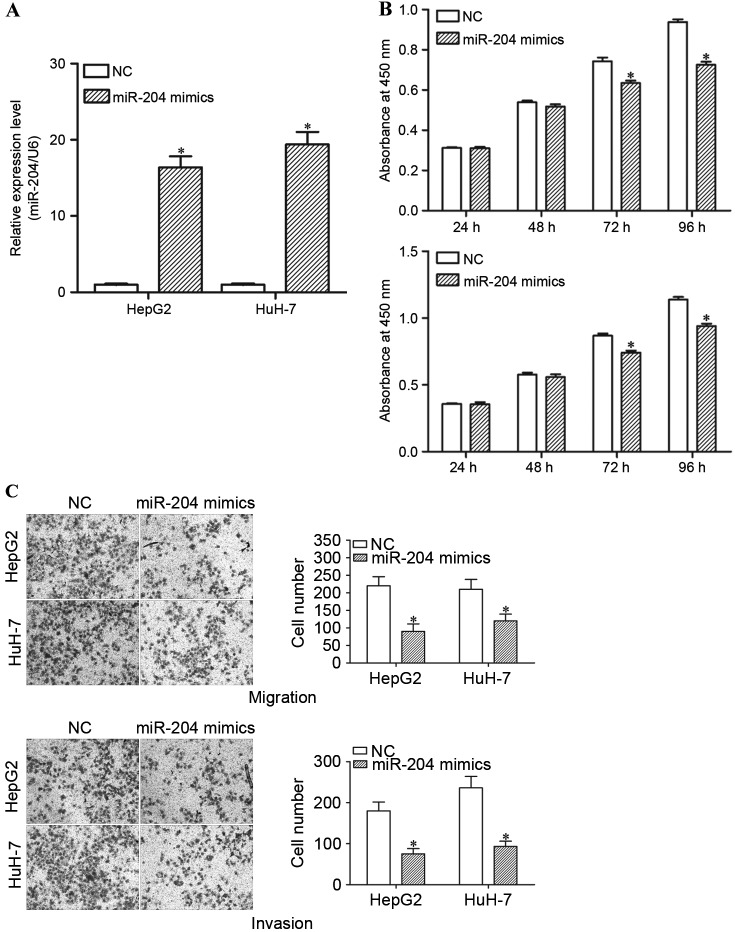
In order to investigate the function of miR-204 in HCC cells, miR-204 mimics or NCs were introduced into HepG2 and HuH-7 cells. RT-qPCR was performed to determine the transfection efficiency. The results demonstrated that miR-204 expression levels are markedly increased in miR-204 mimic-transfected HepG2 and HuH-7 cells, compared with the NC groups (Fig. 4A; P<0.05).
Figure 4.
miR-204 suppresses cell proliferation, migration and invasion of HCC cells. All data are presented as mean ± standard deviation. (A) The relative expression levels of miR-204 expressed in HepG2 and HuH-7 cells following transfection with miR-204 mimics or NC. (B) Cell proliferation assays demonstrated that overexpression of miR-204 suppressed the cell proliferation in HepG2 and HuH-7 cells. (C) The migration and invasion assays indicated that enforced miR-204 expression level suppressed the migration and invasion abilities of HepG2 and HuH-7 cells. *P<0.05, compared with their respective controls. miR-204, microRNA-204; HCC, hepatocellular carcinoma; NC, negative control.
Subsequently, cell proliferation, cell migration and invasion assays were performed in order to investigate the functions of miR-204 in the growth, migration and invasion of HCC cells. The results demonstrated that high expression levels of miR-204 resulted in a significant decrease in the cell proliferation of HepG2 and HuH-7 cells (Fig. 4B; P<0.05). The migration and invasion assays revealed that enforced miR-204 expression suppresses the migration and invasion ability of HepG2 and HuH-7 cells (Fig. 4C; P<0.05). Thus, restoration of miR-204 expression suppresses growth and motility in HCC.
Knockdown of ZEB2 inhibits the proliferation, migration and invasion of HCC cells
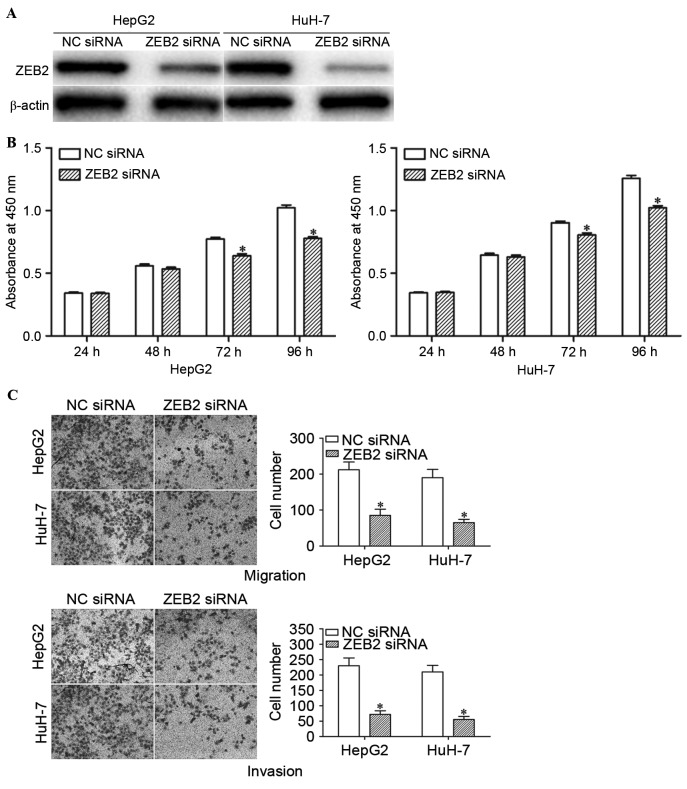
In order to determine whether the functions of miR-204 in HCC cells are mediated by ZEB2, HepG2 and HuH-7 cells were transfected with ZEB2 siRNA or NC siRNA. Western blot analysis confirmed that ZEB2 siRNA significantly decreased the expression levels of ZEB2 in HepG2 and HuH-7 cells (Fig. 5A; P<0.05).
Figure 5.
Knockdown of ZEB2 inhibited the proliferation, migration and invasion of HCC cells. (A) ZEB2 expression levels in HepG2 and HuH-7 cells as determined by western blot analysis following transfection with ZEB2 siRNA or NC siRNA. (B) ZEB2 siRNA inhibited the proliferation of HepG2 and HuH-7 cells. Data are presented as mean ± standard deviation. (C) ZEB2 siRNA suppressed the migration and invasion capacity of HepG2 and HuH-7 cells. Data are presented as mean ± standard deviation. *P<0.05, compared with the respective controls. ZEB2, zinc finger E-box binding homeobox 2; HCC, hepatocellular carcinoma; siRNA, small interfering RNA; NC, negative control.
The effects of ZEB2 siRNA on the proliferation, migration and invasion of HCC cells were determined using cell proliferation, cell migration and invasion assays. Consistently, ZEB2 siRNA suppressed the proliferation, migration and invasion of HepG2 and HuH-7 cells (Fig. 5B and C; P<0.05). These results indicate that miR-204 inhibit the growth, migration and invasion of HCC cells via knockdown of ZEB2 expression.
Discussion
HCC is one of the most common malignant types of cancer worldwide and the second most common cause of cancer-associated mortality in China (25). Therefore, understanding the molecular mechanisms underlying the carcinogenesis and progression of HCC is required for the development of novel therapeutic treatments for patients with HCC, in order to improve the rate of survival. A number of previous studies have demonstrated that abnormal expression levels of miRNAs may be important in HCC initiation and development, suggesting that miRNA may be investigated as a novel direction in the targeted therapy of HCC (26–28).
The present study initially demonstrated that miR-204 was significantly downregulated in HCC tissues and cell lines, indicating that abnormal expression levels of miR-204 may contribute to HCC initiation and development. In addition, TargetScan was used to predict the potential target genes of miR-204. The results revealed that ZEB2 contains a miR-204 seed match at its 3′UTR position. Dual luciferase reporter assays indicated that miR-204 directly targets the 3′UTR of ZEB2. RT-qPCR and western blot analysis demonstrated that ZEB2 mRNA and protein expression levels are decreased in HCC cells transfected with miR-204 mimics. These results indicated that miR-204 negatively regulates ZEB2 expression levels at the post transcription level via directly targeting the 3′UTR of ZEB2. Furthermore, the present study determined the rates of cell growth and metastasis by performing cell proliferation, cell migration and invasion assays, in order to evaluate the association between miR-204 expression and the growth and metastasis capacity of HCC cells. The cell growth, viability and metastasis of HCC cells transfected with the miR-204 mimics was decreased, compared with in the NC groups. Finally, the functions of ZEB2 in HCC cells were determined. The results revealed that the downregulation of ZEB2 may mimic the functions of miR-204 in HCC cells, suggesting that ZEB2 is a functional target of miR-204 in HCC cells.
miR-204 was previously revealed to be downregulated in a number of human tumor subtypes, including thyroid cancer (29), renal cell carcinoma (30), breast cancer (31), glioma (32), acute myeloid leukemia (33), osteosarcoma (34) and ovarian cancer (35). In functional studies, miR-204 was demonstrated to be a tumor suppressor in the aforementioned types of cancer. For example, in thyroid cancer, miR-204 suppressed cell proliferation by targeting high-mobility group AT-hook 2 and insulin like growth factor binding protein 5 (29,36). Wu et al (30) reported that overexpressed miR-204 targeted SRY-Box 4 in order to inhibit the growth, migration and invasion of renal cell carcinoma cells. In breast cancer, low expression levels of miR-204 were revealed to be associated with the tumor-node-metastasis stage, metastasis and chemotherapeutic resistance of patients with breast cancer. In addition, patients with low miR-204 expression levels demonstrated a poorer overall survival time and disease free survival time, compared with those with high miR-204 expression levels (37). miR-204 increased the extent of apoptosis in breast cancer cells via targeting Janus kinase 2 through the signal transducer and activator of transcription 3/B-cell lymphoma 2 signaling pathway (31). Xia et al (32) demonstrated that the upregulation of miR-204 significantly decreased glioma cell growth, migration and invasion in vitro, and suppressed tumorigenesis and increased the overall patient survival rate in vivo by the regulation of RAB22A. Mao et al (38) reported that miR-204 targeted ezrin in order to decrease the migration and invasion abilities of glioma. These findings collectively suggest that miR-204 serves important roles in tumor suppression, and should be investigated as a potential targeted therapeutic treatment for the aforementioned types of cancer.
Identification of miR-204 target mRNAs is required for investigating its functions in carcinogenesis and the progression of HCC, and for the development of novel targeted therapies (39). In the present study, ZEB2 was identified as a functional target gene of miR-204 in HCC cells. ZEB2 is a member of the zinc finger family, and has previously demonstrated upregulation in a number of human cancer subtypes, including breast cancer, gastric cancer, glioma, ovarian cancer and non-small cell lung carcinoma (40–46). ZEB2 has also been demonstrated to be upregulated in HCC, and expression levels of ZEB2 in the nucleus were associated with angiogenesis and metastasis. Patients with HCC and ZEB2 nuclear expression exhibited shorter survival times compared with those without ZEB2 expression. In previous studies, the overexpression of ZEB2 induced motility, invasiveness and angiogenesis of HCC cells (47). By contrast, the knockdown of ZEB2 suppressed HCC cell motility, invasiveness and vasculogenic mimicry formation (47). This was in accordance with the results of the present study.
It has previously been demonstrated that ZEB2 was regulated by numerous miRNAs in a number of human cancer subtypes. For example, in gastric cancer, miR-141 targeted ZEB2 in order to inhibit cancer cell migration (48). Zhou et al (49) reported that miR-153 suppressed cell growth and invasion of ovarian cancer by directly targeting ZEB2. In renal cell carcinoma, miR-205 inhibited cancer cell growth, migration, invasion and induced apoptosis via blockade of ZEB2 (50). These studies indicated that miRNAs may serve as a regulator of ZEB2 in human types of cancer. In the present study, the upregulation of miR-204 in HCC cell lines demonstrated that miR-204 inhibits cell growth, migration and invasion by negatively regulating ZEB2. Therefore, miR-204 maybe investigated as a possible targeted therapy for HCC.
In conclusion, the expression of miR-204 was downregulated in HCC tissues and cell lines. In vitro functional studies revealed that miR-204 acted as a tumor suppressor by inhibiting the proliferation, migration and invasion of HCC. Its numerous tumor suppressor functions are mediated by ZEB2. The downregulation of miR-204 may serve an important role in tumor growth and metastasis, and may be a potential therapeutic target for HCC.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81371902 and 81301923) and the National Natural Science Foundation of Fujian (grant nos. 2011D012, 2015J01561 and 2016J01633).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15:14–22. doi: 10.1634/theoncologist.2010-S4-14. (Suppl 4) [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 6.Asia-Pacific Working Party on Prevention of Hepatocellular Carcinoma: Prevention of hepatocellular carcinoma in the Asia-Pacific region: consensus statements. J Gastroenterol Hepatol. 2010;25:657–663. doi: 10.1111/j.1440-1746.2009.06167.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Dong L, Liu Y, Wen D, Gao D, Sun H, Fan J, Wu W. A c-Myc/miR-17-5p feedback loop regulates metastasis and invasion of hepatocellular carcinoma. Tumour Biol. 2016;37:5039–5047. doi: 10.1007/s13277-015-4355-5. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh MY, Lin ZY, Chen SH, Chuang WL. Risk factors for the leakage of chemotherapeutic agents into systemic circulation after transcatheter arterial chemoembolization of hepatocellular carcinoma. Kaohsiung J Med Sci. 2011;27:431–436. doi: 10.1016/j.kjms.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ, Wu F. Solitary large hepatocellular carcinoma: A specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg. 2009;249:118–123. doi: 10.1097/SLA.0b013e3181904988. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Kowdley KV. MicroRNAs in common human diseases. Genomics Proteomics Bioinformatics. 2012;10:246–253. doi: 10.1016/j.gpb.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 12.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 13.Kozomara A, Griffiths-Jones S. miRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Hara SP, Mott JL, Splinter PL, Gores GJ, LaRusso NF. MicroRNAs: Key modulators of posttranscriptional gene expression. Gastroenterology. 2009;136:17–25. doi: 10.1053/j.gastro.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 16.Lujambio A, Lowe SW. The microcosmos of cancer. Nature. 2012;482:347–355. doi: 10.1038/nature10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33:1126–1133. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang N, Ekanem NR, Sakyi CA, Ray SD. Hepatocellular carcinoma and microRNA: New perspectives on therapeutics and diagnostics. Adv Drug Deliv Rev. 2015;81:62–74. doi: 10.1016/j.addr.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 20.Romero-Cordoba SL, Salido-Guadarrama I, Rodriguez-Dorantes M, Hidalgo-Miranda A. miRNA biogenesis: Biological impact in the development of cancer. Cancer Biol Ther. 2014;15:1444–1455. doi: 10.4161/15384047.2014.955442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan H, Lu H, Wang X, Jin H. MicroRNAs as potential biomarkers in cancer: Opportunities and challenges. Biomed Res Int. 2015;2015:125094. doi: 10.1155/2015/125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petri A, Lindow M, Kauppinen S. MicroRNA silencing in primates: Towards development of novel therapeutics. Cancer Res. 2009;69:393–395. doi: 10.1158/0008-5472.CAN-08-2749. [DOI] [PubMed] [Google Scholar]
- 23.Landi MT, Zhao Y, Rotunno M, Koshiol J, Liu H, Bergen AW, Rubagotti M, Goldstein AM, Linnoila I, Marincola FM, et al. MicroRNA expression differentiates histology and predicts survival of lung cancer. Clin Cancer Res. 2010;16:430–441. doi: 10.1158/1078-0432.CCR-09-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–1134. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Bo L, Lu W, Zhou G, Chen Q. MicroRNA-148b targets Rho-associated protein kinase 1 to inhibit cell proliferation, migration and invasion in hepatocellular carcinoma. Mol Med Rep. 2016;13:477–482. doi: 10.3892/mmr.2015.4500. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Bo L, Zhao X, Chen Q. MicroRNA-133a inhibits cell proliferation, colony formation ability, migration and invasion by targeting matrix metallopeptidase 9 in hepatocellular carcinoma. Mol Med Rep. 2015;11:3900–3907. doi: 10.3892/mmr.2015.3232. [DOI] [PubMed] [Google Scholar]
- 28.Pang X, Huang K, Zhang Q, Zhang Y, Niu J. miR-154 targeting ZEB2 in hepatocellular carcinoma functions as a potential tumor suppressor. Oncol Rep. 2015;34:3272–3279. doi: 10.3892/or.2015.4321. [DOI] [PubMed] [Google Scholar]
- 29.Wu ZY, Wang SM, Chen ZH, Huv SX, Huang K, Huang BJ, Du JL, Huang CM, Peng L, Jian ZX, Zhao G. MiR-204 regulates HMGA2 expression and inhibits cell proliferation in human thyroid cancer. Cancer Biomark. 2015;15:535–542. doi: 10.3233/CBM-150492. [DOI] [PubMed] [Google Scholar]
- 30.Wu D, Pan H, Zhou Y, Zhang Z, Qu P, Zhou J, Wang W. Upregulation of microRNA-204 inhibits cell proliferation, migration and invasion in human renal cell carcinoma cells by downregulating SOX4. Mol Med Rep. 2015;12:7059–7064. doi: 10.3892/mmr.2015.4259. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Qiu W, Zhang G, Xu S, Gao Q, Yang Z. MicroRNA-204 targets JAK2 in breast cancer and induces cell apoptosis through the STAT3/BCl-2/survivin pathway. Int J Clin Exp Pathol. 2015;8:5017–5025. [PMC free article] [PubMed] [Google Scholar]
- 32.Xia Z, Liu F, Zhang J, Liu L. Decreased expression of MiRNA-204-5p contributes to glioma progression and promotes glioma cell growth, migration and invasion. PLoS One. 2015;10:e0132399. doi: 10.1371/journal.pone.0132399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butrym A, Rybka J, Baczynska D, Tukiendorf A, Kuliczkowski K, Mazur G. Low expression of microRNA-204 (miR-204) is associated with poor clinical outcome of acute myeloid leukemia (AML) patients. J Exp Clin Cancer Res. 2015;34:68. doi: 10.1186/s13046-015-0184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Huang J, Zhou J, Liu Y, Fu X, Li Y, Yin G, Wen J. MicroRNA-204 inhibits proliferation, migration, invasion and epithelial-mesenchymal transition in osteosarcoma cells via targeting Sirtuin 1. Oncol Rep. 2015;34:399–406. doi: 10.3892/or.2015.3986. [DOI] [PubMed] [Google Scholar]
- 35.Yan H, Wu W, Ge H, Li P, Wang Z. Up-regulation of miR-204 enhances anoikis sensitivity in epithelial ovarian cancer cell line via brain-derived neurotrophic factor pathway in vitro. Int J Gynecol Cancer. 2015;25:944–952. doi: 10.1097/IGC.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Wang J, Li X, Ma J, Shi C, Zhu H, Xi Q, Zhang J, Zhao X, Gu M. MiR-204-5p suppresses cell proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma. Biochem Biophys Res Commun. 2015;457:621–626. doi: 10.1016/j.bbrc.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Jin X, Zhang Q, Zhang G, Deng X, Ma L. Decreased expression of miR-204 is associated with poor prognosis in patients with breast cancer. Int J Clin Exp Pathol. 2014;7:3287–3292. [PMC free article] [PubMed] [Google Scholar]
- 38.Mao J, Zhang M, Zhong M, Zhang Y, Lv K. MicroRNA-204, a direct negative regulator of ezrin gene expression, inhibits glioma cell migration and invasion. Mol Cell Biochem. 2014;396:117–128. doi: 10.1007/s11010-014-2148-6. [DOI] [PubMed] [Google Scholar]
- 39.Wu D, Niu X, Pan H, Zhou Y, Qu P, Zhou J. MicroRNA-335 is downregulated in bladder cancer and inhibits cell growth, migration and invasion via targeting ROCK1. Mol Med Rep. 2016;13:4379–4385. doi: 10.3892/mmr.2016.5055. [DOI] [PubMed] [Google Scholar]
- 40.Bindels S, Mestdagt M, Vandewalle C, Jacobs N, Volders L, Noël A, van Roy F, Berx G, Foidart JM, Gilles C. Regulation of vimentin by SIP1 in human epithelial breast tumor cells. Oncogene. 2006;25:4975–4985. doi: 10.1038/sj.onc.1209511. [DOI] [PubMed] [Google Scholar]
- 41.Kurashige J, Kamohara H, Watanabe M, Hiyoshi Y, Iwatsuki M, Tanaka Y, Kinoshita K, Saito S, Baba Y, Baba H. MicroRNA-200b regulates cell proliferation, invasion, and migration by directly targeting ZEB2 in gastric carcinoma. Ann Surg Oncol. 2012;19:S656–S664. doi: 10.1245/s10434-012-2217-6. (Suppl 3) [DOI] [PubMed] [Google Scholar]
- 42.Chu PY, Hu FW, Yu CC, Tsai LL, Yu CH, Wu BC, Chen YW, Huang PI, Lo WL. Epithelial-mesenchymal transition transcription factor ZEB1/ZEB2 co-expression predicts poor prognosis and maintains tumor-initiating properties in head and neck cancer. Oral Oncol. 2013;49:34–41. doi: 10.1016/j.oraloncology.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Qi S, Song Y, Peng Y, Wang H, Long H, Yu X, Li Z, Fang L, Wu A, Luo W, et al. ZEB2 mediates multiple pathways regulating cell proliferation, migration, invasion, and apoptosis in glioma. PLoS One. 2012;7:e38842. doi: 10.1371/journal.pone.0038842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai MY, Luo RZ, Chen JW, Pei XQ, Lu JB, Hou JH, Yun JP. Overexpression of ZEB2 in peritumoral liver tissue correlates with favorable survival after curative resection of hepatocellular carcinoma. PLoS One. 2012;7:e32838. doi: 10.1371/journal.pone.0032838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Q, Guo R, Lin M, Zhou B, Wang Y. MicroRNA-200a inhibits CD133/1+ ovarian cancer stem cells migration and invasion by targeting E-cadherin repressor ZEB2. Gynecol Oncol. 2011;122:149–154. doi: 10.1016/j.ygyno.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 46.Gemmill RM, Roche J, Potiron VA, Nasarre P, Mitas M, Coldren CD, Helfrich BA, Garrett-Mayer E, Bunn PA, Drabkin HA. ZEB1-responsive genes in non-small cell lung cancer. Cancer Lett. 2011;300:66–78. doi: 10.1016/j.canlet.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z, Sun B, Li Y, Zhao X, Zhao X, Gu Q, An J, Dong X, Liu F, Wang Y. ZEB2 promotes vasculogenic mimicry by TGF-β1 induced epithelial-to-mesenchymal transition in hepatocellular carcinoma. Exp Mol Pathol. 2015;98:352–359. doi: 10.1016/j.yexmp.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 48.Du Y, Wang L, Wu H, Zhang Y, Wang K, Wu D. MicroRNA-141 inhibits migration of gastric cancer by targeting zinc finger E-box-binding homeobox 2. Mol Med Rep. 2015;12:3416–3422. doi: 10.3892/mmr.2015.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou J, Xie M, Shi Y, Luo B, Gong G, Li J, Wang J, Zhao W, Zi Y, Wu X, Wen J. MicroRNA-153 functions as a tumor suppressor by targeting SET7 and ZEB2 in ovarian cancer cells. Oncol Rep. 2015;34:111–120. doi: 10.3892/or.2015.3952. [DOI] [PubMed] [Google Scholar]
- 50.Chen Z, Tang ZY, He Y, Liu LF, Li DJ, Chen X. miRNA-205 is a candidate tumor suppressor that targets ZEB2 in renal cell carcinoma. Oncol Res Treat. 2014;37:658–664. doi: 10.1159/000368792. [DOI] [PubMed] [Google Scholar]